Enhanced Stimuli-Responsive Electrorheological Property of Poly(ionic liquid)s-Capsulated Polyaniline Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
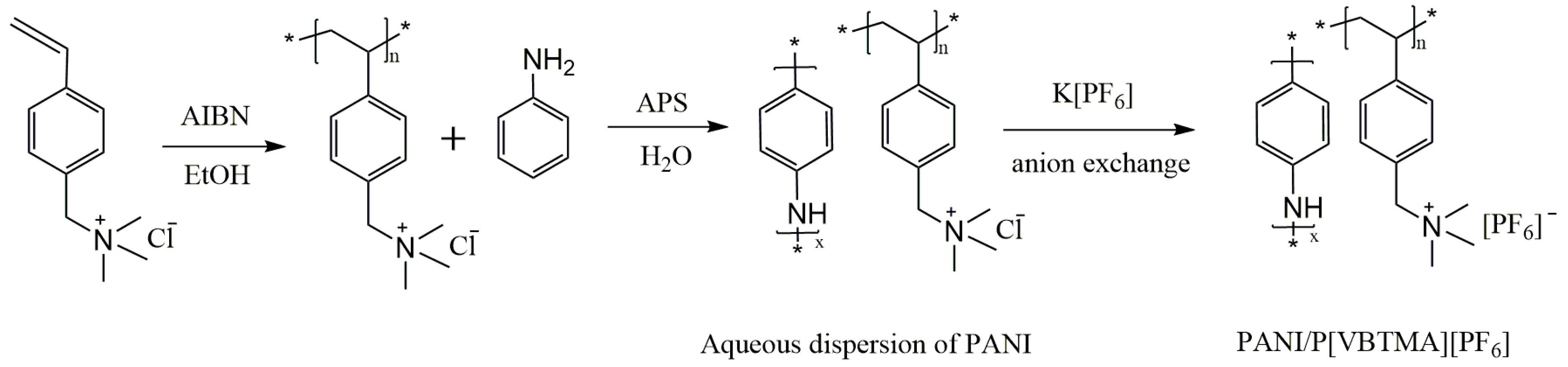
2.2. Preparation of PIL-c-PANI Particles
2.3. Preparation of ER Suspensions
2.4. Characterization and Measurements
3. Results
3.1. Synthesis of PIL-c-PANI Particles
3.2. Characterization of PIL-c-PANI Particles
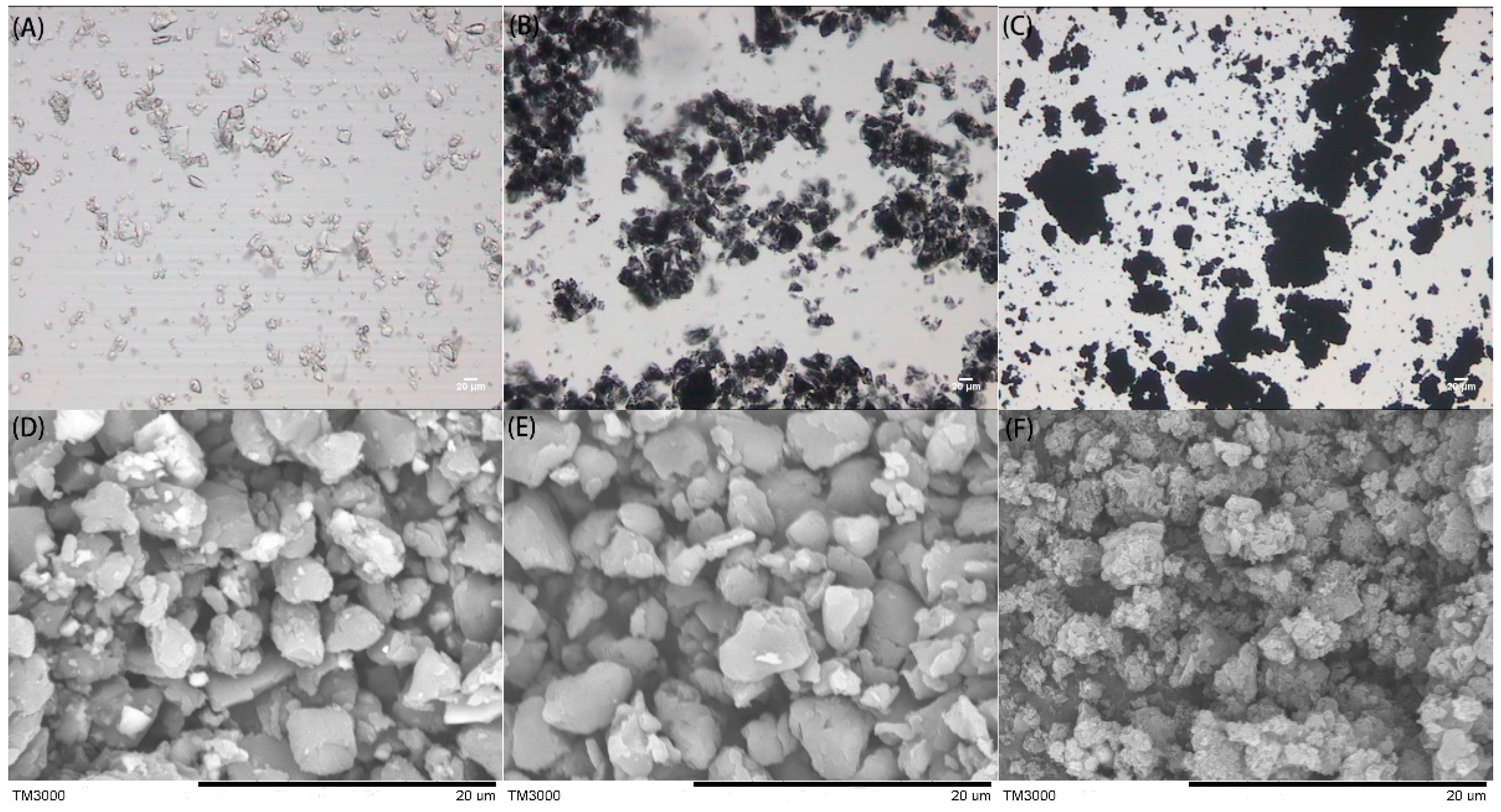
3.2.1. Morphology and Structure Analysis
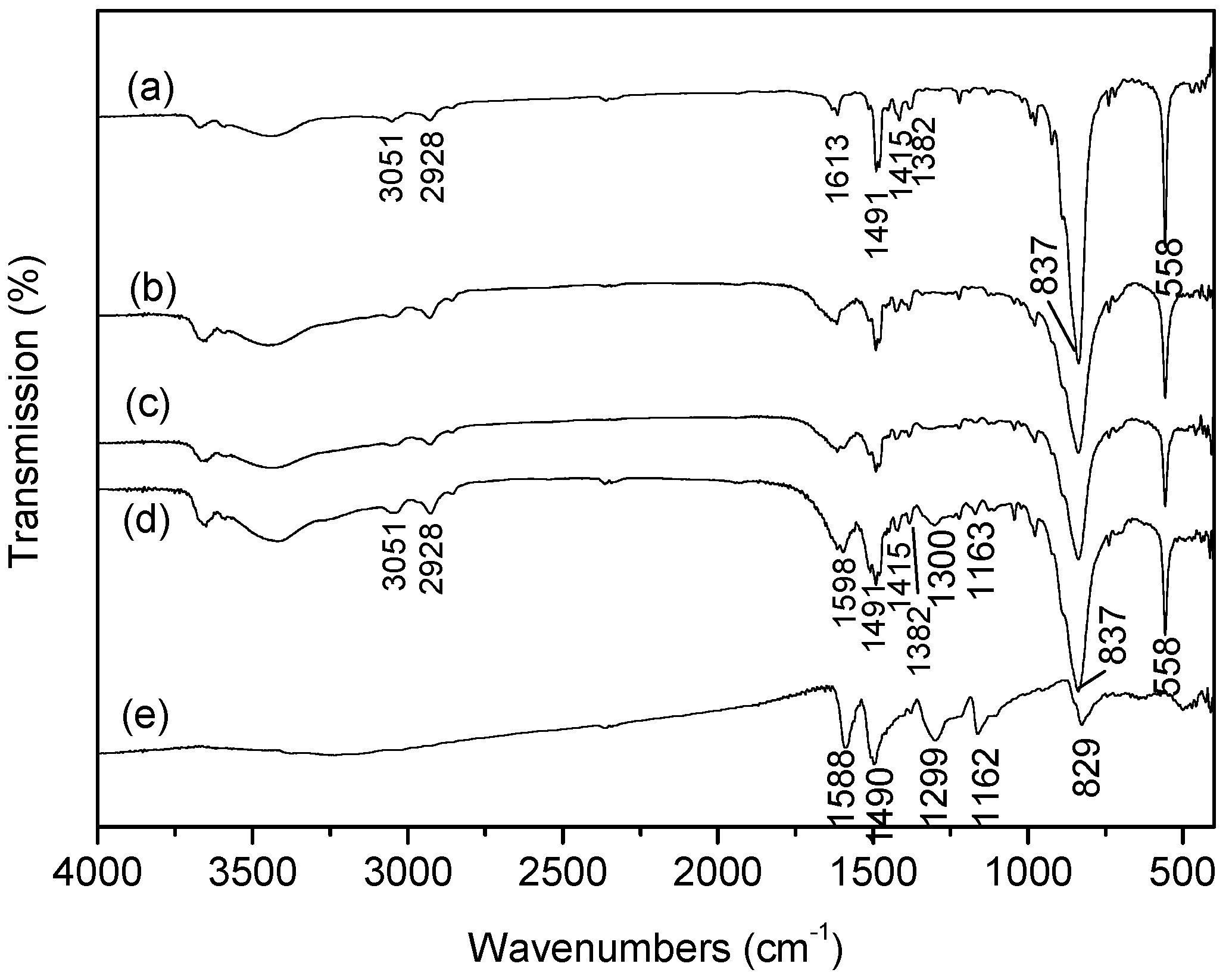
3.2.2. FTIR Analysis
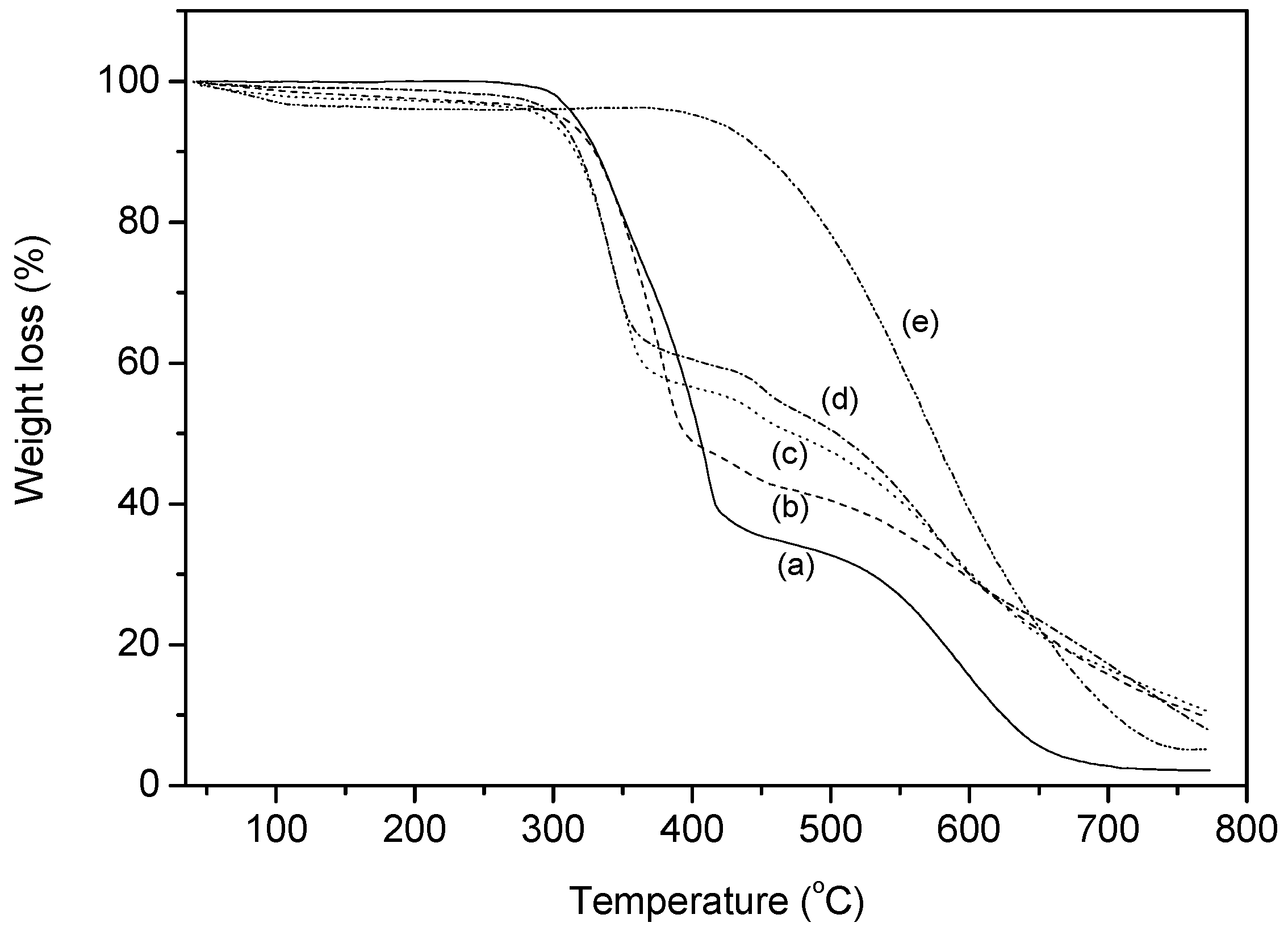
3.2.3. TG Analysis
3.2.4. Zeta Potentials Analysis
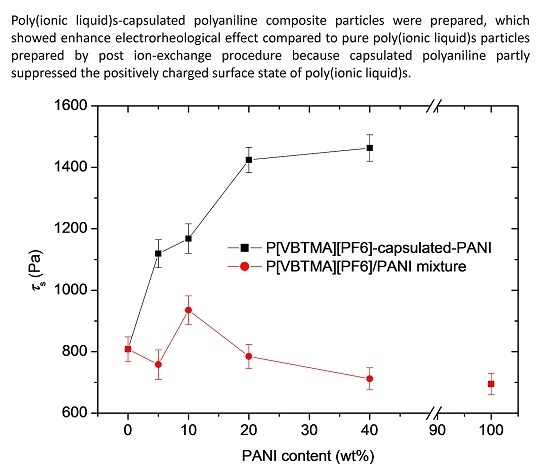
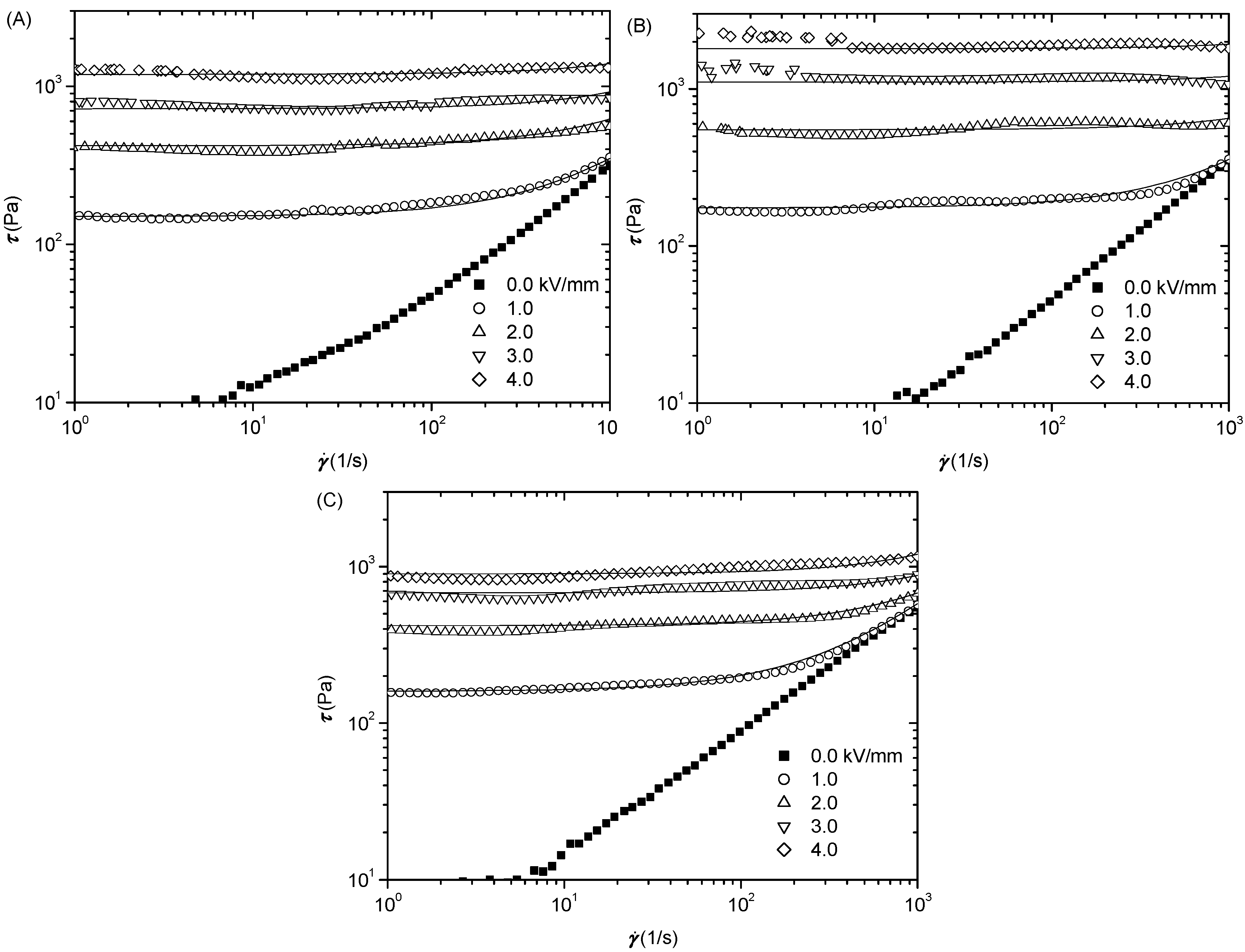
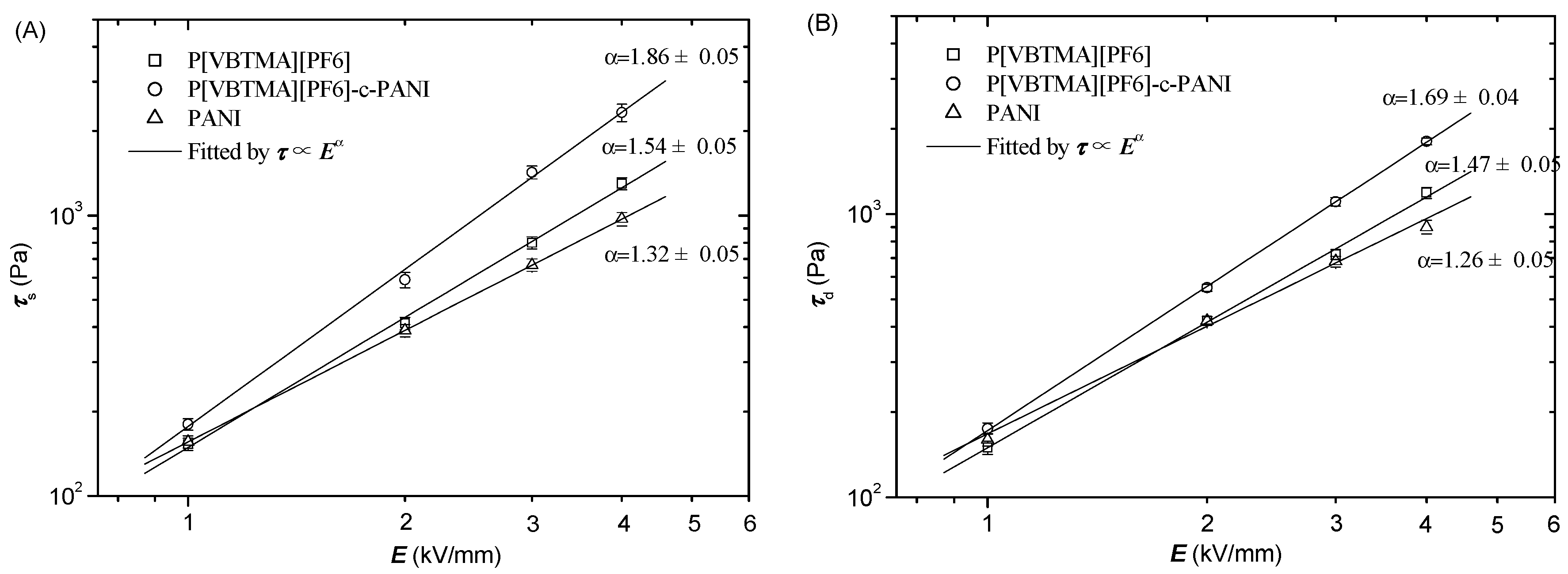
3.3. Electrorheological Property
3.4. Dielectric Properties
3.5. Microscopic ER Structures
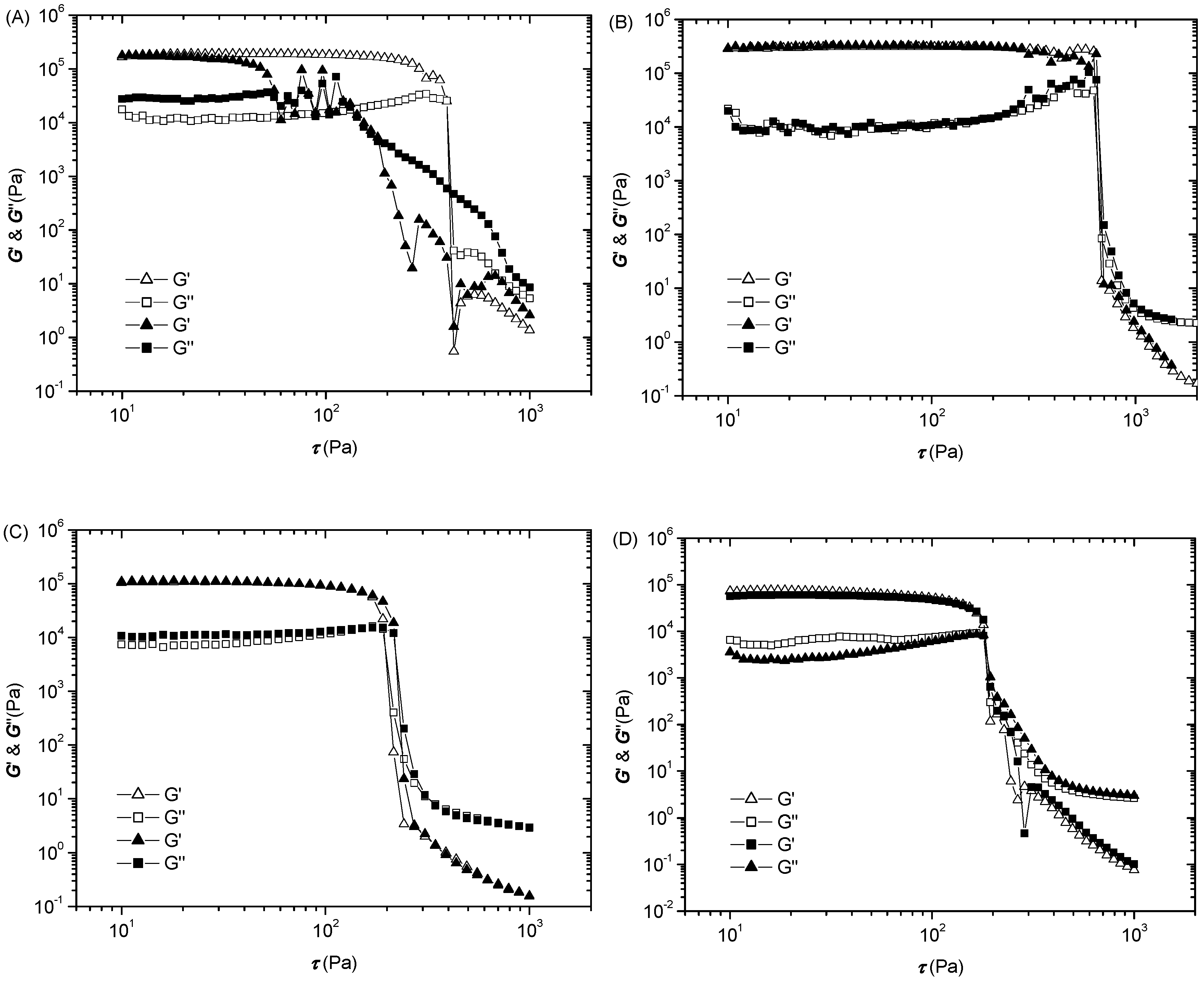
3.6. Oscillatory Study
