Preparation and Characterization of Thermo-Responsive Rod-Coil Diblock Copolymers
Abstract
:1. Introduction
2. Experimental
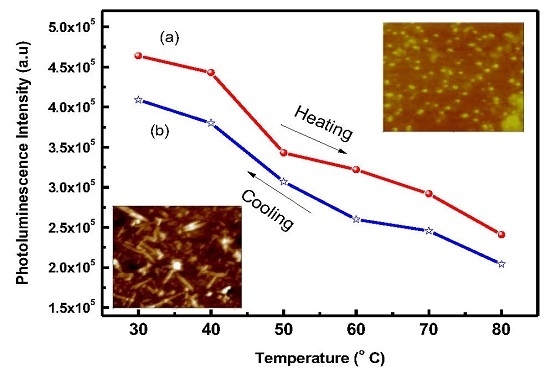
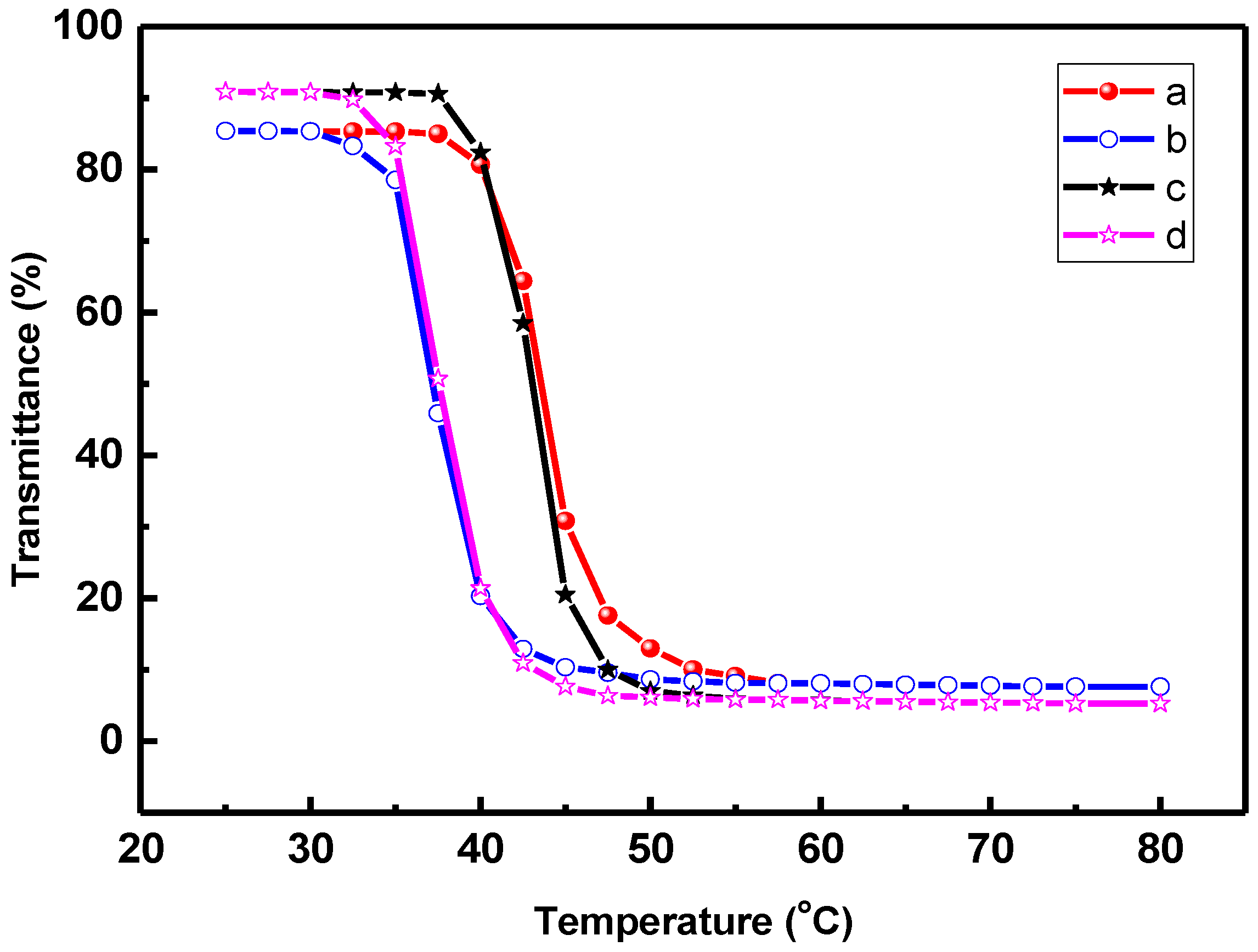
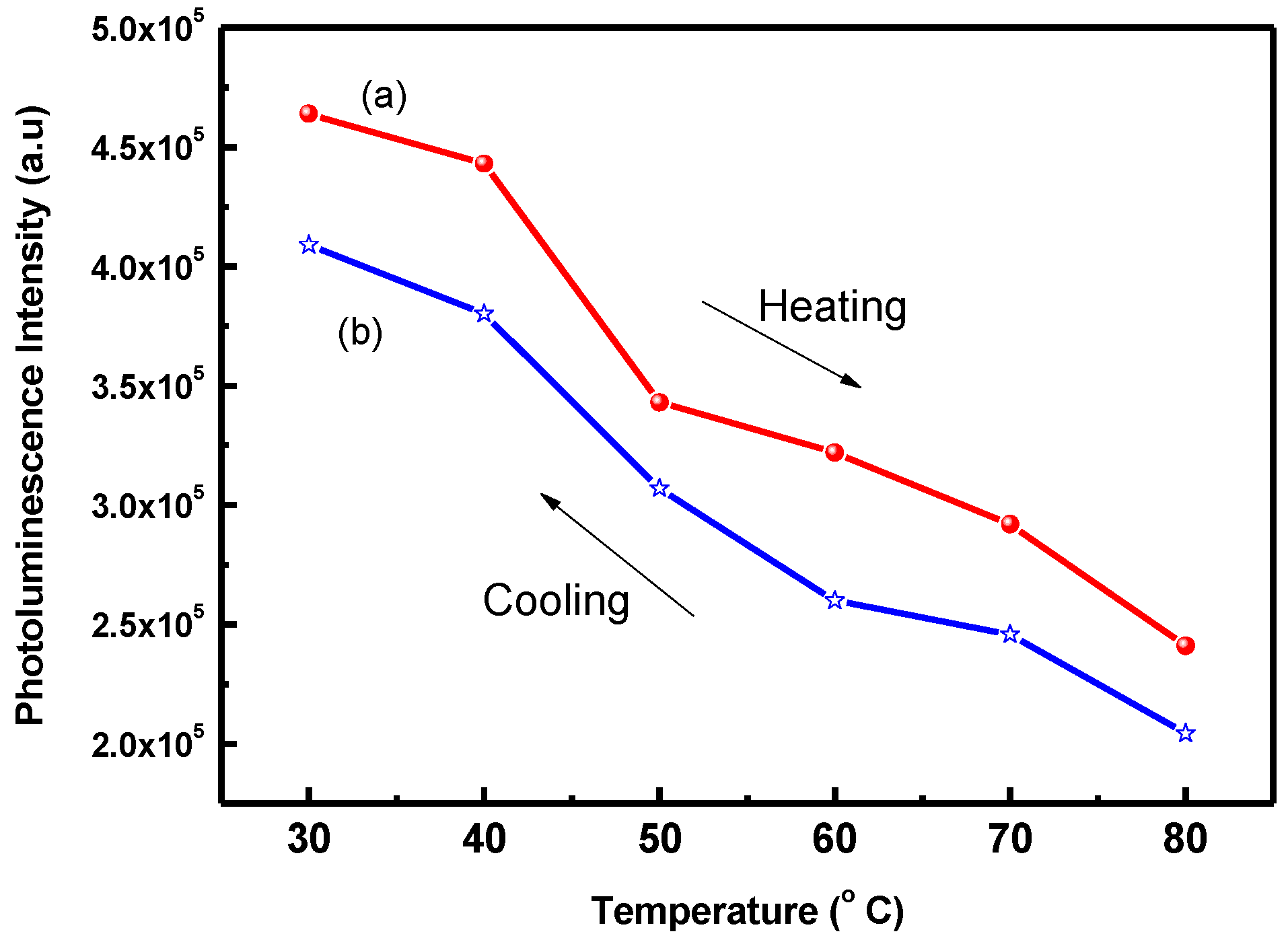
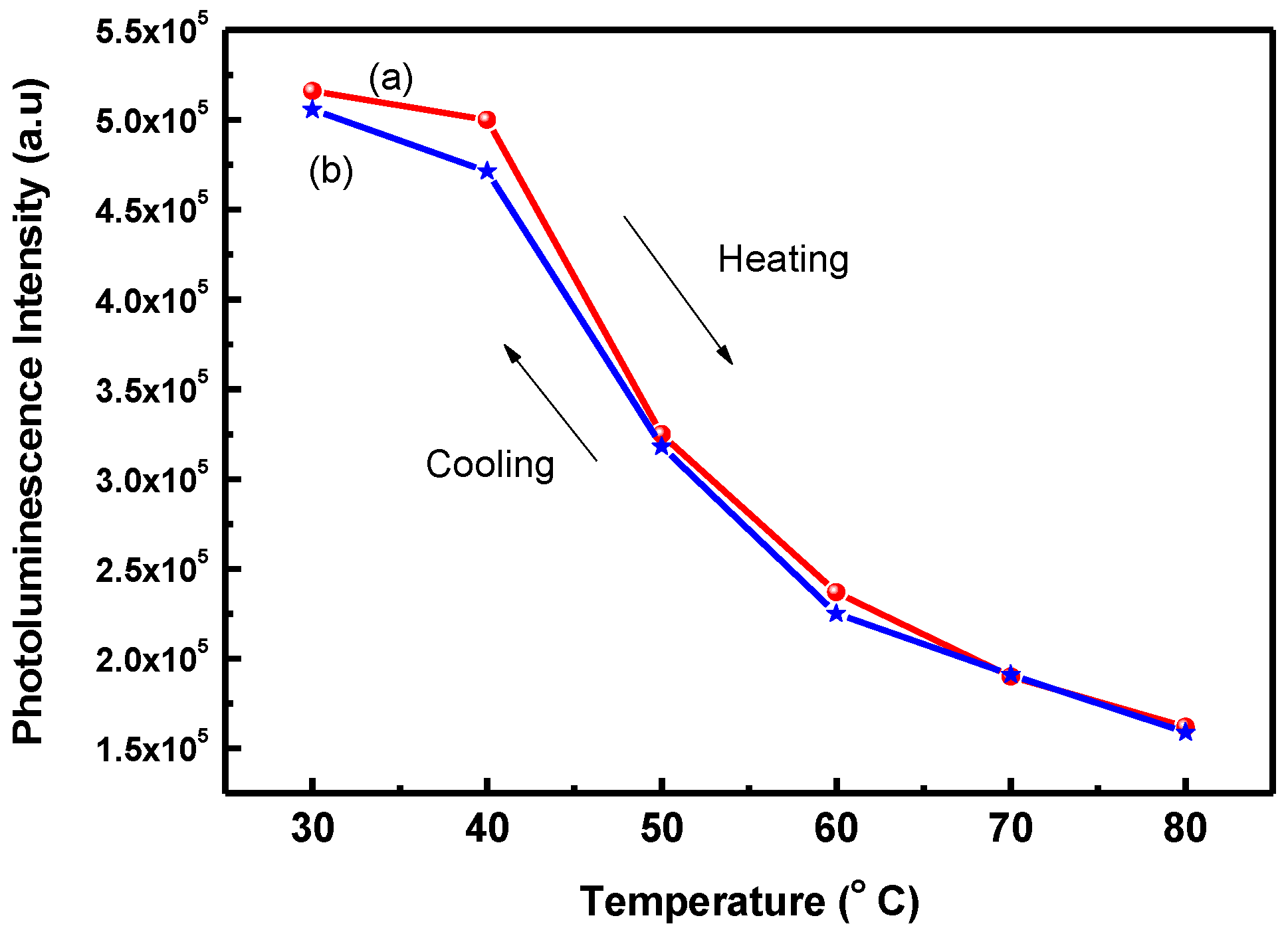
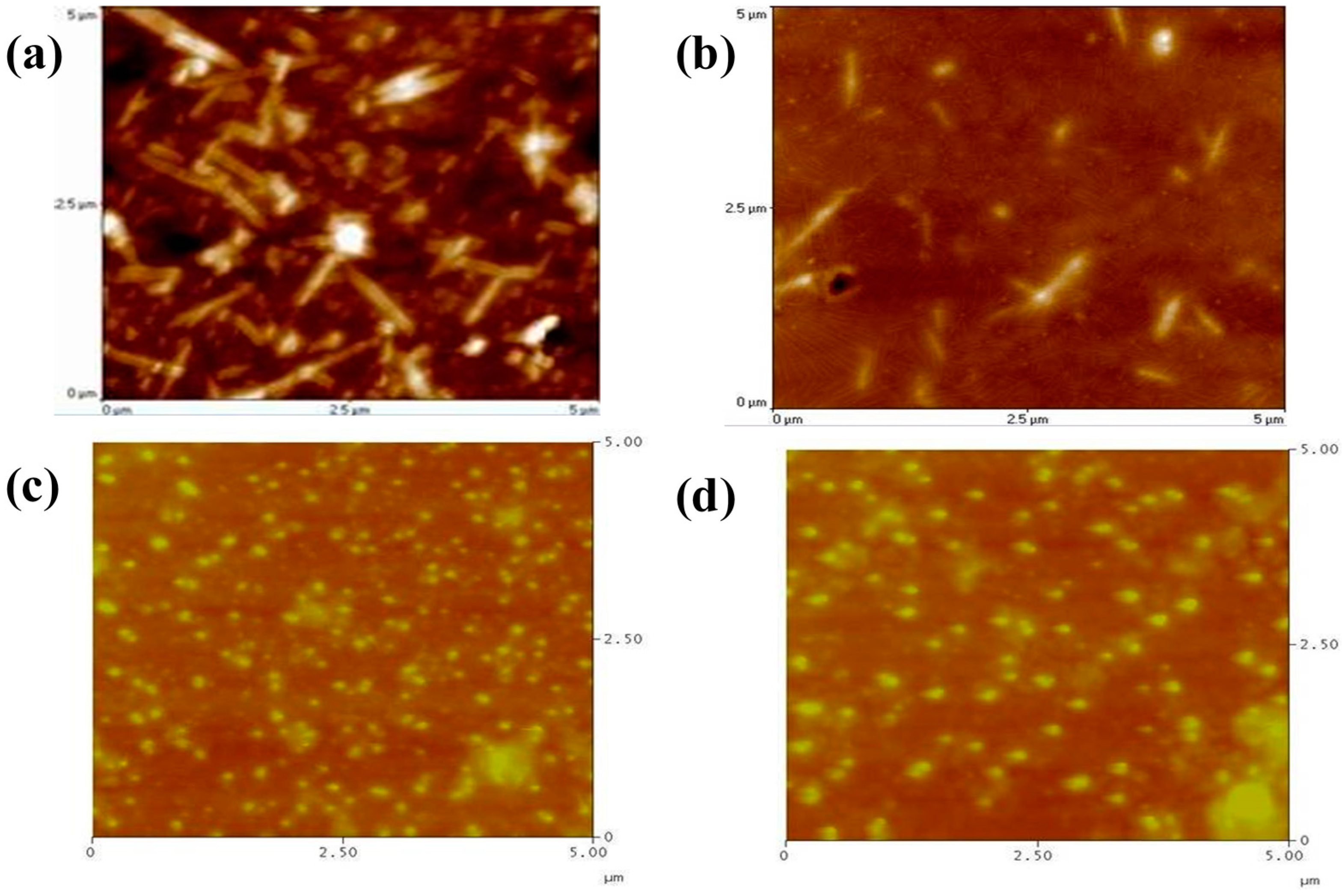
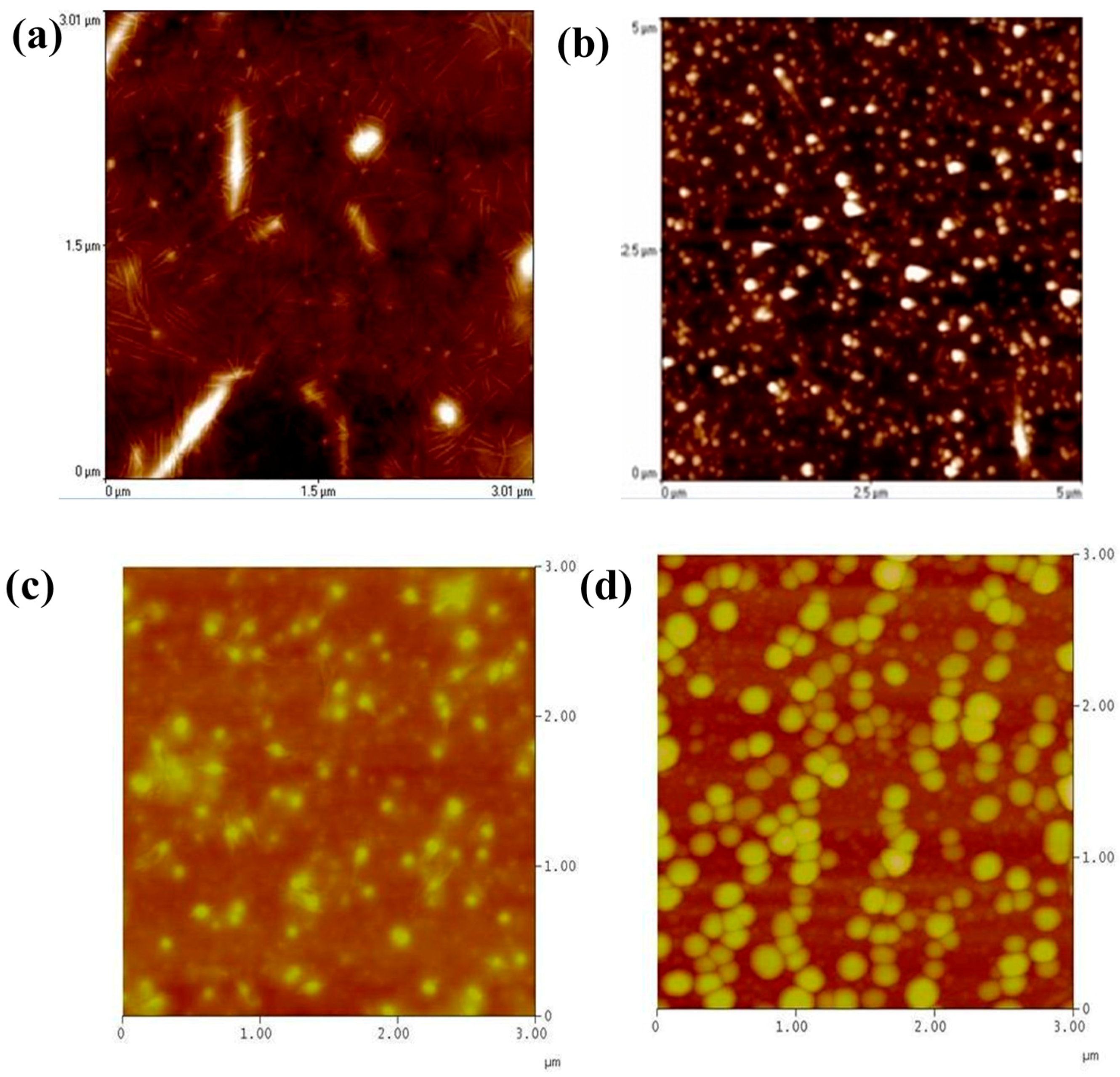
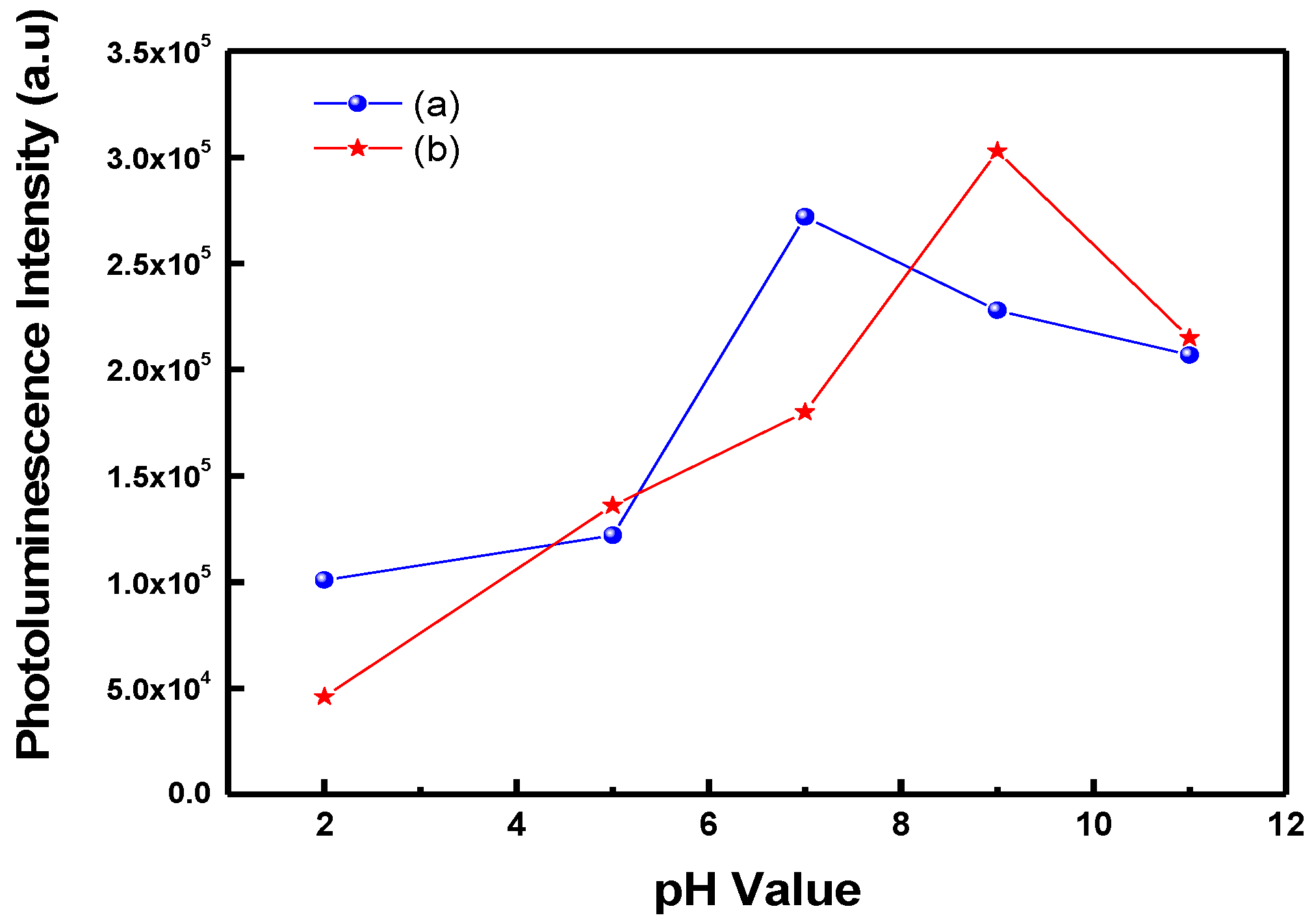
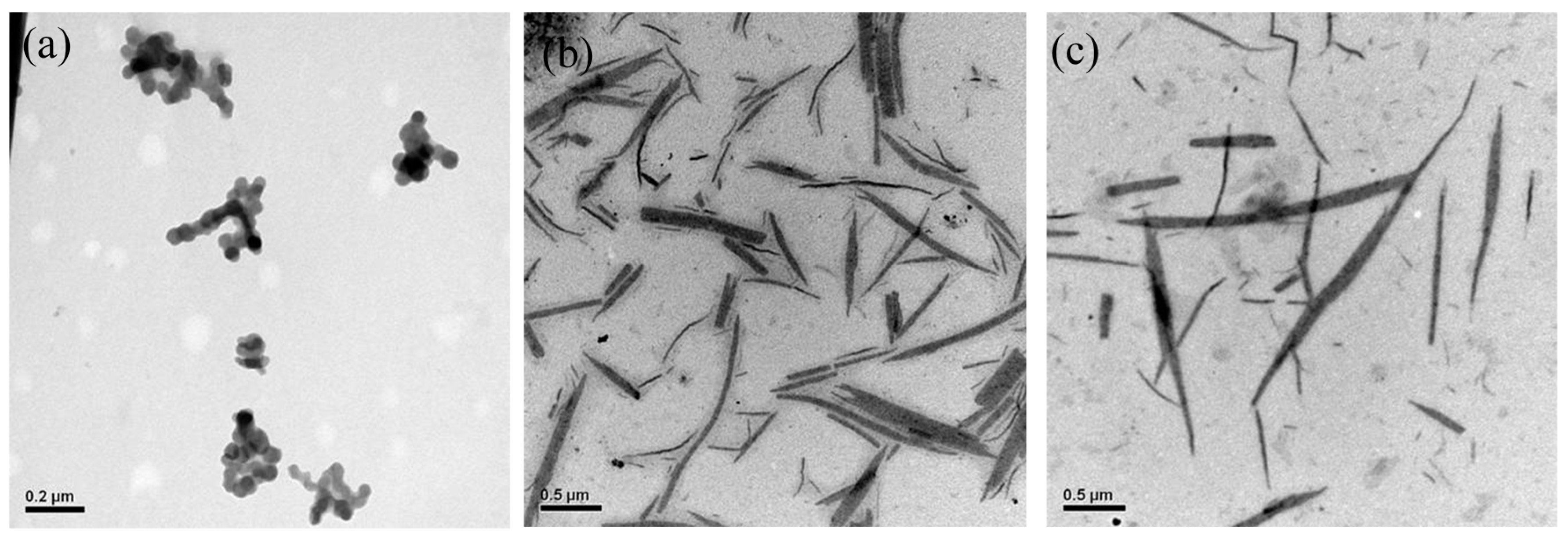
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Véronique, G.; Patrick, L.; Fanny, R.; Nicolas, L.; Cyril, B.; Christoph, H.B.; Sabine, L.; Denis, V.A.; Dimitri, A.I.; Georges, H.; et al. Microstructure and Optoelectronic Properties of P3HT–b–P4VP/PCBM Blends: Impact of PCBM on the Copolymer Self-Assembly. Macromolecular 2013, 46, 8824–8831. [Google Scholar]
- Cheng, L.L.; Chia, H.L.; Chi, C.K.; Sung, T.L.; Wen, C.C. Conjugated rod-coil block copolymers: Synthesis, morphology, photophysical properties, and stimuli-responsive applications. Prog. Polym. Sci. 2011, 36, 603–637. [Google Scholar]
- Wen, C.W.; Ching, Y.C.; Wen, Y.L.; Wen, C.C. Stimuli-responsive conjugated rod-coil block copolymers: Synthesis, morphology, and applications. Polymer 2015, 65, A1–A16. [Google Scholar]
