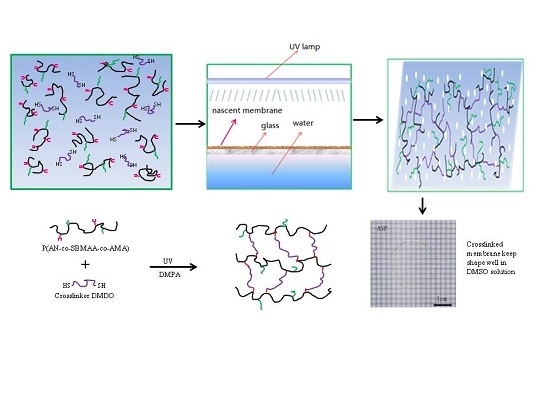
Facile Preparation of Crosslinked PAN Membranes Based on Thiol-Ene Photopolymerization
Abstract
:1. Introduction
2. Experimental
2.1. Materials
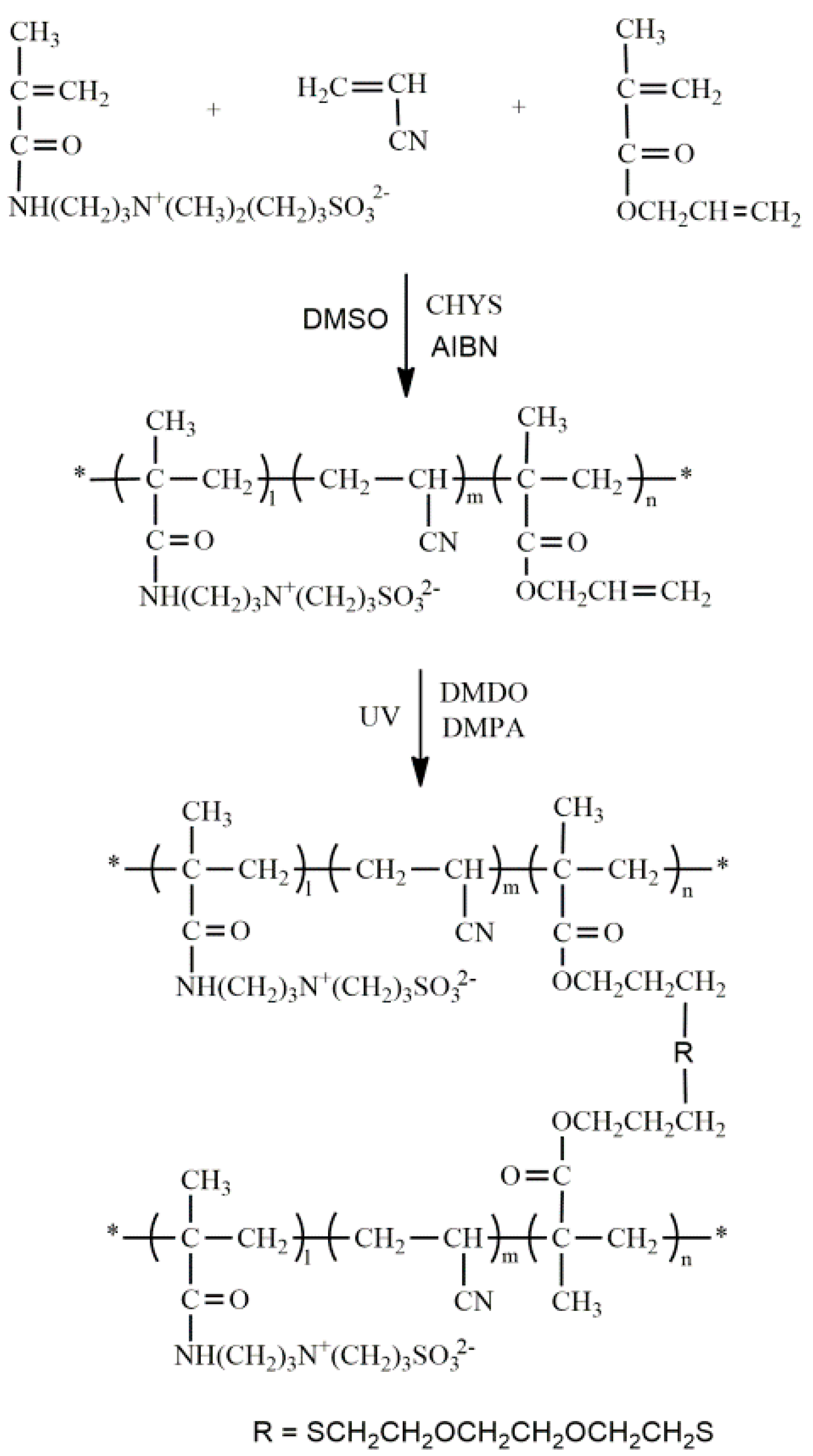
