Isolation and Characterization of Cellulose Nanocrystals from Oil Palm Mesocarp Fiber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cellulose Fibers
2.3. Extraction of Cellulose Nanocrystals
2.4. Characterizations
2.4.1. Chemical Composition of Oil Palm Mesocarp Fiber
2.4.2. Fourier Transform Infrared (FTIR) Spectroscopy
2.4.3. X-ray Diffraction (XRD) Analysis
2.4.4. Thermogravimetric Analysis (TGA)
2.4.5. Scanning Electron Microscopy (SEM)
2.4.6. Transmission Electron Microscopy (TEM)
3. Results
3.1. Chemical Composition of Oil Palm Mesocarp Fiber
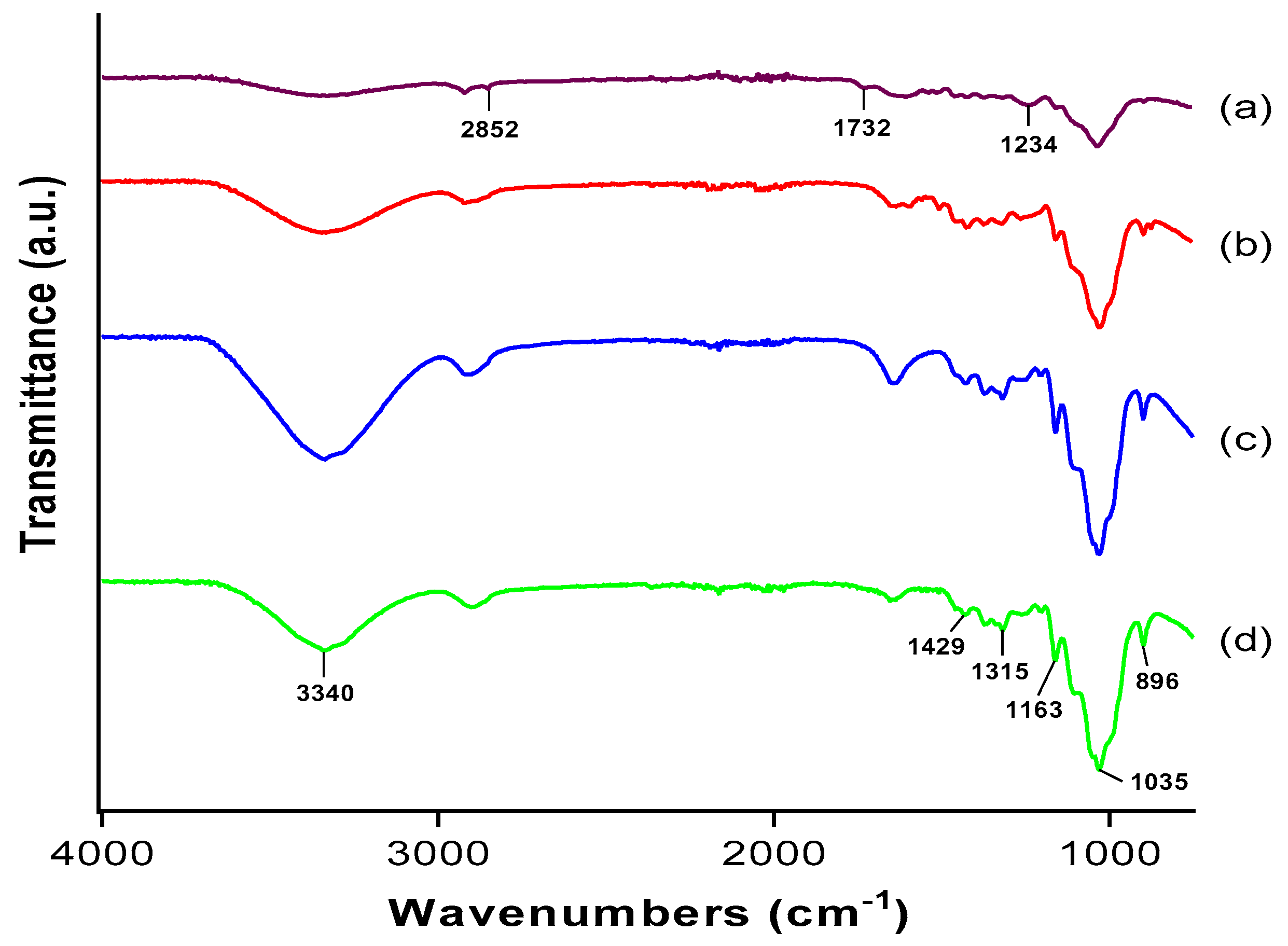
3.2. Fourier Transform Infrared (FTIR) Analysis
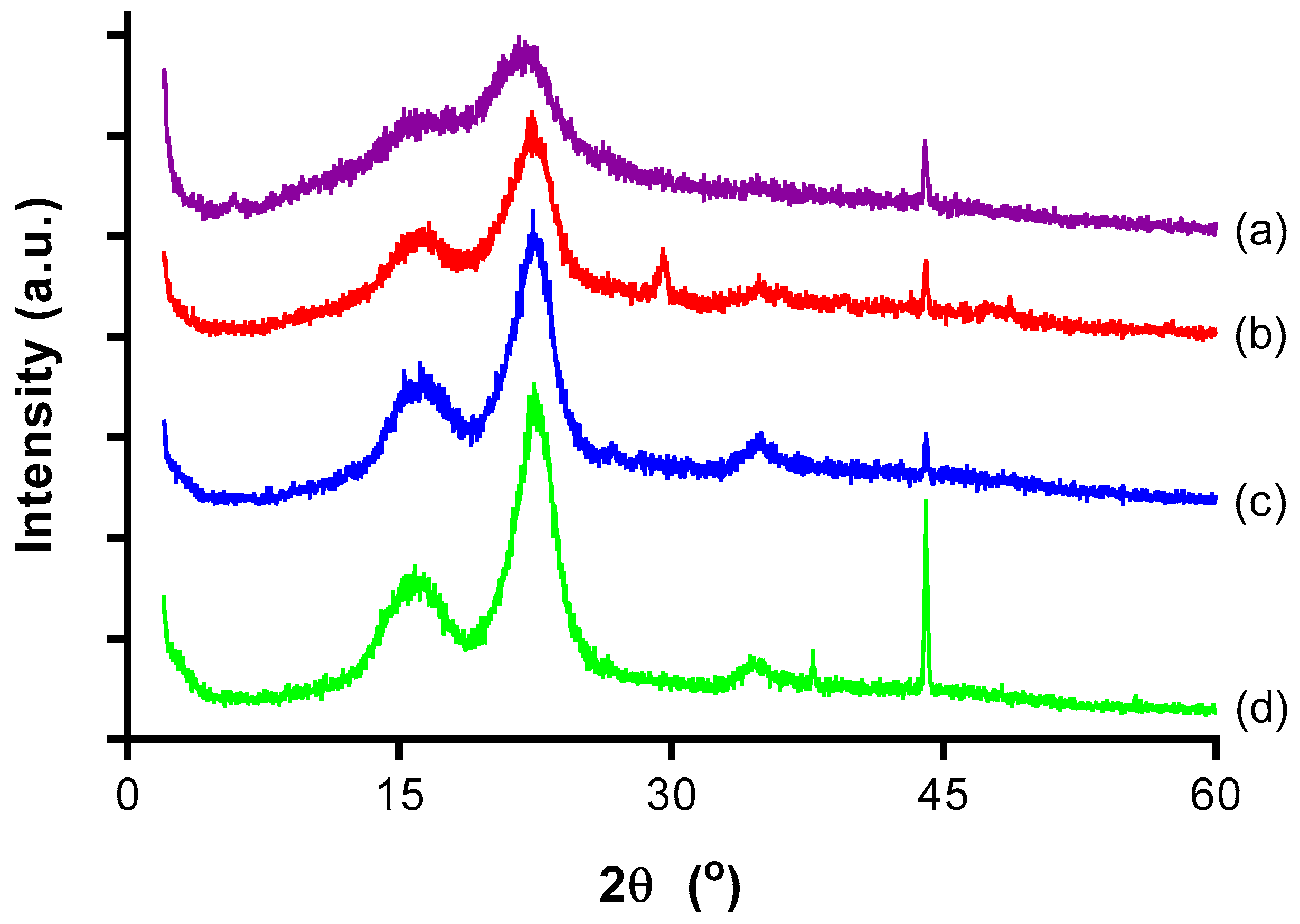
3.3. X-ray Diffraction (XRD) Analysis
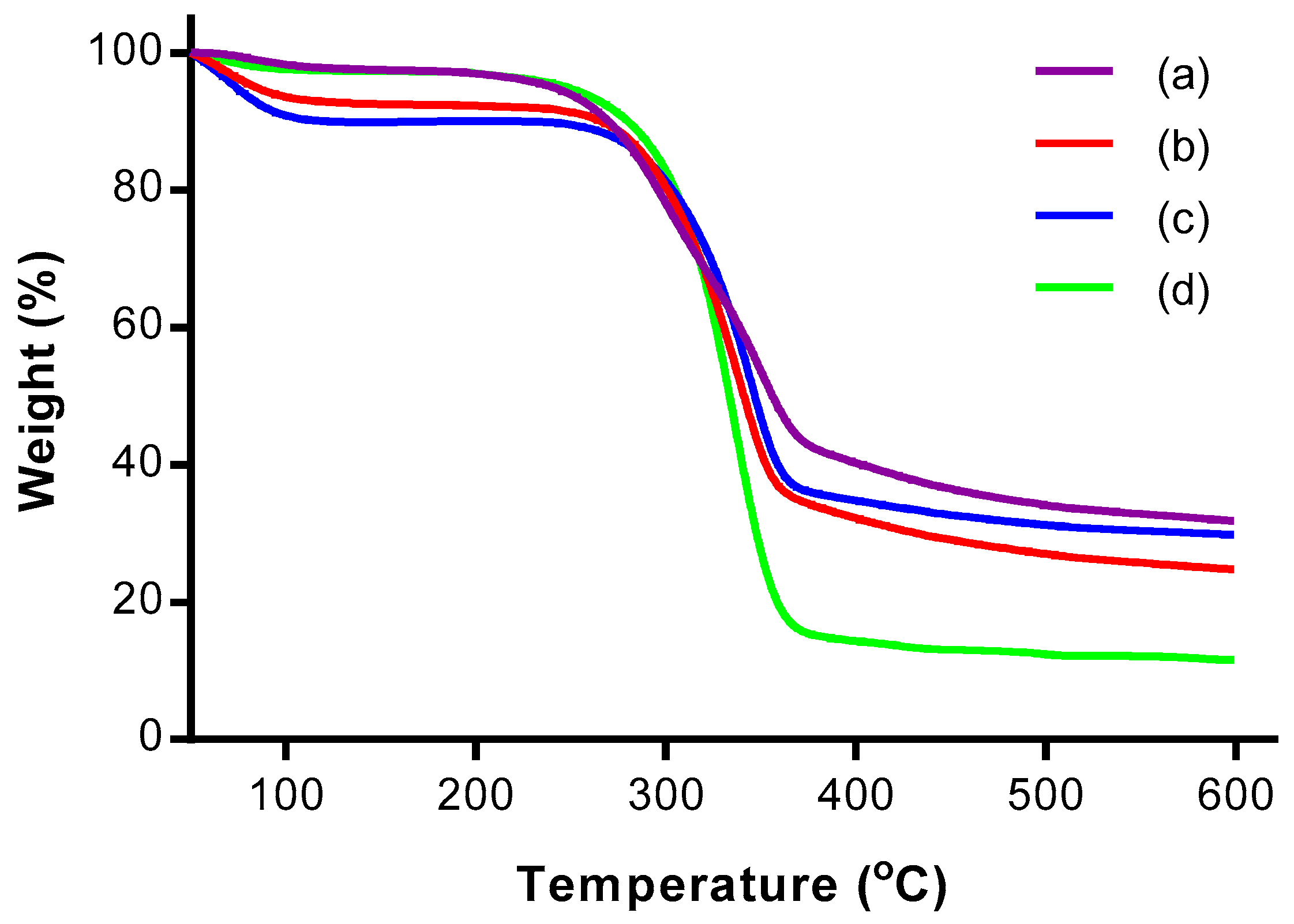
