Chitin-Based Anisotropic Nanostructures of Butterfly Wings for Regulating Cells Orientation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Apparatus
2.3. Methods
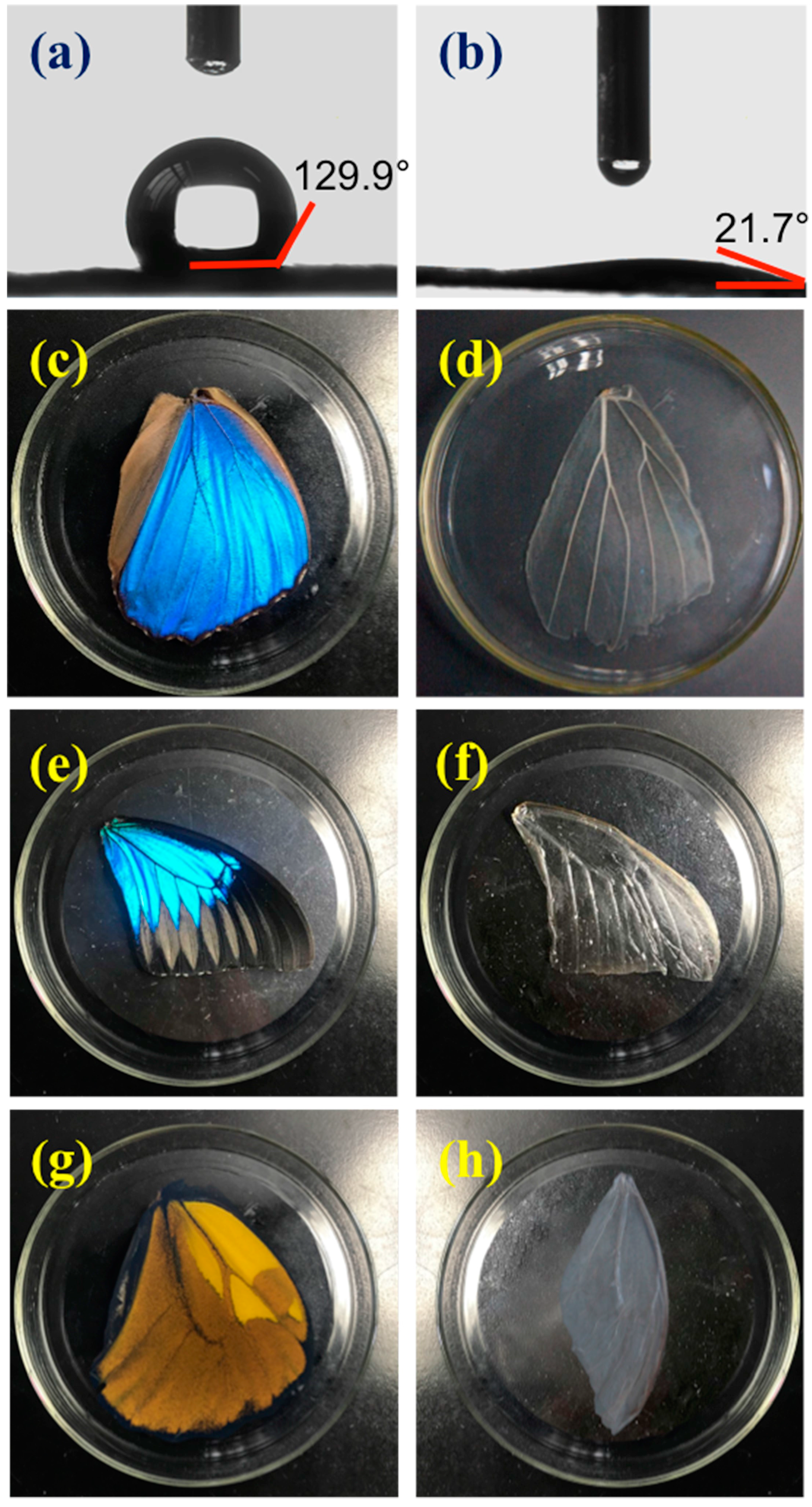
2.3.1. Treatment of Butterfly Wings
2.3.2. Cell Culture
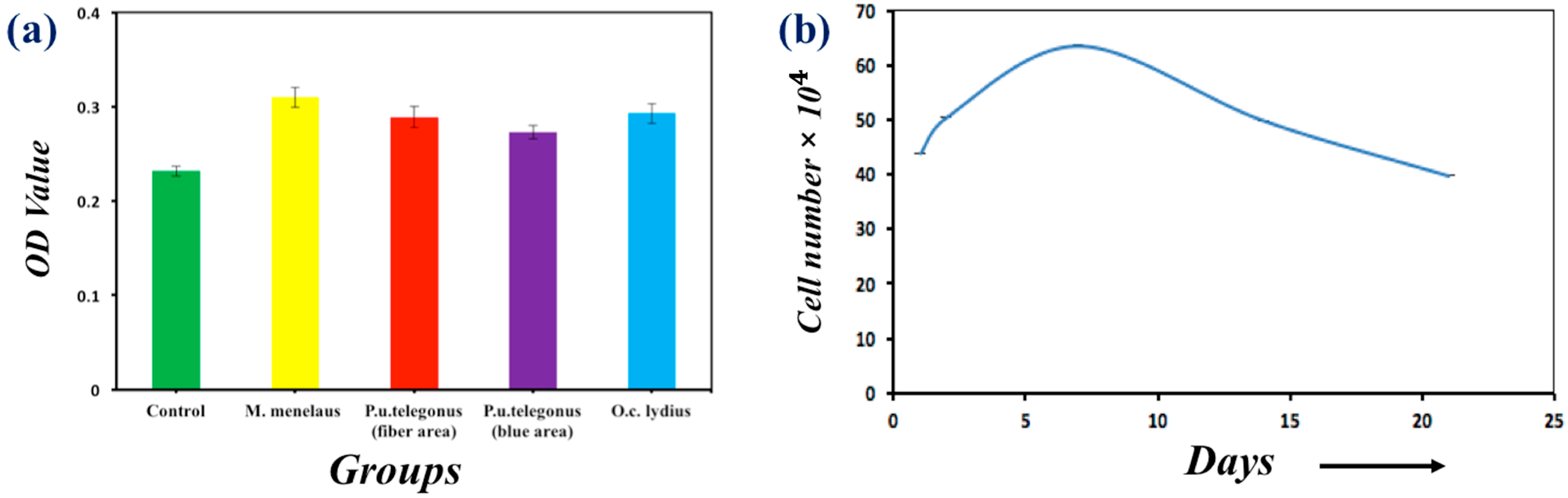
2.3.3. Tetrazolium (MTT) Viability Assay
2.3.4. Fluorescent Viability Staining
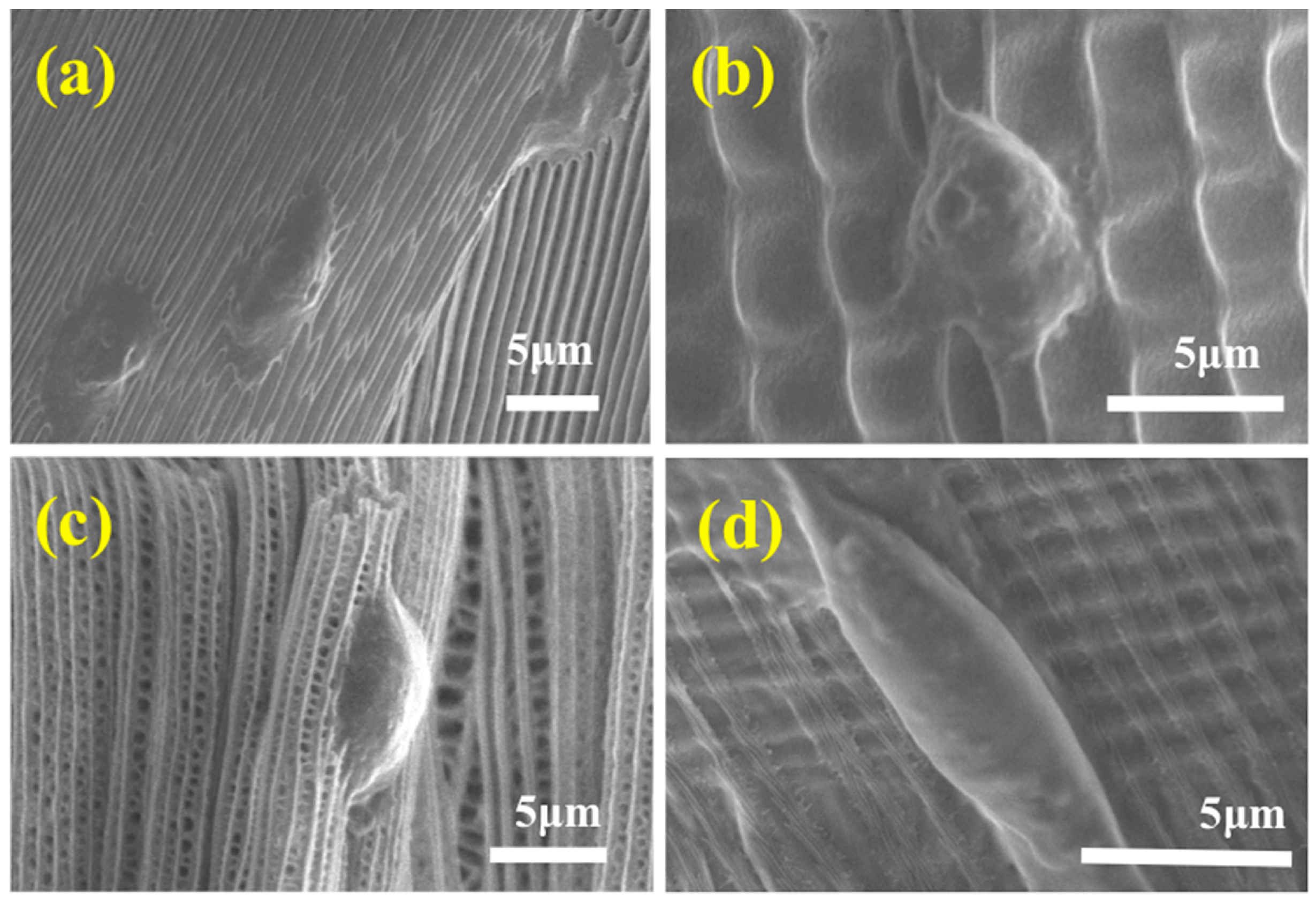
2.3.5. SEM
2.3.6. Statistical Analysis
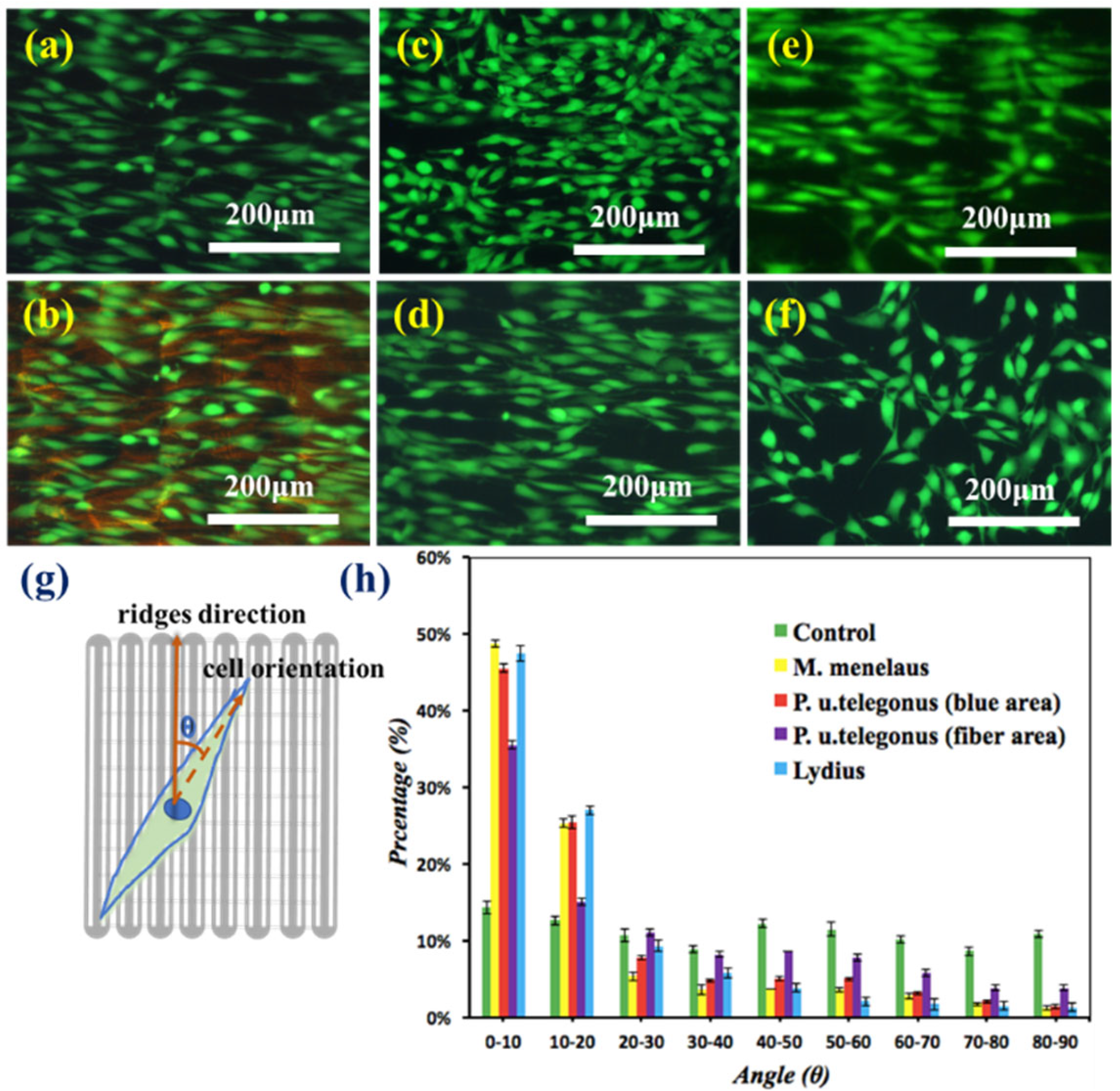
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nano 2011, 6, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Engel, E.; Michiardi, A.; Navarro, M.; Lacroix, D.; Planell, J.A. Nanotechnology in regenerative medicine: The materials side. Trends Biotechnol. 2008, 26, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The Use of Natural Polymers in Tissue Engineering: A Focus on Electrospun Extracellular Matrix Analogues. Polymers 2010, 2, 522. [Google Scholar] [CrossRef]
- Wan, J. Microfluidic-Based Synthesis of Hydrogel Particles for Cell Microencapsulation and Cell-Based Drug Delivery. Polymers 2012, 4, 1084. [Google Scholar] [CrossRef]
- Chen, C.S.; Mrksich, M.; Huang, S.; Whitesides, G.M.; Ingber, D.E. Geometric control of cell life and death. Science 1997, 276, 1425–1428. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zheng, F.; Cheng, Y.; Ding, H.; Zhao, Y.; Gu, Z. Hybrid inverse opals for regulating cell adhesion and orientation. Nanoscale 2014, 6, 10650–10656. [Google Scholar] [CrossRef] [PubMed]
- Mammoto, T.; Ingber, D.E. Mechanical control of tissue and organ development. Development 2010, 137, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Raftery, R.; O’Brien, F.J.; Cryan, S.-A. Chitosan for Gene Delivery and Orthopedic Tissue Engineering Applications. Molecules 2013, 18, 5611–5647. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Singh, D.; Han, S. 3D Printing of Scaffold for Cells Delivery: Advances in Skin Tissue Engineering. Polymers 2016, 8, 19. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, W.; Jiang, X. Precise control of cell adhesion by combination of surface chemistry and soft lithography. Adv. Healthc. Mater. 2013, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, C.J.; Langer, R.; Borenstein, J.T. Engineering Substrate Topography at the Micro- and Nanoscale to Control Cell Function. Angew. Chem. Int. Ed. 2009, 48, 5406–5415. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.H.; Shi, X.L.; Feng, Z.Q.; Gu, Z.Z.; Ding, Y.T. Chitosan nanofiber scaffold enhances hepatocyte adhesion and function. Biotechnol. Lett. 2009, 31, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, N.; Kasahara, Y.; Yamane, S.; Igarashi, T.; Minami, A.; Nisimura, S.-I. Chitosan-Based Hyaluronic Acid Hybrid Polymer Fibers as a Scaffold Biomaterial for Cartilage Tissue Engineering. Polymers 2011, 3, 100–113. [Google Scholar] [CrossRef]
- Lee, J.; Balikov, D.; Yang, J.; Kim, K.; Park, H.; Kim, J.; Kwon, I.; Bellan, L.; Sung, H.-J. Cationic Nanocylinders Promote Angiogenic Activities of Endothelial Cells. Polymers 2016, 8, 15. [Google Scholar] [CrossRef]
- Lu, J.; Zou, X.; Zhao, Z.; Mu, Z.; Zhao, Y.; Gu, Z. Cell Orientation Gradients on an Inverse Opal Substrate. ACS Appl. Mater. Int. 2015, 7, 10091–10095. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Tang, Z.M.; Feng, Z.Q.; Xie, Z.Y.; Gu, Z.Z. Stretched inverse opal colloid crystal substrates-induced orientation of fibroblast. Biomed. Mater. 2010, 5, 35011. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Sun, J.; Ding, J. Critical areas of cell adhesion on micropatterned surfaces. Biomaterials 2011, 32, 3931–3938. [Google Scholar] [CrossRef] [PubMed]