2.2. Synthesis and Characterization of P(AN-co-SBMAA-co-AMA)
2.3. Preparation of PAN-Based Crosslinked Membranes
2.4. Characterization and Measurement Methods
2.4.1. Characterization of P(AN-co-SBMAA-co-AMA)
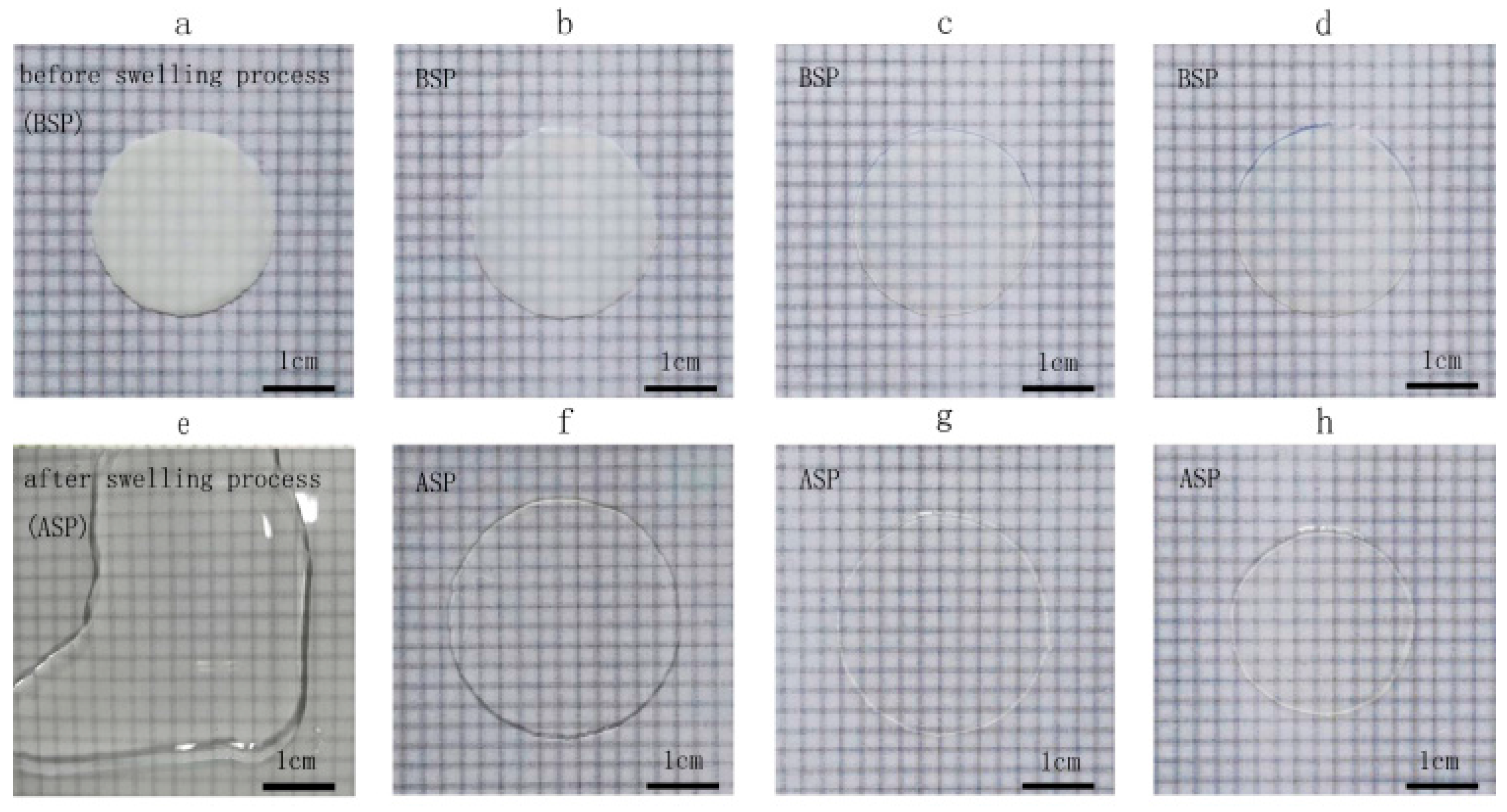
2.4.2. Evaluation of the Crosslinking Density of Membranes
2.4.3. Membrane Morphologies
2.4.4. Permeability Measurements
3. Results and Discussion
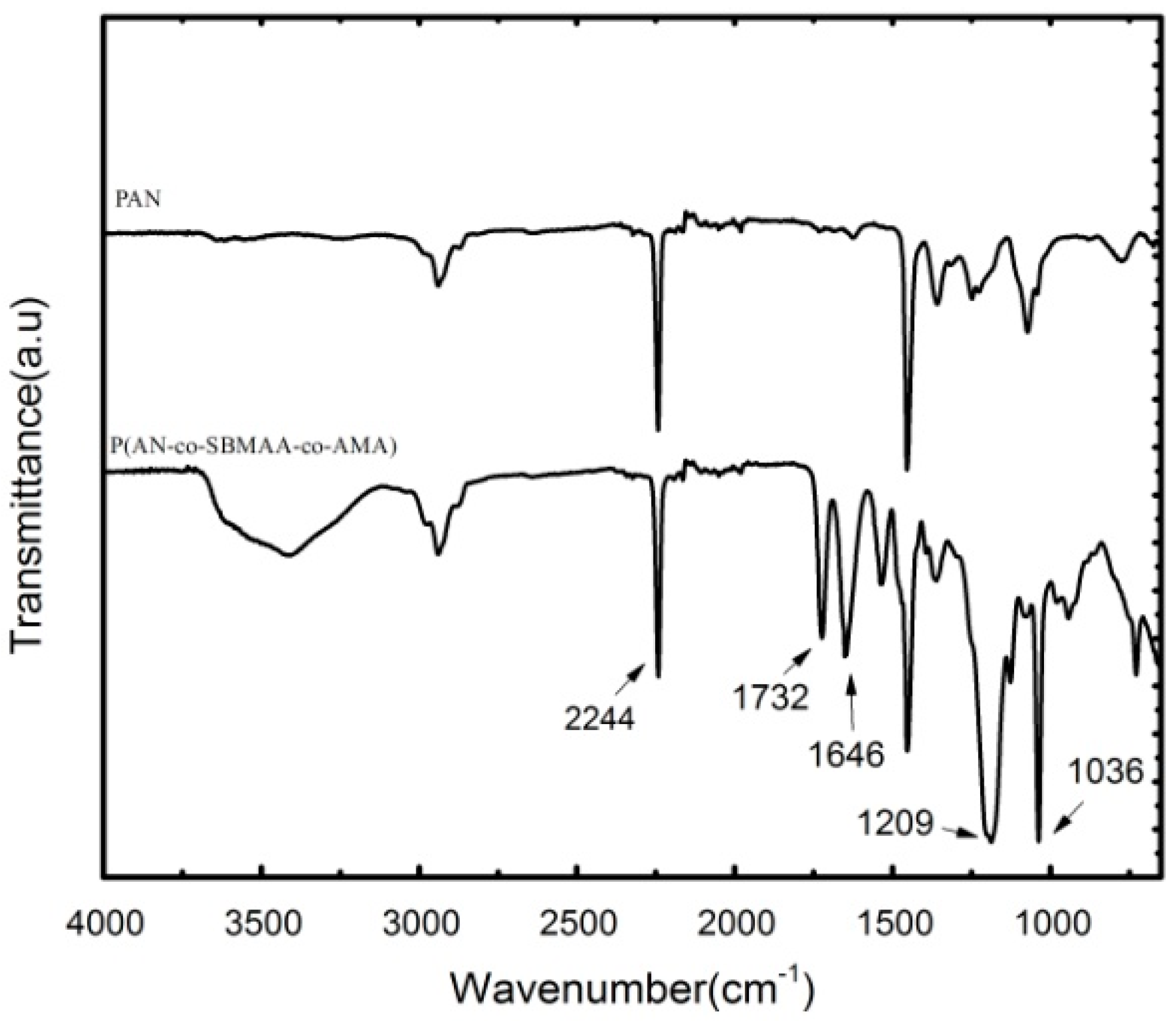