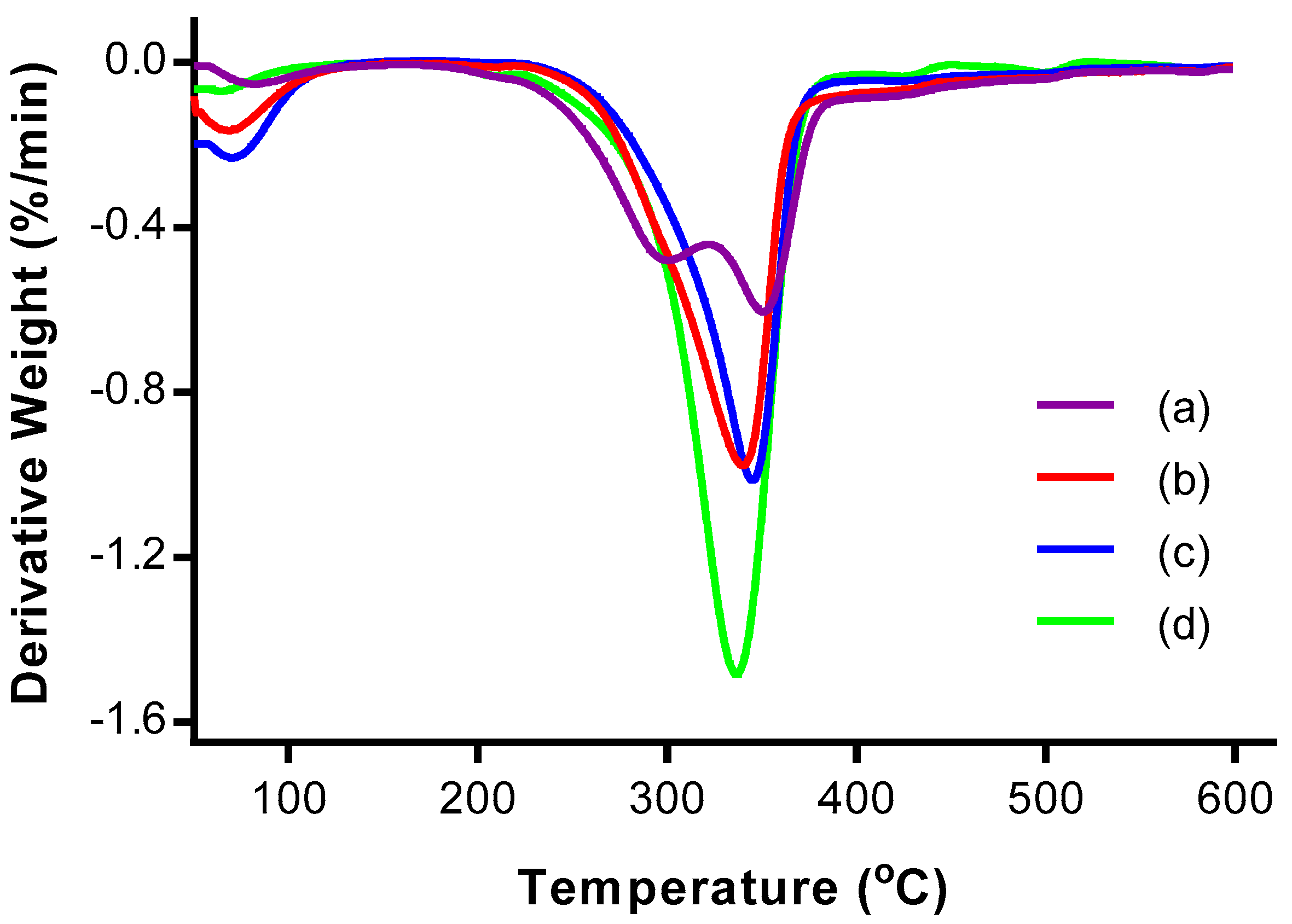
3.4. Thermogravimetric Analysis (TGA)
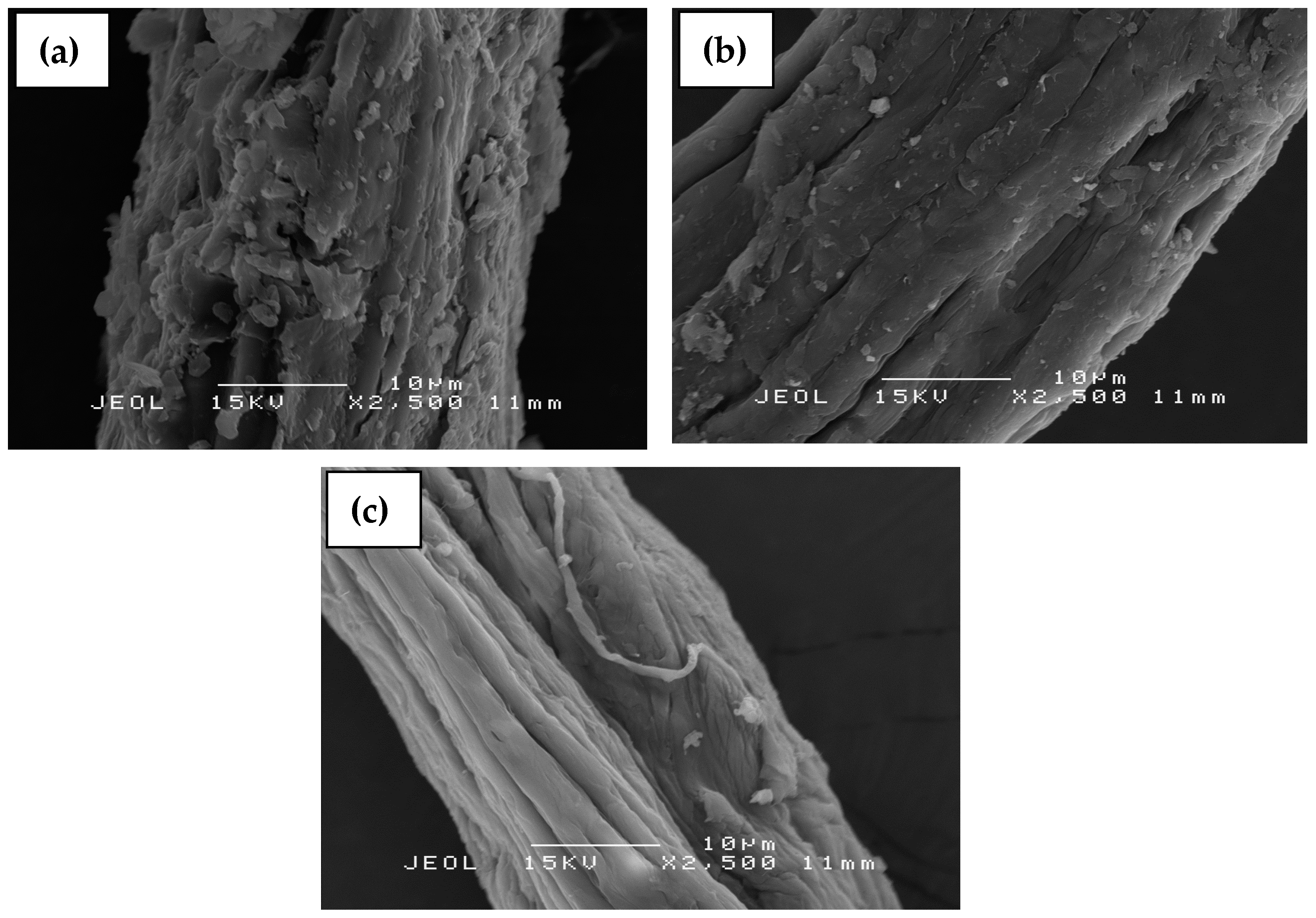
3.5. Scanning Electron Microscopy (SEM)
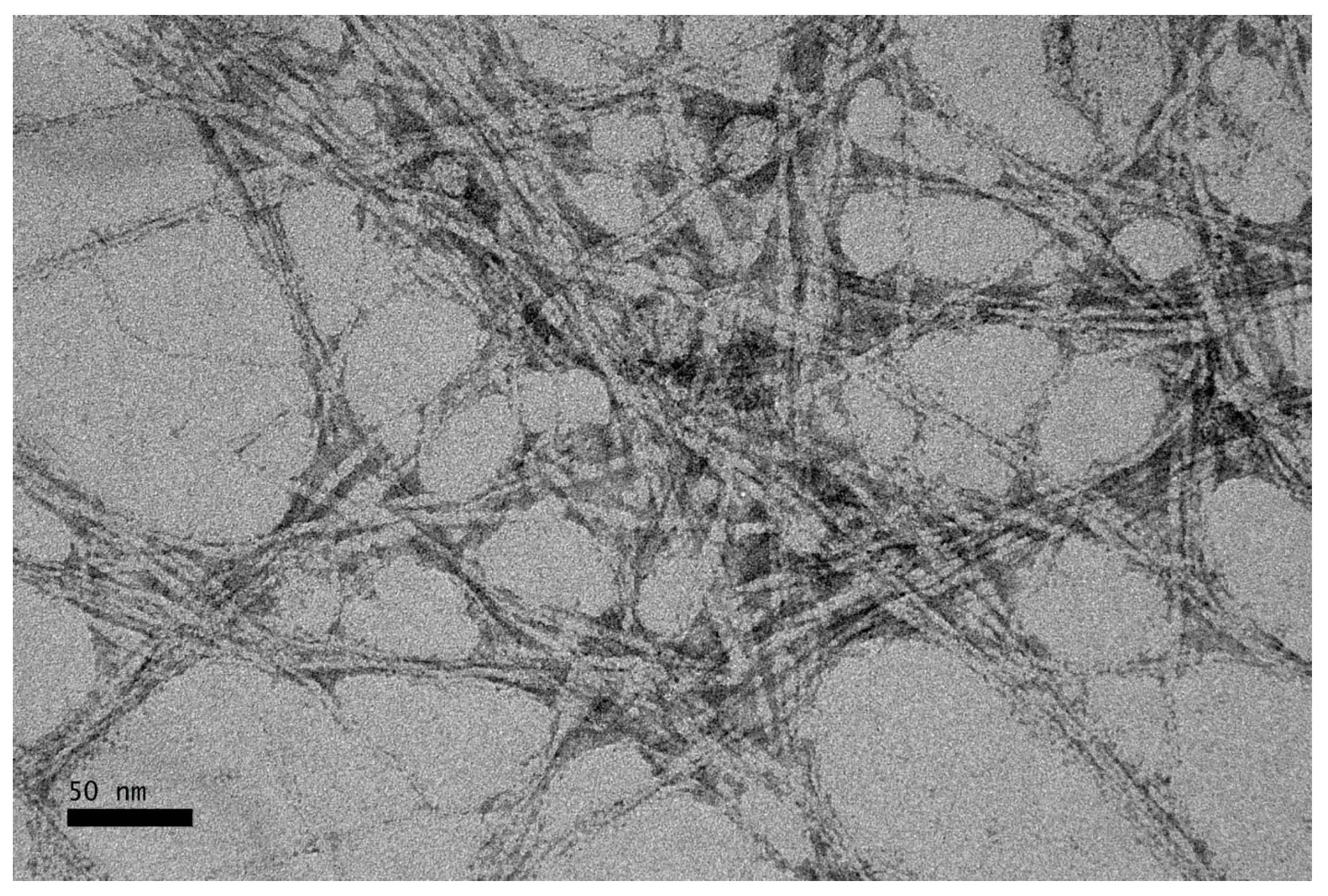
3.6. Transmission Electrion Microscopy (TEM)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Awalludin, M.F.; Sulaiman, O.; Hashim, R.; Nadhari, W.N.A.W. An overview of the oil palm industry in malaysia and its waste utilization through thermochemical conversion, specifically via liquefaction. Renew. Sustain. Energy Rev. 2015, 50, 1469–1484. [Google Scholar] [CrossRef]
- Koay, A. Green Wealth in Oil Palm. Available online: http://www.thestar.com.my/news/environment/2014/03/24/green-wealth-in-oil-palm/ (accessed on 24 March 2014).