- Egles, C.; Zonari, A.; Novikoff, S.; Electo, N.R.P.; Breyner, N.M.; Gomes, D.A.; Martins, A.; Neves, N.M.; Reis, R.L.; Goes, A.M. Endothelial Differentiation of Human Stem Cells Seeded onto Electrospun Polyhydroxybutyrate/Polyhydroxybutyrate-Co-Hydroxyvalerate Fiber Mesh. PLoS ONE 2012, 7, e35422. [Google Scholar]
- Lee, Y.-S.; Livingston Arinzeh, T. Electrospun Nanofibrous Materials for Neural Tissue Engineering. Polymers 2011, 3, 413–426. [Google Scholar] [CrossRef]
- Lu, H.; Feng, Z.; Gu, Z.; Liu, C. Growth of outgrowth endothelial cells on aligned PLLA nanofibrous scaffolds. J. Mater. Sci. Mater. Med. 2009, 20, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Popa, E.; Santo, V.; Rodrigues, M.; Gomes, M. Magnetically-Responsive Hydrogels for Modulation of Chondrogenic Commitment of Human Adipose-Derived Stem Cells. Polymers 2016, 8, 28. [Google Scholar] [CrossRef]
- Schulte, V.A.; Díez, M.; Möller, M.; Lensen, M.C. Surface Topography Induces Fibroblast Adhesion on Intrinsically Nonadhesive Poly(ethylene glycol) Substrates. Biomacromolecules 2009, 10, 2795–2801. [Google Scholar] [CrossRef] [PubMed]
- Udenni Gunathilake, T.; Ching, Y.; Ching, K.; Chuah, C.; Abdullah, L. Biomedical and Microbiological Applications of Bio-Based Porous Materials: A Review. Polymers 2017, 9, 160. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, H. Biodegradability and Biocompatibility Study of Poly(Chitosan-g-lactic Acid) Scaffolds. Molecules 2012, 17, 3243–3258. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Arazi, N.; Domb, A.J.; Katzhendler, J. New Biocompatible Polyesters Derived from α-Amino Acids: Hydrolytic Degradation Behavior. Polymers 2010, 2, 418–439. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, J.; Ro, H.W.; Wang, Z.; Zhou, J.; Lin, N.J.; Cicerone, M.T.; Soles, C.L.; Lin-Gibson, S. Thermodynamic Underpinnings of Cell Alignment on Controlled Topographies. Adv. Mater. 2011, 23, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Liu, X.; Liu, Y.; Zhang, L. Enhancing the Compatibility, Hydrophilicity and Mechanical Properties of Polysulfone Ultrafiltration Membranes with Lignocellulose Nanofibrils. Polymers 2016, 8, 349. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Negahi Shirazi, A.; Dehghani, F. Biomedical Applications of Biodegradable Polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef]