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Capadona, J.R.; Shanmuganathan, K.; Tyler, D.J.; Rowan, S.J.; Weder, C. Stimuli-responsive polymer nanocomposites inspired by the sea cucumber dermis. Science 2008, 319, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Lampert, C.M. Chromogenic smart materials. Mater. Today 2004, 7, 28–35. [Google Scholar] [CrossRef]
- Halsey, T.C. Electrorheological fluids. Science 1992, 258, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.D.; Choi, H.J. Electrorheological fluids: Smart soft matter and characteristics. Soft Matter 2012, 8, 11961–11978. [Google Scholar] [CrossRef]
- Coulter, J.P.; Weiss, K.D.; Carlson, J.D. Engineering applications of electrorheological materials. J. Intell. Mater. Syst. Struct. 1993, 4, 248–259. [Google Scholar] [CrossRef]
- Sheng, P.; Wen, W. Electrorheological fluids: Mechanisms, dynamics, and microfluidics applications. Annu. Rev. Fluid Mech. 2012, 44, 143–174. [Google Scholar] [CrossRef]
- Tao, R.; Tang, H.; Tawhid-Al-Islam, K.; Du, E.; Kim, J. Electrorheology leads to healthier and tastier chocolate. Proc. Natl. Acad. Sci. USA 2016, 113, 7399–7402. [Google Scholar] [CrossRef] [PubMed]
- Winslow, W.M. Induced fibration of suspensions. J. Appl. Phys. 1949, 20, 1137–1140. [Google Scholar] [CrossRef]
- Hao, T. Electrorheological fluids. Adv. Mater. 2001, 13, 1847–1857. [Google Scholar] [CrossRef]
- Wen, W.; Huang, X.; Yang, S.; Lu, K.; Sheng, P. The giant electrorheological effect in suspensions of nanoparticles. Nat. Mater. 2003, 2, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Shen, R.; Wang, X.; Sun, G.; Wen, W. The electrorheological fluids with high shear stress. Int. J. Mod. Phys. B 2005, 19, 1065–1070. [Google Scholar] [CrossRef]
- See, H. Advances in modelling the mechanisms and rheology of electrorheological fluids. Korea-Aust. Rheol. J. 1999, 11, 169–195. [Google Scholar]
- Weiss, K.D.; Carlson, J.D.; Coulter, J.P. Review: Material aspects of electrorheological systems. J. Intell. Mater. Syst. Struct. 1993, 4, 13–34. [Google Scholar] [CrossRef]
