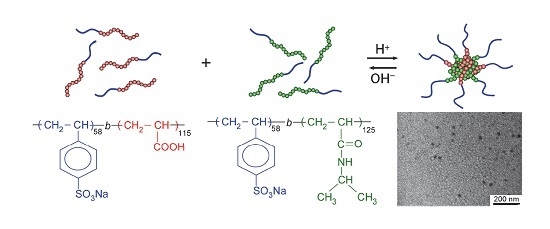
pH-Induced Association and Dissociation of Intermolecular Complexes Formed by Hydrogen Bonding between Diblock Copolymers
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of PNaSS Macro-Chain Transfer Agent (PNaSS Macro–CTA)
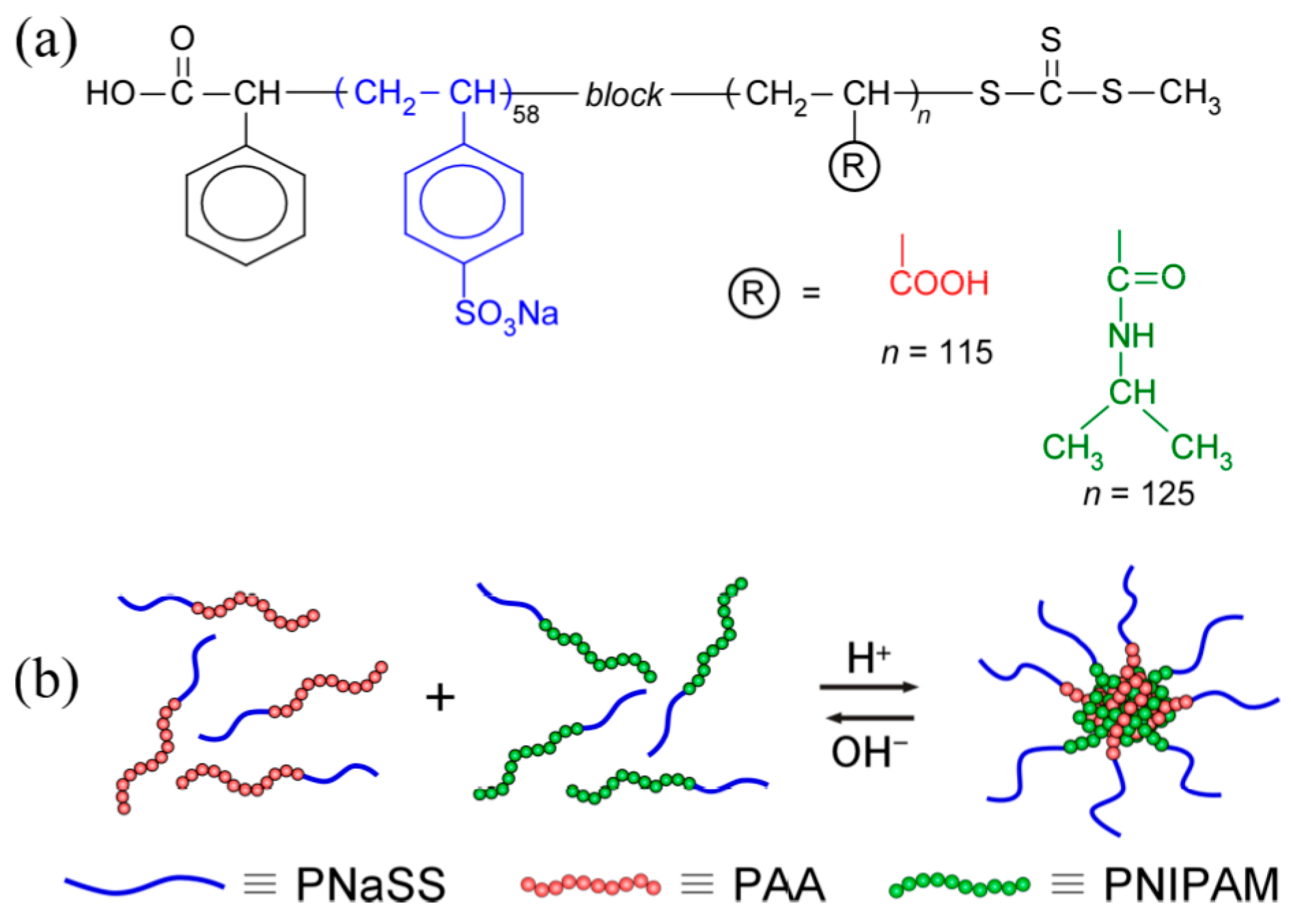
2.3. Preparation of PNaSS58–b–PAA125
2.4. Preparation of PNaSS58–b–PNIPAM115
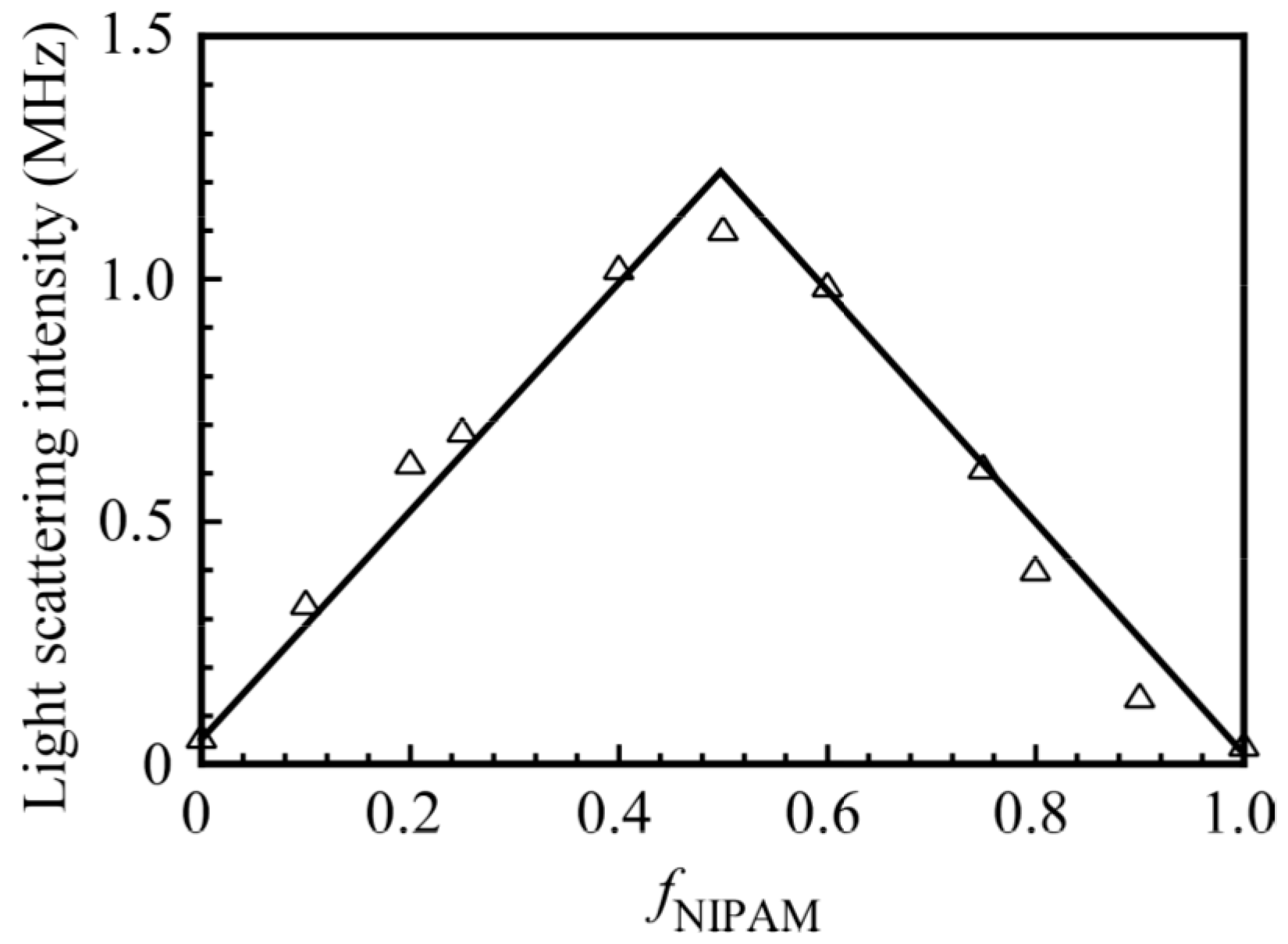
2.5. Preparation of the Water-Soluble Complex
2.6. Measurements
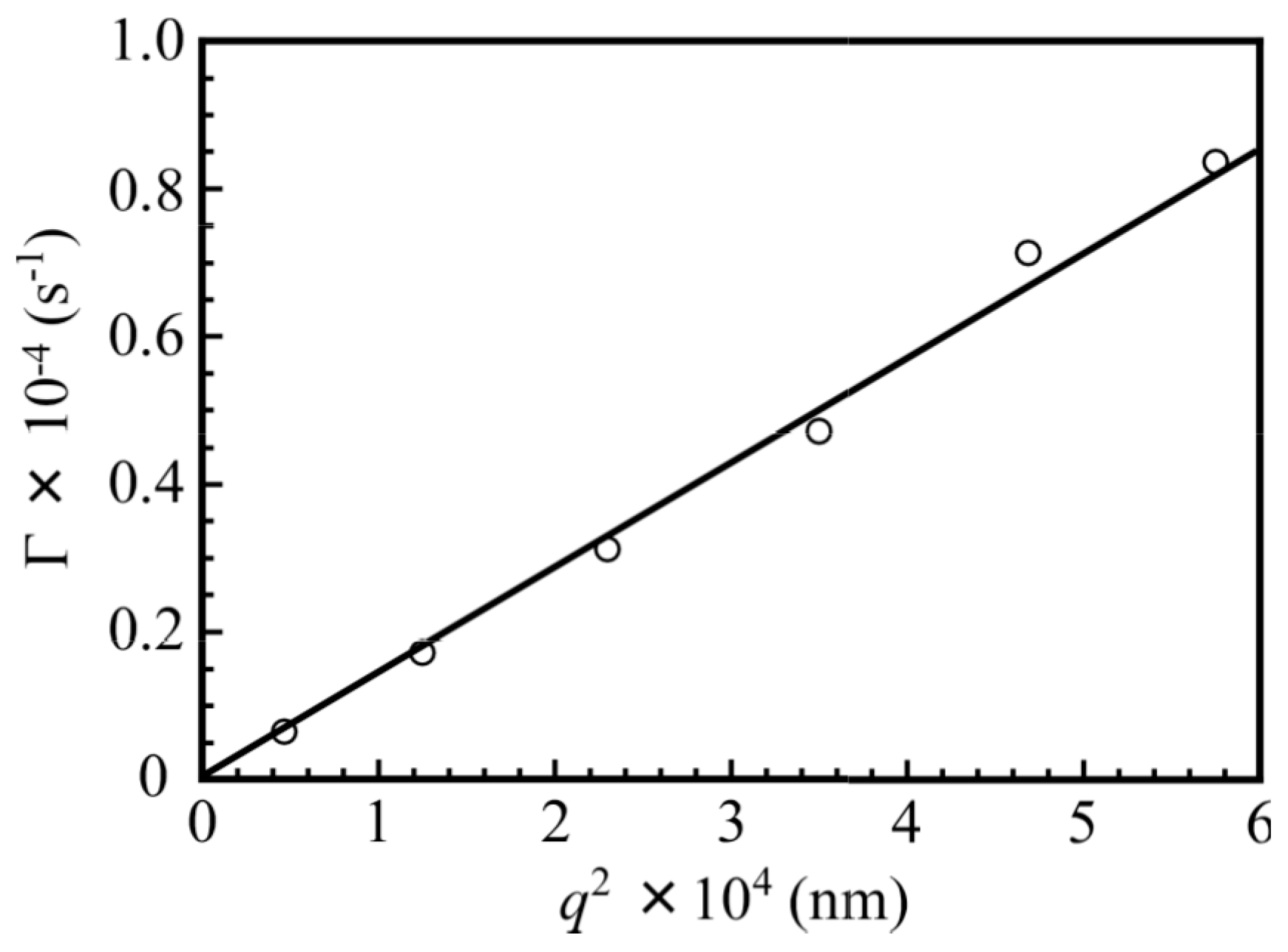
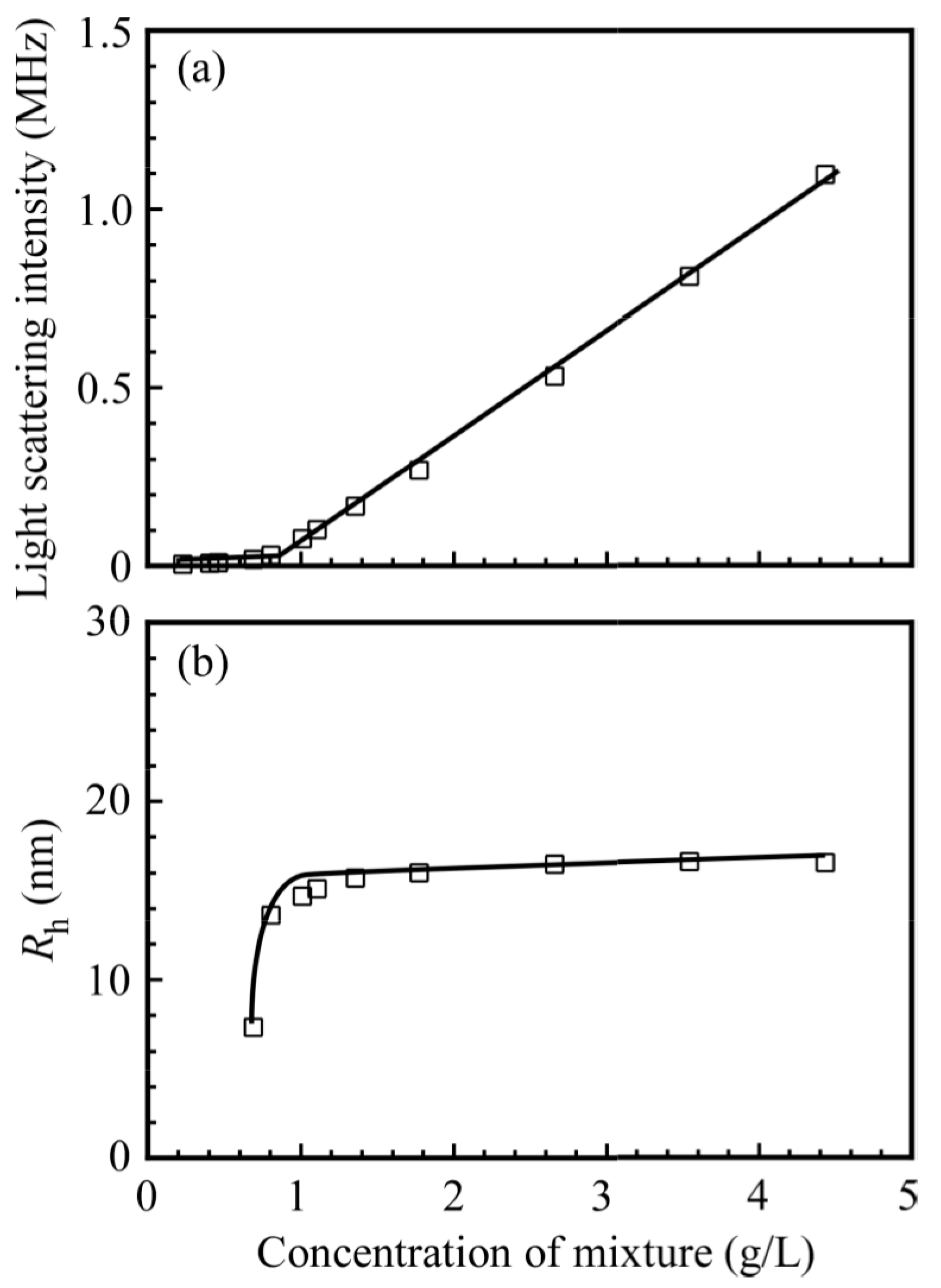
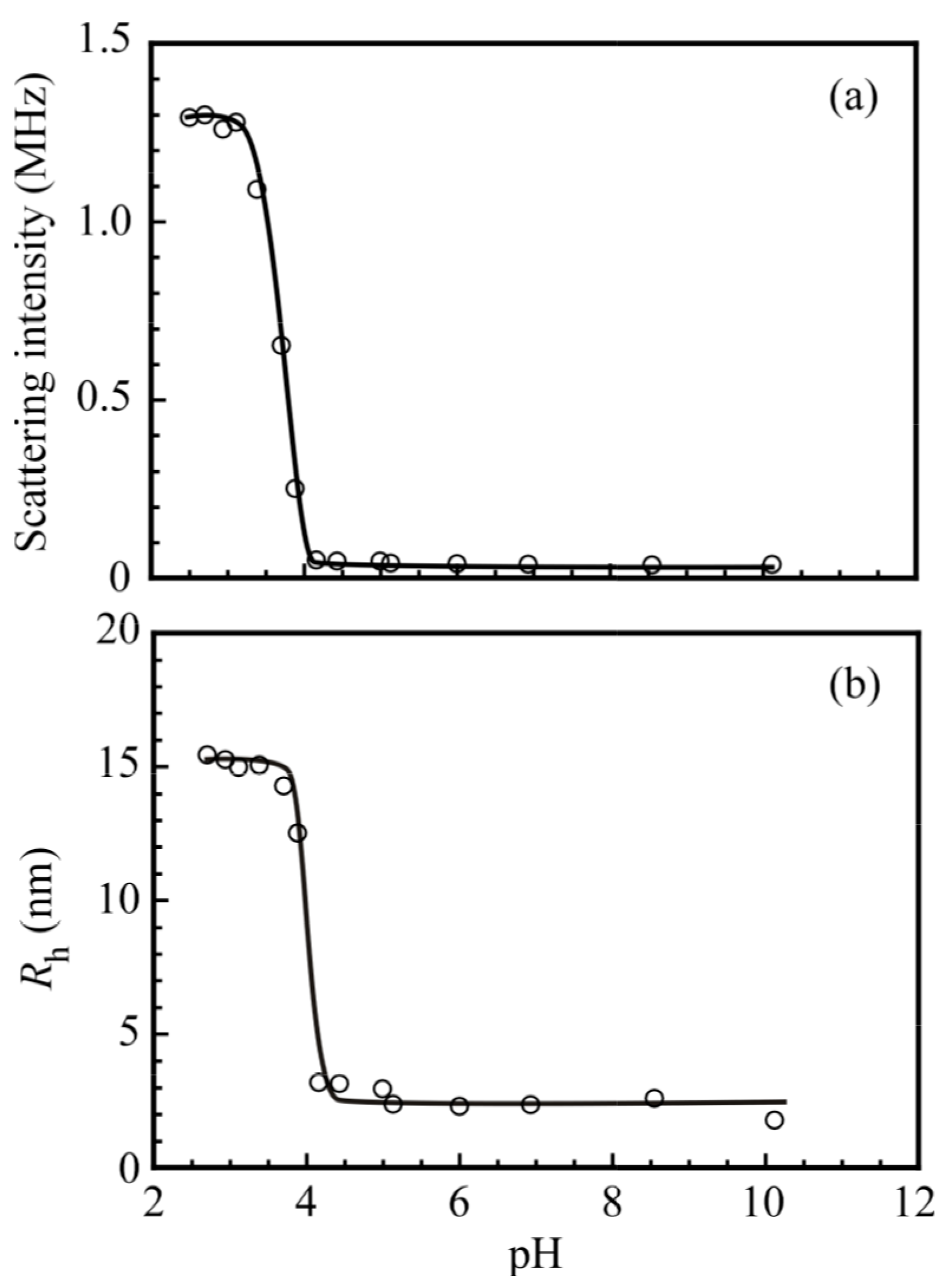
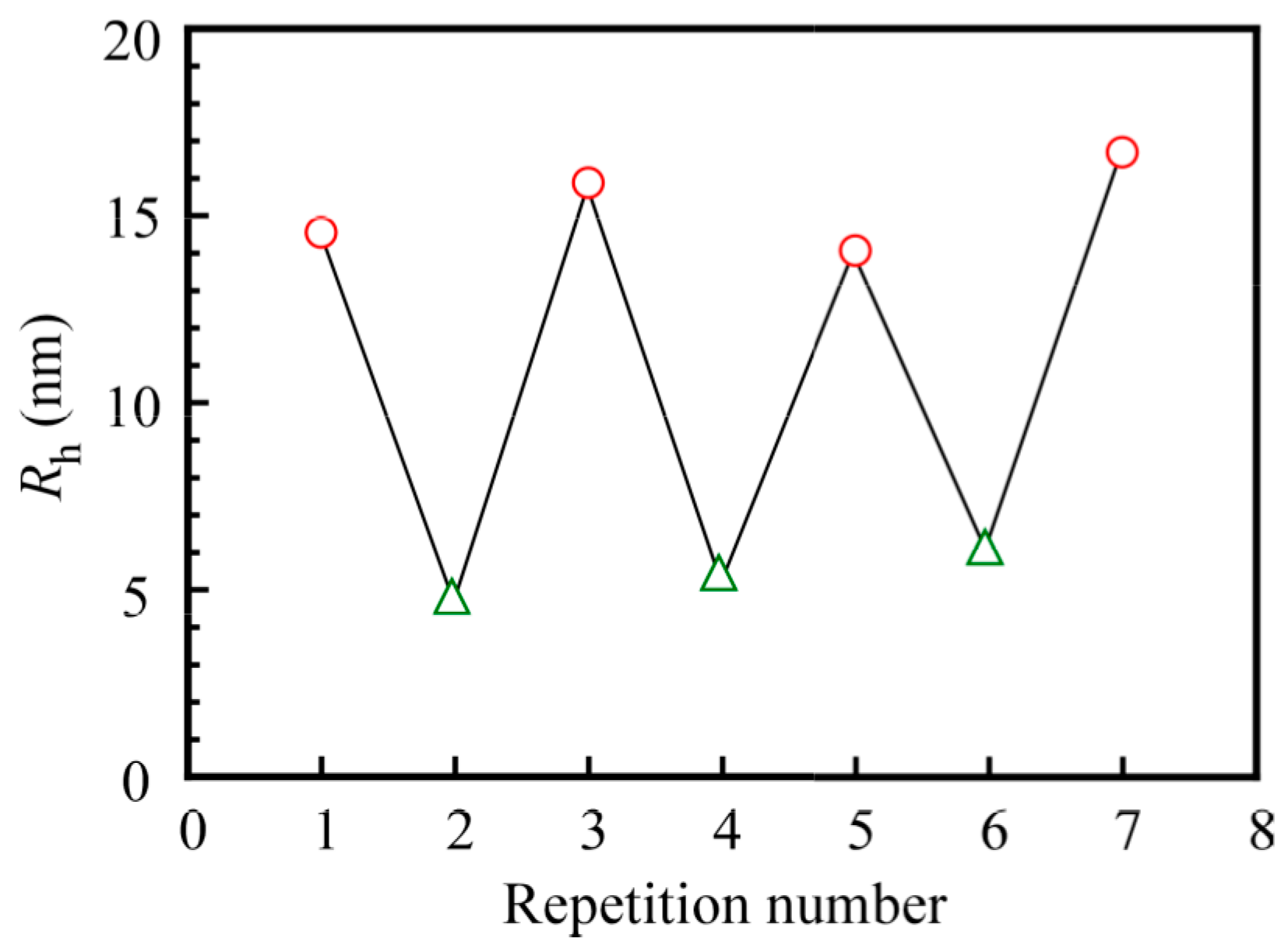
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xu, R.; Winnik, M.A.; Riess, G.; Croucher, M.D. Micellization of polystylene-poly(ethylene oxide) block copolymers in water. 5. A test of the star and mean-field models. Macromolecules 1992, 25, 644–652. [Google Scholar] [CrossRef]