- Mhd Haniffa, M.; Ching, Y.; Abdullah, L.; Poh, S.; Chuah, C. Review of Bionanocomposite Coating Films and Their Applications. Polymers 2016, 8, 246. [Google Scholar] [CrossRef]
- Park, S.; Park, K. Engineered Polymeric Hydrogels for 3D Tissue Models. Polymers 2016, 8, 23. [Google Scholar] [CrossRef]
- Haidar, Z.S. Bio-Inspired/-Functional Colloidal Core-Shell Polymeric-Based NanoSystems: Technology Promise in Tissue Engineering, Bioimaging and NanoMedicine. Polymers 2010, 2, 323. [Google Scholar] [CrossRef]
- Liu, W.; Shang, L.; Zheng, F.; Lu, J.; Qian, J.; Zhao, Y.; Gu, Z. Photonic crystal encoded microcarriers for biomaterial evaluation. Small 2014, 10, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Nilsen-Nygaard, J.; Strand, S.; Vårum, K.; Draget, K.; Nordgård, C. Chitosan: Gels and Interfacial Properties. Polymers 2015, 7, 552. [Google Scholar] [CrossRef]
- Sharma, J.; Lizu, M.; Stewart, M.; Zygula, K.; Lu, Y.; Chauhan, R.; Yan, X.; Guo, Z.; Wujcik, E.; Wei, S. Multifunctional Nanofibers towards Active Biomedical Therapeutics. Polymers 2015, 7, 186. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, Q.; Shi, L.; Zhang, X.; Zhang, K.-Q. Bio-inspired sensors based on photonic structures of Morpho butterfly wings: A review. J. Mater. Chem. C 2016, 4, 1752–1763. [Google Scholar] [CrossRef]
- Tao, L.; Wenhong, P.; Shenmin, Z.; Di, Z. Bio-inspired fabrication of stimuli-responsive photonic crystals with hierarchical structures and their applications. Nanotechnology 2016, 27, 122001. [Google Scholar]