- Yin, J.B.; Zhao, X.P. Preparation and electrorheological activity of mesoporous rare-earth-doped TiO2. Chem. Mater. 2002, 14, 4633–4640. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, C.A.; Jang, W.H.; Choi, H.J.; Jhon, M.S. Synthesis and electrorheological characteristics of microencapsulated polyaniline particles with melamine–formaldehyde resins. Polymer 2001, 42, 8277–8283. [Google Scholar] [CrossRef]
- Cao, Y.; Choi, H.J.; Zhang, W.L.; Wang, B.; Hao, C.; Liu, J. Eco-friendly mass production of poly(p-phenylenediamine)/graphene oxide nanoplatelet composites and their electrorheological characteristics. Compos. Sci. Technol. 2016, 122, 36–41. [Google Scholar] [CrossRef]
- Wang, B.X.; Liu, C.J.; Yin, Y.C.; Yu, S.; Chen, K.; Liu, P.; Liang, B. Double template assisting synthesized core–shell structured titania/polyaniline nanocomposite and its smart electrorheological response. Compos. Sci. Technol. 2013, 86, 89–100. [Google Scholar] [CrossRef]
- Quadrat, O.; Stejskal, J. Polyaniline in electrorheology. J. Ind. Eng. Chem. 2006, 12, 352–358. [Google Scholar]
- Bloodworth, R.; Wendt, E. Materials for ER fluids. Int. J. Mod. Phys. B 1996, 10, 2951–2964. [Google Scholar] [CrossRef]
- Dong, Y.Z.; Yin, J.B.; Zhao, X.P. Microwave-synthesized poly(ionic liquid) particles: A new material with high electrorheological activity. J. Mater. Chem. A 2014, 2, 9812–9819. [Google Scholar] [CrossRef]
- Mecerreyes, D. Polymeric ionic liquids: Broadening the properties and applications of polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648. [Google Scholar] [CrossRef]
- Dong, Y.Z.; Yin, J.B.; Yuan, J.H.; Zhao, X.P. Microwave-assisted synthesis and high-performance anhydrous electrorheological characteristic of monodisperse poly (ionic liquid) particles with different size of cation/anion parts. Polymer 2016, 97, 408–417. [Google Scholar] [CrossRef]