- Dian, H.L.; Chia, H.L.; Ching, Y.C.; Jung, C.H.; Yu, C.C.; Wei, T.C.; Wen, C.C. Poly[2,7-(9,9-dihexylfluorene)]-block-Poly(2-vinylpyridine) Rod-Coil Star-block Copolymers: Synthesis, Micellar Structures, and Photophysical Properties. Macromol. Chem. Phys. 2011, 212, 297–304. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Hsu, C.Y.; Li, G.Y. Synthesis and Morphological Transformation of Conjugated Amphiphilic Diblock Copolymers in Mixed Solvents. J. Nanomater. 2013, 2013. [Google Scholar] [CrossRef]
- Kumari, P.; Bera, M.K.; Malik, S.; Kuila, B.K. Amphiphilic and thermo-responsive Conjugated Block Copolymer with Its Solvent Dependent Optical and Photoluminescence Properties: Toward Sensing Applications. ACS Appl. Mater. Interfaces 2015, 7, 12348–12354. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Yang, Y.L.; Yen, W.C.; Su, W.F.; Dai, C.A. Solution self-assembly and phase transformations of form II crystals in nanoconfined poly(3-hexyl thiophene) based rod-coil block copolymers. Nanoscale 2014, 6, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Vriezema, D.M.; Hoogboom, J.; Velonia, K.; Takazawa, K.; Christianen, P.; Maan, J.; Rowan, A.E.; Nolte, R.J. Vesicles and polymerized vesicles from thiophene-containing rod-coil block copolymers. Angew. Chem. 2003, 42, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Seo, M.; Kim, S.Y. Synthesis