- Ye, B.; Ding, H.; Cheng, Y.; Gu, H.; Zhao, Y.; Xie, Z.; Gu, Z. Photonic Crystal Microcapsules for Label-free Multiplex Detection. Adv. Mater. 2014, 26, 3270–3274. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xie, Z.; Gu, H.; Zhu, C.; Gu, Z. Bio-inspired variable structural color materials. Chem. Soc. Rev. 2012, 41, 3297–3317. [Google Scholar] [CrossRef] [PubMed]
- Cary, A.T.; Yulan, F.; Anne-Martine, J.; Eugenii, U.D.; Rene, L. Reproduction and optical analysis of Morpho -inspired polymeric nanostructures. J. Opt. 2016, 18, 065105. [Google Scholar]
- Garrett, N.L.; Sekine, R.; Dixon, M.W.A.; Tilley, L.; Bambery, K.R.; Wood, B.R. Bio-sensing with butterfly wings: naturally occurring nano-structures for SERS-based malaria parasite detection. Phys Chem. Chem. Phys. 2015, 17, 21164–21168. [Google Scholar] [CrossRef] [PubMed]
- Potyrailo, R.A.; Ghiradella, H.; Vertiatchikh, A.; Dovidenko, K.; Cournoyer, J.R.; Olson, E. Morpho butterfly wing scales demonstrate highly selective vapour response. Nat. Photonics 2007, 1, 123–128. [Google Scholar] [CrossRef]
- Potyrailo, R.A.; Starkey, T.A.; Vukusic, P.; Ghiradella, H.; Vasudev, M.; Bunning, T.; Naik, R.R.; Tang, Z.; Larsen, M.; Deng, T.; et al. Discovery of the surface polarity gradient on iridescent Morpho butterfly scales reveals a mechanism of their selective vapor response. Proc. Natl. Acad. Sci. USA 2013, 110, 15567–15572. [Google Scholar] [CrossRef] [PubMed]
- Siddique, R.H.; Faisal, A.; Hünig, R.; Bartels, C.; Wacker, I.; Lemmer, U.; Hölscher, H. Utilizing laser interference lithography to fabricate hierarchical optical active nanostructures inspired by the blue Morpho butterfly. Proc. SPIE 2014, 9187, 91870E-1. [Google Scholar]
- Zhang, S.; Chen, Y. Nanofabrication and coloration study of artificial Morpho butterfly wings with aligned lamellae layers. Sci. Rep. 2015, 5, 16637. [Google Scholar] [CrossRef] [PubMed]
- Cortese, L.; Pattelli, L.; Utel, F.; Vignolini, S.; Burresi, M.; Wiersma, D.S. Anisotropic Light Transport in White Beetle Scales. Adv. Opt. Mater. 2015, 3, 1337–1341. [Google Scholar] [CrossRef]