- Yuan, J.Y.; Mecerreyes, D.; Antonietti, M. Poly(ionic liquid)s: An update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Men, Y.J.; Kuzmicz, D.; Yuan, J.Y. Poly (ionic liquid) colloidal particles. Curr. Opin. Colloid Interface Sci. 2014, 19, 76–83. [Google Scholar] [CrossRef]
- Marcilla, R.; Ochoteco, E.; Pozo-Gonzalo, C.; Grande, H.; Pomposo, J.A.; Mecerreyes, D. New Organic Dispersions of Conducting Polymers Using Polymeric Ionic Liquids as Stabilizers. Macromol. Rapid Commun. 2005, 26, 1122–1126. [Google Scholar] [CrossRef]
- Yu, G.R.; Li, Q.Z.; Li, N.; Man, Z.W.; Pu, C.H.; Asumana, C.; Chen, X.C. Synthesis of new crosslinked porous ammonium-based poly (ionic liquid) and application in CO2 adsorption. Polym. Eng. Sci. 2014, 54, 59–63. [Google Scholar] [CrossRef]
- Block, H.; Kelly, J.P. Electro-rheology. J. Phys. D Appl. Phys. 1988, 21, 1661. [Google Scholar] [CrossRef]
- Block, H.; Rattray, P. Recent developments in ER fluids. In Progress in Electrorheology, 1st ed.; Havelka, K.O., Filisko, F.E., Eds.; Springer: New York, NY, USA, 1995; pp. 19–42. [Google Scholar]