- Harada, A.; Kataoka, K. Formation of polyion complex micelles in an aqueous milieu from a pair of oppositely-charged block copolymers with poly(ethylene glycol) segments. Macromolecules 1995, 28, 5294–5299. [Google Scholar] [CrossRef]
- Kabanov, V.A.; Zezin, A.B.; Kasaikin, V.A.; Zakharova, J.A.; Litmanovich, E.A.; Ivleva, E.M. Self-assembly of ionic amphiphiles on polyelectrolyte chains. Polym. Int. 2003, 52, 1566–1572. [Google Scholar] [CrossRef]
- Foreman, M.B.; Coffman, J.P.; Murcia, M.J.; Naumann, C.A.; Cesana, S.; Jordan, R.; Smith, G.S.; Naumann, C.A. Gelation of amphiphilic lipopolymers at the air-water interface: 2D analogue to 3D gelation of colloidal systems with grafted polymer chains. Langmuir 2003, 19, 326–332. [Google Scholar] [CrossRef]
- Jeon, S.H.; Ree, T. Characterization of poly(carboxylic acid)/(poly(ethylene oxide) blends formed through hydrogen bonding by spectroscopic and calorimetric analyses. J. Polym. Sci. A 1999, 26, 1419–1428. [Google Scholar] [CrossRef]