- Ehrlich, H.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; Schupp, P.; et al. Three-Dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Int. J. Biol. Macromol. 2010, 47, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Schröder-Turk, G.E.; Wickham, S.; Averdunk, H.; Brink, F.; Fitz Gerald, J.D.; Poladian, L.; Large, M.C.J.; Hyde, S.T. The chiral structure of porous chitin within the wing-scales of Callophrys rubi. J. Struct. Biol. 2011, 174, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Petrenko, I.; Stelling, A.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan Chitin as a Versatile Template for Extreme Biomimetics. Polymers 2015, 7, 235. [Google Scholar] [CrossRef]
- Saranathan, V.; Osuji, C.O.; Mochrie, S.G.; Noh, H.; Narayanan, S.; Sandy, A.; Dufresne, E.R.; Prum, R.O. Structure, function, and self-assembly of single network gyroid (I4132) photonic crystals in butterfly wing scales. Proc. Natl. Acad. Sci. USA 2010, 107, 11676–11681. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Chennazhi, K.P.; Srinivasan, S.; Nair, S.V.; Furuike, T.; Tamura, H. Chitin scaffolds in tissue engineering. Int. J. Mol. Sci. 2011, 12, 1876–1887. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.Q.; Chu, X.; Huang, N.P.; Wang, T.; Wang, Y.; Shi, X.; Ding, Y.; Gu, Z.Z. The effect of nanofibrous galactosylated chitosan scaffolds on the formation of rat primary hepatocyte aggregates and the maintenance of liver function. Biomaterials 2009, 30, 2753–2763. [Google Scholar] [CrossRef] [PubMed]
- Mu, Z.; Zhao, X.; Xie, Z.; Zhao, Y.; Zhong, Q.; Bo, L.; Gu, Z. In situ synthesis of gold nanoparticles (AuNPs) in butterfly wings for surface enhanced Raman spectroscopy (SERS). J. Mater. Chem. B 2013, 1, 1607–1613. [Google Scholar] [CrossRef]