of Well-Defined Rod-Coil Block Copolymers Containing Trifluoromethylated Poly(phenylene oxide)s by Chain-Growth Condensation Polymerization and Atom Transfer Radical Polymerization. J. Polym. Sci. A 2010, 48, 1049–1057. [Google Scholar] [CrossRef]
- Marsitzky, D.; Brand, T.; Geerts, Y.; Klapper, M.; Müllen, K. Synthesis of rod-coil block copolymers via end-functionalized poly(p-phenylene)s. Macromol. Rapid Commun. 1998, 19, 385–389. [Google Scholar] [CrossRef]
- Schmücker, S.; Kuckling, D. Enhanced Preparation of Alkoxyamine-Functionalized Poly(p-phenylene)s and Their Use as Macroinitiators for the Synthesis of Stimuli-Responsive Coil-Rod-Coil Block Copolymers. Macromol. Chem. Phys. 2012, 213, 1725–1734. [Google Scholar] [CrossRef]
- Kakogianni, S.; Kourkouli, S.N.; Andreopoulou, A.K.; Kallitsis, J.K. A versatile approach for creating hybrid semiconducting polymer-fullerene architectures for organic electronics. J. Mater. Chem. A 2014, 2, 8110–8117. [Google Scholar] [CrossRef]
- Park, J.; Moon, M.; Seo, M.; Choi, H.; Kim, S.Y. Well-Defined Star-Shaped Rod-Coil Diblock Copolymers as a New Class of Unimolecular Micelles: Encapsulation of Guests and thermo-responsive Phase Transition. Macromolecules 2010, 43, 8304–8313. [Google Scholar] [CrossRef]