- Lee, W.; Chang, J.; Ju, S. Hydrogen-bond structure at the interfaces between water/poly(methyl methacrylate), water/poly(methacrylic acid), and water/poly(2-aminoethyl-methacrylate). Langmuir 2010, 26, 12640–12647. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Koide, M.; Tsuchida, E. Selective complexation of macromolecules. Macromolecules 1977, 10, 1259–1264. [Google Scholar] [CrossRef]
- Velda, J.L.; Liu, Y.; Huglin, M.B. Effect of pH on the swelling behaviour of hydrogels based on N-isopropylacrylamide with acidic comonomers. Macromol. Chem. Phys. 1998, 199, 1127–1134. [Google Scholar] [CrossRef]
- Erbil, C.; Akpinar, F.D.; Uyanik, N. Investigation of the thermal aggregation in aqueous poly(N-isopropylacrylamide-co-itaconic acid) solutions. Macromol. Chem. Phys. 1999, 200, 2448–2453. [Google Scholar] [CrossRef]
- Shieh, Y.-T.; Lin, P.-Y.; Chen, T.; Kuo, S.-W. Temperature, pH- and CO2-sensitive poly(N-isopropylacryl amide-co-acrylic acid) copolymers with high glass transition temperatures. Polymers 2016, 8, 434–449. [Google Scholar] [CrossRef]