- Howling, G.I.; Dettmar, P.W.; Goddard, P.A.; Hampson, F.C.; Dornish, M.; Wood, E.J. The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro. Biomaterials 2001, 22, 2959–2966. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Mutsenko, V.V.; Bazhenov, V.V.; Rogulska, O.; Tarusin, D.N.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; Meißner, H.; Lode, A.; et al. 3D chitinous scaffolds derived from cultivated marine demosponge Aplysina aerophoba for tissue engineering approaches based on human mesenchymal stromal cells. Int. J. Biol. Macromol. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Mutsenko, V.V.; Gryshkov, O.; Lauterboeck, L.; Rogulska, O.; Tarusin, D.N.; Bazhenov, V.V.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; et al. Novel chitin scaffolds derived from marine sponge Ianthella basta for tissue engineering approaches based on human mesenchymal stromal cells: Biocompatibility and cryopreservation. Int. J. Biol. Macromol. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, T.; Majima, T.; Suenaga, N.; Iwasaki, N.; Yamane, S.; Minami, A. Rotator cuff regeneration using chitin fabric as an acellular matrix. J. Shoulder Elbow Surg. 2006, 15, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Sowmya, S.; Kumar, P.T.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbaz, A.; Lu, J.; Gao, B.; Zheng, F.; Mu, Z.; Zhao, Y.; Gu, Z. Chitin-Based Anisotropic Nanostructures of Butterfly Wings for Regulating Cells Orientation. Polymers 2017, 9, 386. https://doi.org/10.3390/polym9090386
Elbaz A, Lu J, Gao B, Zheng F, Mu Z, Zhao Y, Gu Z. Chitin-Based Anisotropic Nanostructures of Butterfly Wings for Regulating Cells Orientation. Polymers. 2017; 9(9):386. https://doi.org/10.3390/polym9090386
Chicago/Turabian StyleElbaz, Abdelrahman, Jie Lu, Bingbing Gao, Fuyin Zheng, Zhongde Mu, Yuanjin Zhao, and Zhongze Gu. 2017. "Chitin-Based Anisotropic Nanostructures of Butterfly Wings for Regulating Cells Orientation" Polymers 9, no. 9: 386. https://doi.org/10.3390/polym9090386