- Tung, Y.C.; Chen, W.C. Poly[2,7-(9,9-dihexylfluorene)]-block-poly[3-(trimethoxysilyl)propyl methacrylate] (PF-b-PTMSPMA) rod-coil block copolymers: Synthesis, morphology and photophysical properties in mixed solvents. React. Funct. Polym. 2009, 69, 507–518. [Google Scholar] [CrossRef]
- Yang, G.Z.; Chen, X.L.; Wang, L.M.; Shi, J.G.; Li, C.Z.; Liu, T. Synthesis and characterization of fluorene-based rod-coil liquid crystal polymers. Polym. Adv. Technol. 2009, 20, 104–110. [Google Scholar] [CrossRef]
- Yao, J.H.; Mya, K.Y.; Shen, L.; He, B.P.; Li, L.; Li, Z.H.; Chen, Z.K.; Li, X.; Loh, K.P. Fluorescent nanoparticles comprising amphiphilic rod-coil graft copolymers. Macromolecules 2008, 41, 1438–1443. [Google Scholar] [CrossRef]
- Rubatat, L.; Kong, X.; Jenekhe, S.A.; Ruokolainen, J.; Hojeij, M.; Mezzenga, R. Self-assembly of polypeptide/pi-conjugated polymer/polypeptide triblock copolymers in rod-rod-rod and coll-rod-coil conformations. Macromolecules 2008, 41, 1846–1852. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.F.; Wei, H.B.; Wan, X.H. Tunable assembly of amphiphilic rod-coil block copolymers in solution. Chem. Soc. Rev. 2013, 42, 9127–9154. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.C.; Tian, Y.; Chen, C.Y.; Lee, C.S.; Sheng, Y.J.; Chen, W.C.; Alex, K.Y.J. Theoretical and experimental studies on the surface structures of conjugated rod-coil block copolymer brushes. Langmuir 2007, 23, 2805–2814. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, X.; Wu, K.; Lu, M. Synthesis and self-assembly of a dual thermal and pH-responsive ternary graft copolymer for sustained release drug delivery. RSC Adv. 2016, 6, 2571–2581. [Google Scholar] [CrossRef]
- Jiang, F.; Chen, S.; Cao, Z.; Wang, G. A photo, temperature, and pH responsive spiropyran-functionalized polymer: Synthesis, self-assembly and controlled release. Polymer 2016, 83, 85–91. [Google Scholar] [CrossRef]
- Billing, M.; Rudolph, T.; Täuscher, E.; Beckert, R.; Schacher, F.H. Synthesis and Complexation of Well-Defined Labeled Poly(N,N-dimethylaminoethyl methacrylate)s (PDMAEMA). Polymers 2015, 7, 2478–2493. [Google Scholar] [CrossRef]
- Lin, S.T.; Tung, Y.C.; Chen, W.C. Synthesis, structures and multifunctional sensory properties of poly[2,7-(9,9-dihexylfluorene)]-block-poly[2-(dimethylamino)ethyl methacrylate] rod-coil diblock copolymers. J. Mater. Chem. 2008, 18, 3985–3992. [Google Scholar] [CrossRef]
- Huang, K.K.; Fang, Y.K.; Hsu, J.C.; Kuo, C.C.; Chang, W.H.; Chen, W.C. Synthesis, Micellar Structures, and Multifunctional Sensory Properties of Poly(3-hexylthiophene)-block-poly(2-(dimethylamino)ethyl methacrylate) Rod-Coil Diblock Copolymers. J. Polym. Sci. A 2011, 49, 147–155. [Google Scholar] [CrossRef]