- Bian, F.; Liu, M. Complexation between poly(N,N-diethylacrylamide) and poly(acrylic acid) in aqueous solution. Euro. Polym. J. 2003, 39, 1867–1874. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Yusa, S. Water-soluble complexes formed from hydrogen bonding interactions between a poly(ethylene glycol)-containing triblock copolymer and poly(methacrylic acid). Polym. J. 2013, 45, 985–992. [Google Scholar] [CrossRef]
- Yusa, S.; Shimada, Y.; Mitsukami, Y.; Yamamoto, T.; Morishima, Y. pH-Responsive micellization of amphiphilic diblock copolymers synthesized via reversible addition-fragmentation chain transfer polymerization. Macromolecules 2003, 36, 4208–4215. [Google Scholar] [CrossRef]
- Yusa, S.; Shimada, Y.; Mitsukami, Y.; Yamamoto, T.; Morishima, Y. Heat-induced association and dissociation behavior of amphiphilic diblock copolymers synthesized via reversible addition-fragmentation chain transfer radical polymerization. Macromolecules 2004, 37, 7507–7513. [Google Scholar] [CrossRef]
- Yusa, S.; Endo, T.; Ito, M. Synthesis of thermo-responsive 4-arm star-shaped porphyrin-centered poly(N,N-diethylacrylamide) via reversible addition-fragmentation chain transfer radical polymerization. J. Poly. Sci. A 2009, 47, 6827–6838. [Google Scholar] [CrossRef]
- Jakeš, J. Testing of the constrained regularization method of inverting Laplace transform on simulated very wide quasielastic light scattering autocorrelation functions. Czech. J. Phys. B 1988, 38, 1305–1316. [Google Scholar] [CrossRef]
- Brown, W.; Nicolai, T.; Hvidt, S.; Stepanek, P. Relaxation time distributions of entangled polymer solutions from dynamic light scattering and dynamic mechanical measurements. Macromolecules 1990, 23, 357–359. [Google Scholar] [CrossRef]
- Zimm, B.H. Apparatus and methods for measurement and interpretation of the angular variation of light scattering; preliminary results on polystyrene solutions. J. Chem. Phys. 1948, 16, 1099–1116. [Google Scholar] [CrossRef]
- Yusa, S.; Fukuda, K.; Yamamoto, T.; Ishihara, K.; Morishima, Y. Synthesis of well-defined amphiphilic block copolymers having phospholipid polymer sequences as a novel biocompatible