- Lee, H.V.; Hamid, S.B.A.; Zain, S.K. Conversion of lignocellulosic biomass to nanocellulose: Structure and chemical process. Sci. World J. 2014, 2014, 20. [Google Scholar] [CrossRef] [PubMed]
- Kelly-Yong, T.L.; Lee, K.T.; Mohamed, A.R.; Bhatia, S. Potential of hydrogen from oil palm biomass as a source of renewable energy worldwide. Energy Policy 2007, 35, 5692–5701. [Google Scholar] [CrossRef]
- Nordin, N.; Ariffin, H.; Andou, Y.; Hassan, M.; Shirai, Y.; Nishida, H.; Yunus, W.; Karuppuchamy, S.; Ibrahim, N.A. Modification of oil palm mesocarp fiber characteristics using superheated steam treatment. Molecules 2013, 18, 9132–9146. [Google Scholar] [CrossRef] [PubMed]
- Saka, S.; Munusamy, M.V.; Shibata, M.; Tono, Y.; Miyafuji, H. Chemical Constituents of the Different Anatomical Parts of the Oil Palm (Elaeis Guineensis) for Their Sustainable Utilization; Natural Resources & Energy Environment JSPS-VCC Program on Environmental Science, Engineering and Ethics (Group IX): Kyoto, Japan, 2008; pp. 19–34. [Google Scholar]
- Lamaming, J.; Hashim, R.; Sulaiman, O.; Leh, C.P.; Sugimoto, T.; Nordin, N.A. Cellulose nanocrystals isolated from oil palm trunk. Carbohydr. Polym. 2015, 127, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Lani, N.S.; Ngadi, N.; Johari, A.; Jusoh, M. Isolation, characterization, and application of nanocellulose from oil palm empty fruit bunch fiber as nanocomposites. J. Nanomater. 2014, 2014, 9. [Google Scholar] [CrossRef]
- Song, Y.K.; Leng Chew, I.M.; Yaw Choong, T.S.; Tan, J.; Tan, K.W. Isolation of nanocrystalline cellulose from oil palm empty fruit bunch—A response surface methodology study. MATEC Web Conf. 2016, 60, 04009. [Google Scholar] [CrossRef]
- Jonoobi, M.; Khazaeian, A.; Tahir, P.M.; Azry, S.S.; Oksman, K. Characteristics of cellulose nanofibers isolated from rubberwood and empty fruit bunches of oil palm using chemo-mechanical process. Cellulose 2011, 18, 1085–1095. [Google Scholar] [CrossRef]
- Fahma, F.; Iwamoto, S.; Hori, N.; Iwata, T.; Takemura, A. Isolation, preparation, and characterization of nanofibers from oil palm empty-fruit-bunch (opefb). Cellulose 2010, 17, 977–985. [Google Scholar] [CrossRef]
- Mazlita, Y.; Lee, H.V.; Hamid, S.B.A. Preparation of cellulose nanocrystals bio-polymer from agro- industrial wastes: Separation and characterization. Polym. Polym. Compos. 2016, 24, 719–728. [Google Scholar]
- Siqueira, G.; Abdillahi, H.; Bras, J.; Dufresne, A. High reinforcing capability cellulose nanocrystals extracted from syngonanthus nitens (capim dourado). Cellulose 2010, 17, 289–298. [Google Scholar] [CrossRef]
- Then, Y.Y.; Ibrahim, N.A.; Zainuddin, N.; Ariffin, H.; Wan Yunus, W.M.Z.; Chieng, B.W. Surface modifications of oil palm mesocarp fiber by superheated steam, alkali, and superheated steam-alkali for biocomposite applications. BioResources 2014, 9, 7467–7483. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Mamun, A.A.; Volk, J. Physical, chemical and surface properties of wheat husk, rye husk and soft wood and their polypropylene composites. Compos. Part A 2010, 41, 480–488. [Google Scholar] [CrossRef]
- Lamaming, J.; Hashim, R.; Leh, C.P.; Sulaiman, O.; Sugimoto, T.; Nasir, M. Isolation and characterization of cellulose nanocrystals from parenchyma and vascular bundle of oil palm trunk (elaeis guineensis). Carbohydr. Polym. 2015, 134, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Sreekala, M.S.; Kumaran, M.G.; Thomas, S. Oil palm fibers: Morphology, chemical composition, surface modification, and mechanical properties. J. Appl. Polym. Sci. 1997, 66, 821–835. [Google Scholar] [CrossRef]