- Huang, K.K.; Fang, Y.K.; Hsu, J.C.; Kuo, C.C.; Chang, W.H.; Chen, W.C. Thermoresponsive Luminescent Electrospun Fibers Prepared From Poly(DMAEMA-co-SA-co-StFl) Multifunctional Random Copolymers. ACS Appl. Mater. Interfaces 2010, 2, 3340–3347. [Google Scholar]
- Bruce, C.; Javakhishvili, I.; Fogelström, L.; Carlmark, A.; Hvilsted, S.; Malmström, E. Well-defined ABA- and BAB-type block copolymers of PDMAEMA and PCL. RSC Adv. 2014, 4, 25809–25818. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Li, Y.; Zhai, X.; Xu, B.; Cao, Z.; Liu, W. Polycation-b-Polyzwitterion Copolymer Grafted Luminescent Carbon Dots as a Multifunctional Platform for Serum-Resistant Gene Delivery and Bioimaging. ACS Appl. Mater. Interfaces 2014, 6, 20487–20497. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Fan, Q.L.; Chua, S.J.; Huang, W. Synthesis of conjugated—Ionic block copolymers by controlled radical polymerization. Macromolecules 2003, 36, 304–310. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, J.; Li, W.; Wang, W.; Liu, C.; Griffith, M.; Liu, W. Cationic polymer brush grafted-nanodiamond via atom transfer radical polymerization for enhanced gene delivery and bioimaging. J. Mater. Chem. 2011, 21, 7755–7764. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, X.; Fan, Q.; Hu, W.; Huang, W. Conjugated polyelectrolyte brushes with extremely high charge density for improved energy transfer and fluorescence quenching applications. Polym. Chem. 2011, 2, 2369–2377. [Google Scholar] [CrossRef]
- Gao, X.; Lu, P.; Ma, Y. Ultrasound-assisted Suzuki coupling reaction for rapid synthesis of polydihexylfluorene. Polymer 2014, 55, 3083–3086. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, J.; Bao, F.; Jiang, S.; Zhang, X.; Ma, R. Covalent functionalization of graphene with polythiophene through a Suzuki coupling reaction. RSC Adv. 2015, 5, 42754–42761. [Google Scholar] [CrossRef]
- Lin, S.T.; Fuchise, K.; Chen, Y.; Sakai, R.; Satoh, T.; Kakuchi, T.; Chen, W.C. Synthesis, thermomorphic characteristics, and fluorescent properties of poly[2,7-(9,9-dihexylfluorene)]-block-poly(N-isopropylacrylamide)-block-poly(N-hydroxyethylacrylamide) rod-coil-coil triblock copolymers. Soft Matter 2009, 5, 3761–3770. [Google Scholar] [CrossRef]
- Sun, B.; Lin, Y.; Wu, P.; Siesler, H.W. A FTIR and 2D-IR spectroscopic study on the microdynamics phase separation mechanism of the poly(N-isopropylacrylamide) aqueous solution. Macromolecules 2008, 41, 1512–1520. [Google Scholar] [CrossRef]