polymer micelle reagent. Biomacromolecules 2005, 6, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Yusa, S.; Konishi, Y.; Mitsukami, Y.; Yamamoto, T.; Morishima, Y. pH-responsive micellization of amine-containing cationic diblock copolymers prepared by reversible addition-fragmentation chain transfer (RAFT) radical polymerization. Polym. J. 2005, 37, 480–488. [Google Scholar] [CrossRef]
- Xu, R.; Winnik, M.A.; Hallet, F.R.; Riess, G.; Croucher, M.D. Light-scattering study of the association behavior of styrene-ethylene oxide block copolymers in aqueous solution. Macromolecules 1991, 24, 87–93. [Google Scholar] [CrossRef]
- Huber, K.; Bantle, S.; Lutz, P.; Burchard, W. Hydrodynamic and thermodynamic behavior of short-chain polystyrene in toluene and cyclohexane at 34.5 °C. Macromolecules 1985, 18, 1461–1467. [Google Scholar] [CrossRef]
- Akcasu, A.Z.; Han, C.C. Molecular weight and temperature dependence of polymer dimensions in solution. Macromolecules 1979, 12, 276–280. [Google Scholar] [CrossRef]
- Konishi, T.; Yoshizaki, T.; Yamakawa, H. On the “universal constants” ρ and Φ of flexible polymers. Macromolecules 1991, 24, 5614–5622. [Google Scholar] [CrossRef]
- Yin, X.; Stöver, H.D.H. Thermosensitive and pH-sensitive polymers based on maleic anhydride copolymers. Macromolecules 2002, 35, 10178–10181. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, J.E. Solution properties of poly(N-isopropylacrylamide). J. Macromol. Sci. A 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Winnik, F.M. Fluorescence studies of aqueous solutions of poly(N-isopropylacrylamide) below and above their LCST. Macromolecules 1990, 23, 233–242. [Google Scholar] [CrossRef]
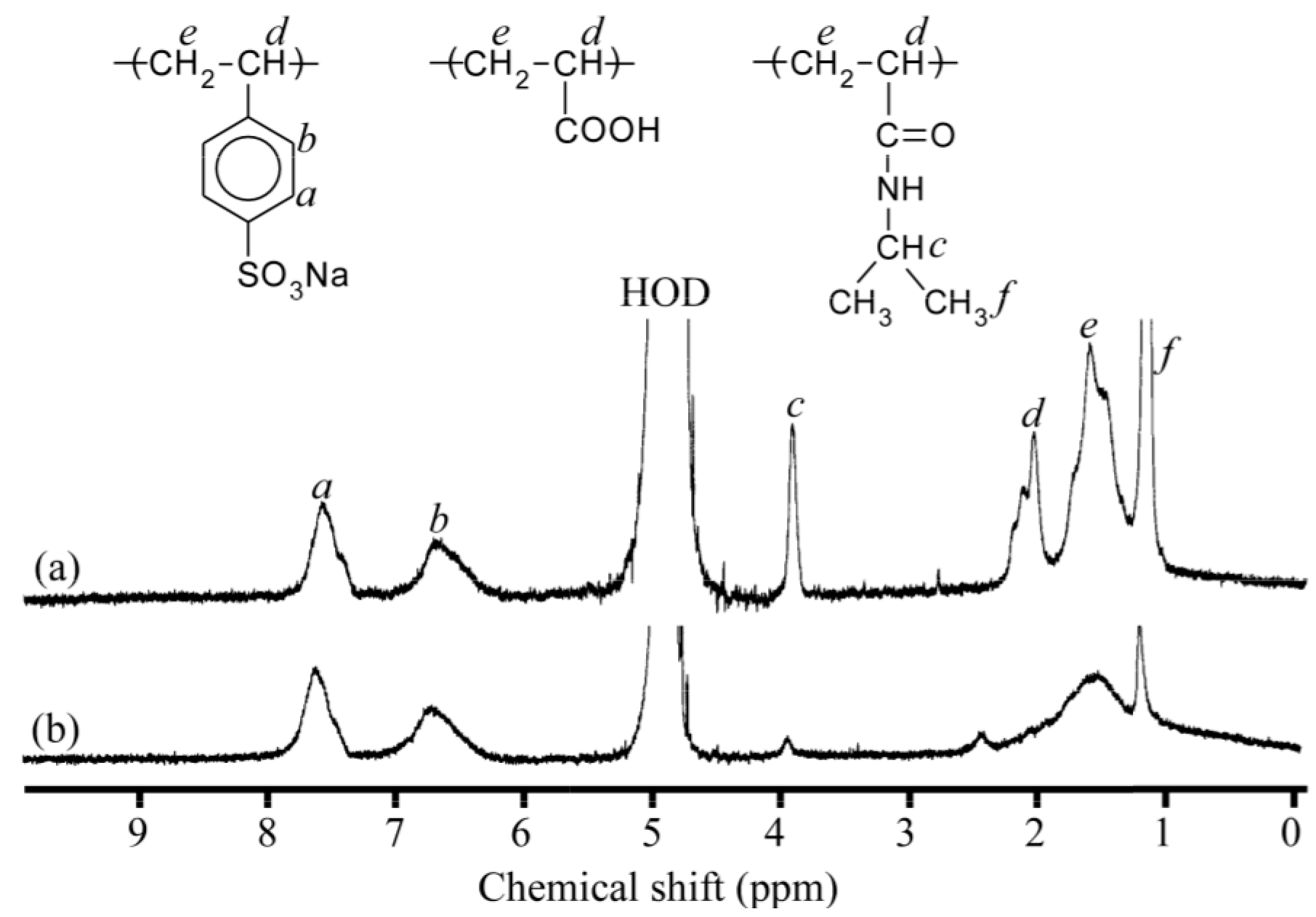



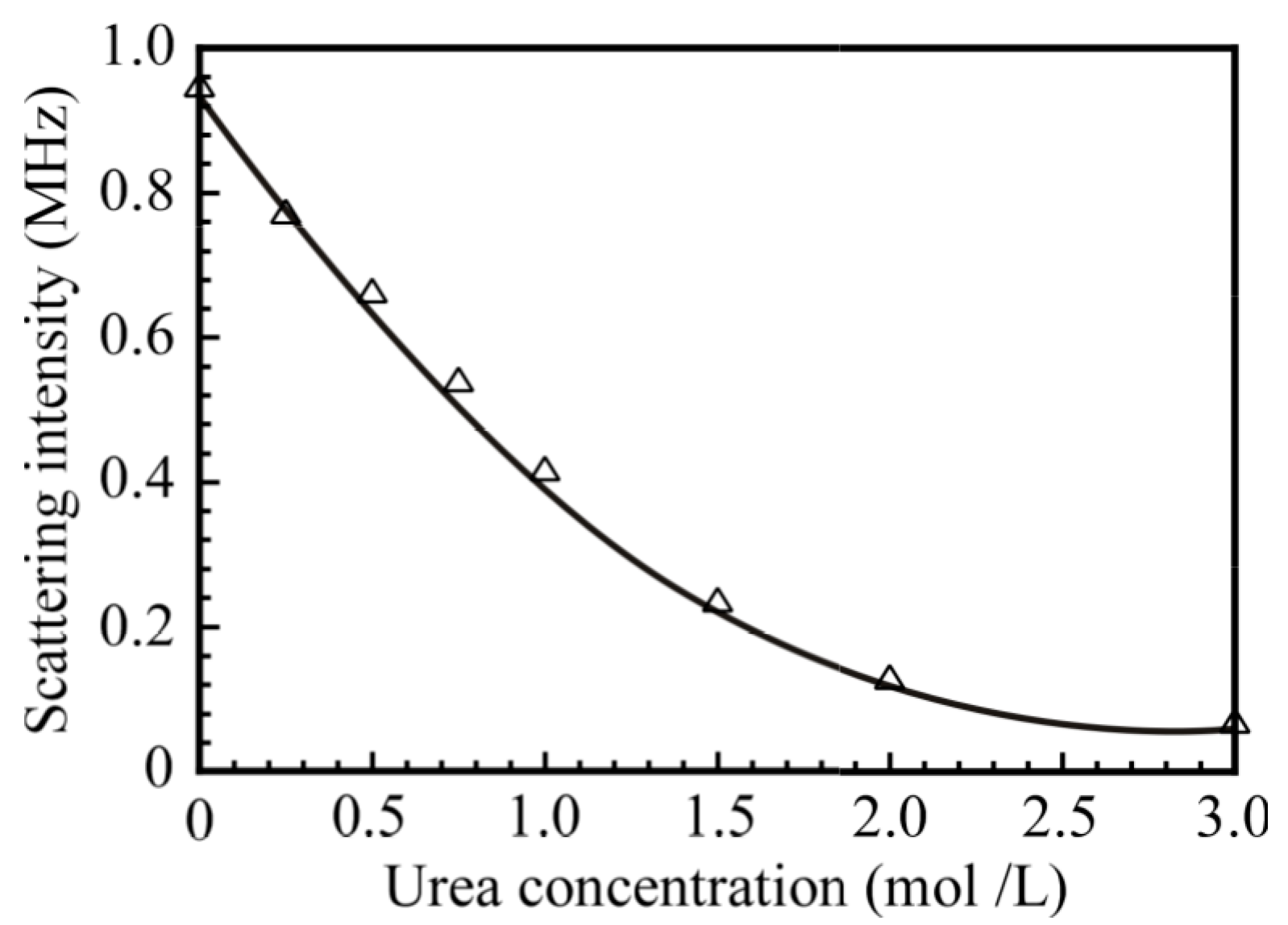
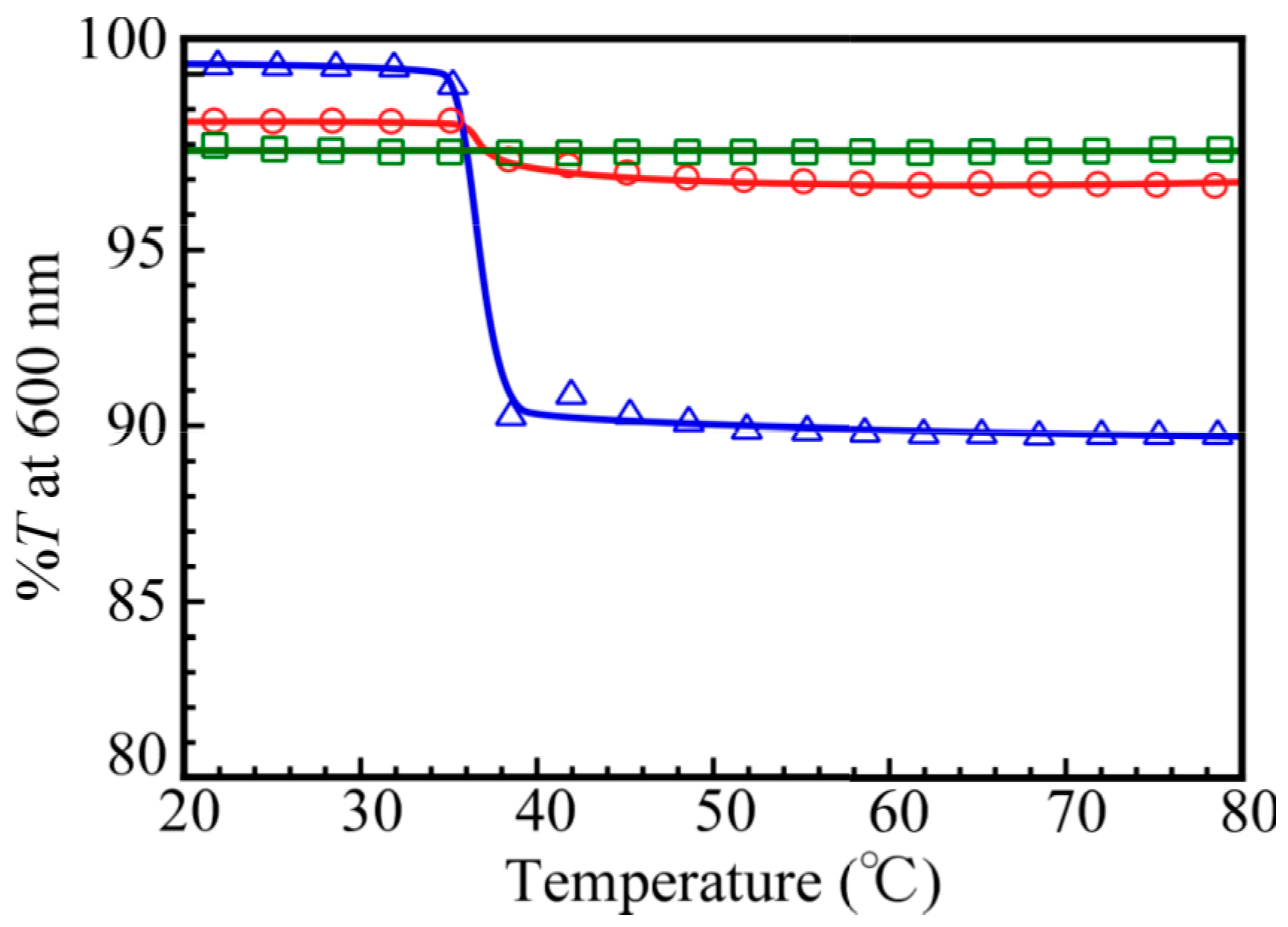
| Samples | DP of PNaSS a | DP of PAA b | DP of PNIPAM b | Mn(theo) c × 10−4 | Mn(NMR) b × 10−4 | Mn(GPC) a × 10−4 | Mw/Mn a |
|---|---|---|---|---|---|---|---|
| PNaSS58–b–PAA125 | 58 | 125 | 2.07 | 2.12 | 3.51 | 1.18 | |
| PNaSS58–b–PNIPAM115 | 58 | 115 | 2.27 | 2.52 | 1.21 | 1.39 |