- Ikazaki, F.; Kawai, A.; Uchida, K.; Kawakami, T.; Edamura, K.; Sakurai, K.; Anzai, H.; Asako, Y. Mechanisms of electrorheology: The effect of the dielectric property. J. Phys. D Appl. Phys. 1998, 31, 336. [Google Scholar] [CrossRef]
- Hanaoka, R.; Hotta, K.; Anzai, H.; Sakurai, K.; Kuroda, S. Internal structure and ER properties in ER suspensions of disperse system under DC electric field. Electr. Eng. Jpn. 2000, 132, 9–18. [Google Scholar] [CrossRef]
- Chin, B.D.; Park, O.O. Rheology and microstructures of electrorheological fluids containing both dispersed particles and liquid drops in a continuous phase. J. Rheol. 2000, 44, 397–412. [Google Scholar] [CrossRef]



| Sample | P[VBTMA][PF6] | P[VBTMA][PF6]-c-PANI | PANI |
|---|---|---|---|
| Zeta potentials (mV) | +134 | +110 | +39 |
| Sample | ε0 (a) | ε∞ (b) | ∆ε (c) | τ(s) (d) | α (e) | σp (S/cm) (f) |
|---|---|---|---|---|---|---|
| P[VBTMA][PF6] | 4.31 | 2.91 | 1.40 | 0.0022 | 1.26 | ~1.0 × 10−9 |
| PANI | 4.28 | 2.68 | 1.60 | 0.0040 | 1.20 | ~7.5 × 10−10 |
| P[VBTMA][PF6]-c-PANI | 4.35 | 2.65 | 1.70 | 0.0023 | 1.24 | ~2.6 × 10−10 |
| P[VBTMA][PF6]/PANI | 4.48 | 2.8 | 1.68 | 0.0030 | 1.12 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, C.; Dong, Y.; Liu, Y.; Zhao, X.; Yin, J. Enhanced Stimuli-Responsive Electrorheological Property of Poly(ionic liquid)s-Capsulated Polyaniline Particles. Polymers 2017, 9, 385. https://doi.org/10.3390/polym9090385
Zheng C, Dong Y, Liu Y, Zhao X, Yin J. Enhanced Stimuli-Responsive Electrorheological Property of Poly(ionic liquid)s-Capsulated Polyaniline Particles. Polymers. 2017; 9(9):385. https://doi.org/10.3390/polym9090385
Chicago/Turabian StyleZheng, Chen, Yuezhen Dong, Yang Liu, Xiaopeng Zhao, and Jianbo Yin. 2017. "Enhanced Stimuli-Responsive Electrorheological Property of Poly(ionic liquid)s-Capsulated Polyaniline Particles" Polymers 9, no. 9: 385. https://doi.org/10.3390/polym9090385