- Souza, N.F.; Pinheiro, J.A.; Brígida, A.I.S.; Morais, J.P.S.; de Souza Filho, M.d.s.M.; de Freitas Rosa, M. Fibrous residues of palm oil as a source of green chemical building blocks. Ind. Crops Prod. 2016, 94, 480–489. [Google Scholar] [CrossRef]
- Do Nascimento, D.M.; Almeida, J.S.; do S. Vale, M.; Leitão, R.C.; Muniz, C.R.; de Figueirêdo, M.C.B.; Morais, J.P.S.; de F. Rosa, M. A comprehensive approach for obtaining cellulose nanocrystal from coconut fiber. Part I: Proposition of technological pathways. Ind. Crops Prod. 2016, 93, 66–75. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Fatah, I.; Khalil, H.; Hossain, M.; Aziz, A.; Davoudpour, Y.; Dungani, R.; Bhat, A. Exploration of a chemo-mechanical technique for the isolation of nanofibrillated cellulosic fiber from oil palm empty fruit bunch as a reinforcing agent in composites materials. Polymers 2014, 6, 2611. [Google Scholar] [CrossRef]
- Sonia, A.; Dasan, K.P. Chemical, morphology and thermal evaluation of cellulose microfibers obtained from hibiscus sabdariffa. Carbohydr. Polym. 2013, 92, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Moriana, R.; Vilaplana, F.; Ek, M. Cellulose nanocrystals from forest residues as reinforcing agents for composites: A study from macro- to nano-dimensions. Carbohydr. Polym. 2016, 139, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Kallel, F.; Bettaieb, F.; Khiari, R.; García, A.; Bras, J.; Chaabouni, S.E. Isolation and structural characterization of cellulose nanocrystals extracted from garlic straw residues. Ind. Crops Prod. 2016, 87, 287–296. [Google Scholar] [CrossRef]
- Norul Izani, M.A.; Paridah, M.T.; Anwar, U.M.K.; Mohd Nor, M.Y.; H’ng, P.S. Effects of fiber treatment on morphology, tensile and thermogravimetric analysis of oil palm empty fruit bunches fibers. Compos. Part B 2013, 45, 1251–1257. [Google Scholar] [CrossRef]
- Liu, H.; Liu, D.; Yao, F.; Wu, Q. Fabrication and properties of transparent polymethylmethacrylate/cellulose nanocrystals composites. Bioresour. Technol. 2010, 101, 5685–5692. [Google Scholar] [CrossRef] [PubMed]
- Flauzino Neto, W.P.; Silvério, H.A.; Dantas, N.O.; Pasquini, D. Extraction and characterization of cellulose nanocrystals from agro-industrial residue—Soy hulls. Ind. Crops Prod. 2013, 42, 480–488. [Google Scholar] [CrossRef]

| OPMF | Treated OPMF | |
|---|---|---|
| Cellulose (%) | 32.22 ± 1.54 | 81.11 ± 0.45 |
| Hemicellulose (%) | 31.62 ± 0.46 | 12.65 ± 0.14 |
| Lignin (%) | 23.89 ± 1.12 | 5.09 ± 1.23 |
| Samples | 2θ (Amorphous) (°) | 2θ (002) (°) | CrI (%) | ||
|---|---|---|---|---|---|
| Degree | Intensity (Iam) | Degree | Intensity (I002) | ||
| OPMF | 18.76 | 298 | 21.58 | 538 | 44.61 |
| Alkaline treated OPMF | 18.44 | 214 | 22.28 | 612 | 65.03 |
| Bleached OPMF | 18.96 | 198 | 22.36 | 786 | 74.81 |
| CNC | 18.58 | 190 | 22.46 | 856 | 77.80 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chieng, B.W.; Lee, S.H.; Ibrahim, N.A.; Then, Y.Y.; Loo, Y.Y. Isolation and Characterization of Cellulose Nanocrystals from Oil Palm Mesocarp Fiber. Polymers 2017, 9, 355. https://doi.org/10.3390/polym9080355
Chieng BW, Lee SH, Ibrahim NA, Then YY, Loo YY. Isolation and Characterization of Cellulose Nanocrystals from Oil Palm Mesocarp Fiber. Polymers. 2017; 9(8):355. https://doi.org/10.3390/polym9080355
Chicago/Turabian StyleChieng, Buong Woei, Syn Huey Lee, Nor Azowa Ibrahim, Yoon Yee Then, and Yuet Ying Loo. 2017. "Isolation and Characterization of Cellulose Nanocrystals from Oil Palm Mesocarp Fiber" Polymers 9, no. 8: 355. https://doi.org/10.3390/polym9080355