| pH | Rh a (nm) | Mw(SLS) b × 10−5 | Rg b (nm) | Rg/Rh | Nagg c |
|---|---|---|---|---|---|
| 3 | 15.0 | 9.23 | 12.9 | 0.86 | 46 |
| 10 | 3.7 | 0.20 | 13.6 | 3.7 | 1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizusaki, M.; Endo, T.; Nakahata, R.; Morishima, Y.; Yusa, S.-i. pH-Induced Association and Dissociation of Intermolecular Complexes Formed by Hydrogen Bonding between Diblock Copolymers. Polymers 2017, 9, 367. https://doi.org/10.3390/polym9080367
Mizusaki M, Endo T, Nakahata R, Morishima Y, Yusa S-i. pH-Induced Association and Dissociation of Intermolecular Complexes Formed by Hydrogen Bonding between Diblock Copolymers. Polymers. 2017; 9(8):367. https://doi.org/10.3390/polym9080367
Chicago/Turabian StyleMizusaki, Masanobu, Tatsuya Endo, Rina Nakahata, Yotaro Morishima, and Shin-ichi Yusa. 2017. "pH-Induced Association and Dissociation of Intermolecular Complexes Formed by Hydrogen Bonding between Diblock Copolymers" Polymers 9, no. 8: 367. https://doi.org/10.3390/polym9080367