- Han, X.; Zhang, X.; Zhu, H.; Yin, Q.; Liu, H.; Hu, Y. Effect of Composition of PDMAEMA-b-PAA Block Copolymers on Their pH- and Temperature-Responsive Behaviors. Langmuir 2013, 29, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.L.; Wu, W.B.; Zhu, L.; Zhu, W.Y.; Dai, H.Q. pH-Responsive Cellulose Papers Prepared via ARGET ATRP Grafting of Poly(2-(dimethylamino)ethyl methacrylate). Acta Polym. Sin. 2015, 10, 1151–1157. [Google Scholar]
- Liu, M.; Zhao, L.; Li, S.; Ye, H.; An, H.; Zhang, Y. pH-responsive ethylene vinyl alcohol copolymer membrane based on porphyrin supramolecular self-assembly. RSC Adv. 2016, 6, 10704–10712. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.-Y.; Chien, W.-C.; Tsai, C.-L. Preparation and Characterization of Thermo-Responsive Rod-Coil Diblock Copolymers. Polymers 2017, 9, 340. https://doi.org/10.3390/polym9080340
Yu Y-Y, Chien W-C, Tsai C-L. Preparation and Characterization of Thermo-Responsive Rod-Coil Diblock Copolymers. Polymers. 2017; 9(8):340. https://doi.org/10.3390/polym9080340
Chicago/Turabian StyleYu, Yang-Yen, Wen-Chen Chien, and Chia-Liang Tsai. 2017. "Preparation and Characterization of Thermo-Responsive Rod-Coil Diblock Copolymers" Polymers 9, no. 8: 340. https://doi.org/10.3390/polym9080340