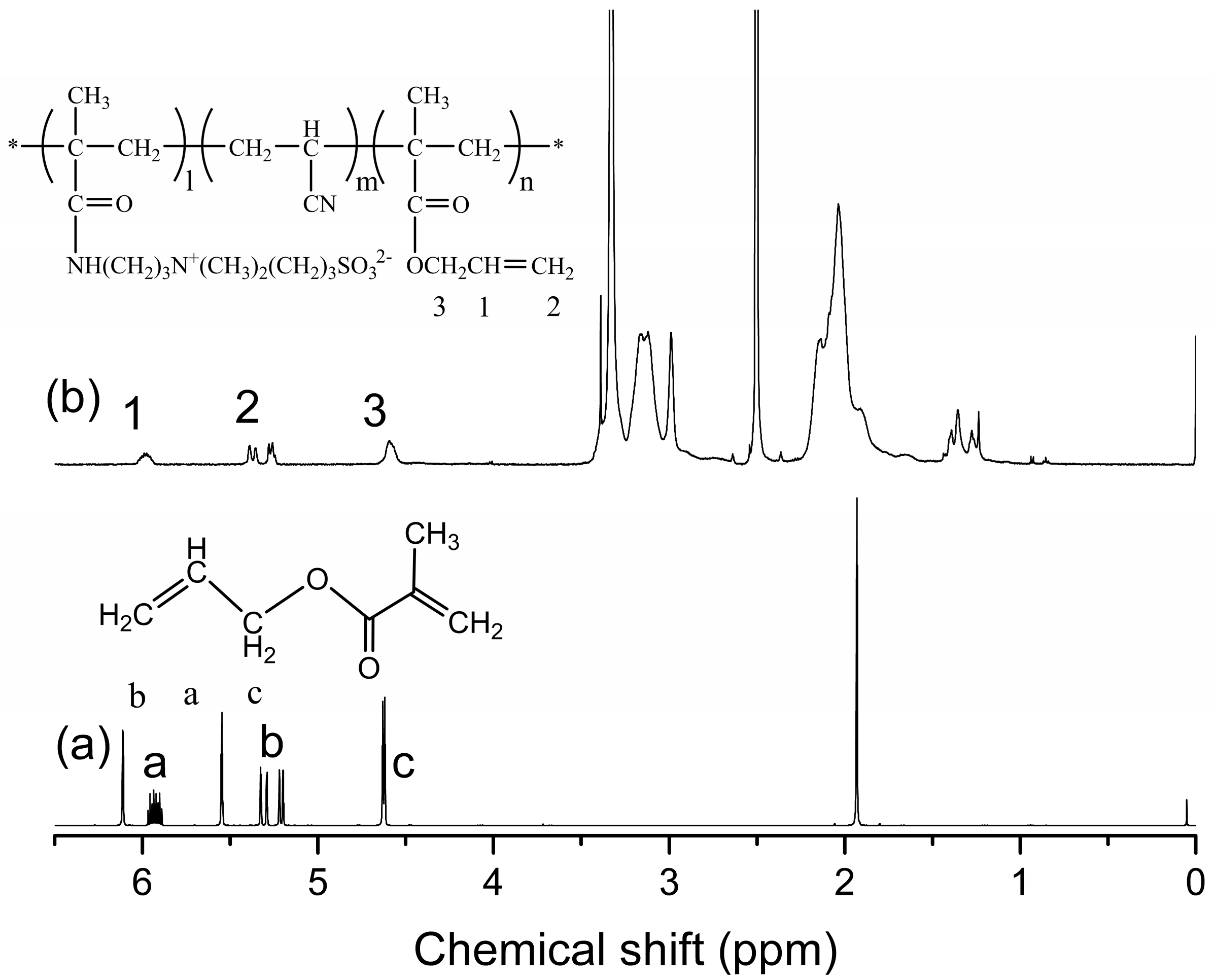
3.1. Characterization of the P(AN-co-SBMAA-co-AMA) Copolymer
3.2. Evaluation of Membrane Crosslinking Density
3.3. Evaluation of Membrane Crosslinking Density
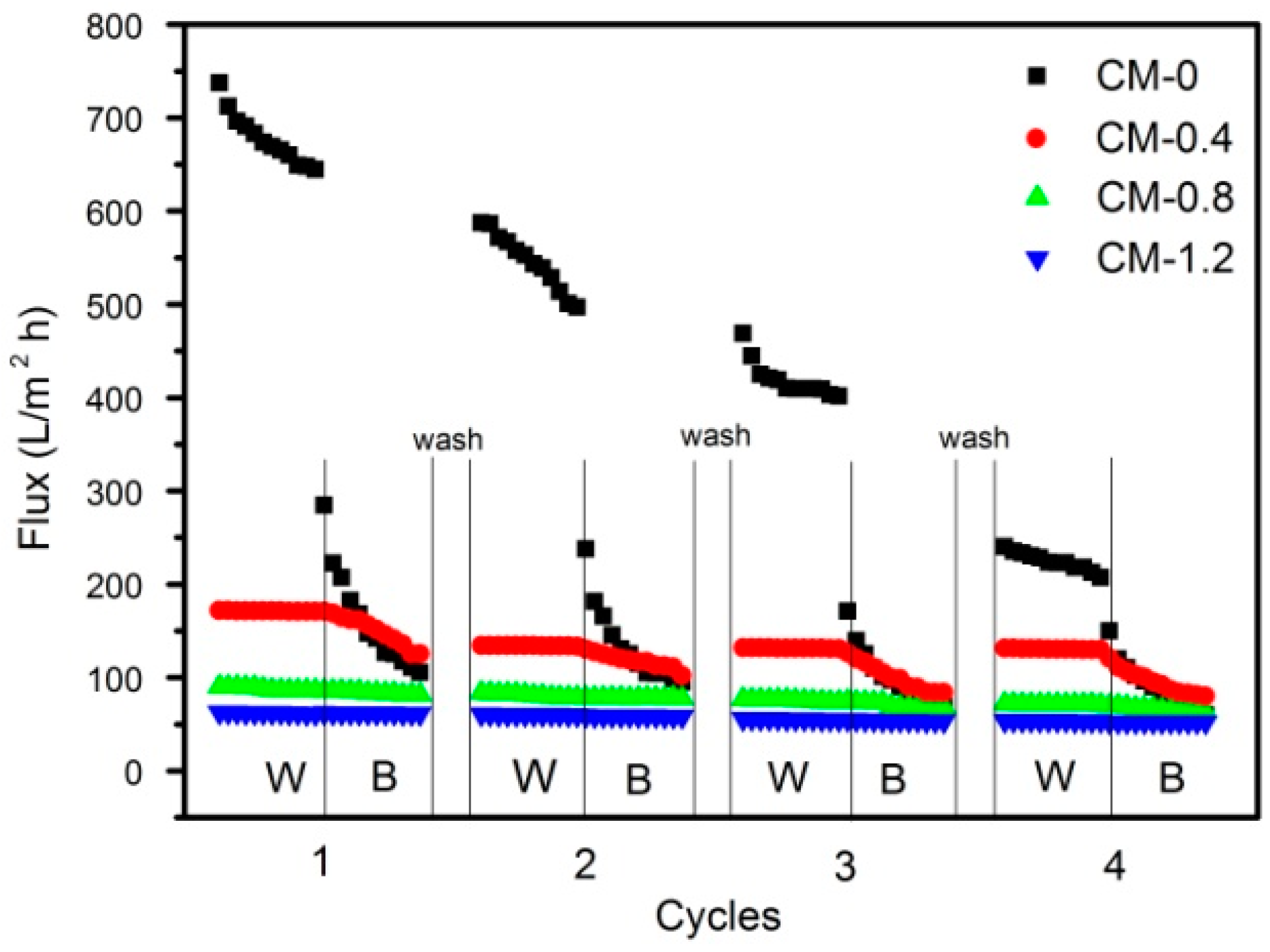
3.4. Permeability Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ognibene, G.; Cristaldi, D.A.; Fiorenza, R.; Blanco, I.; Cicala, G.; Scire, S.; Fragala, M.E. Photoactivity of hierarchically nanostructured ZnO-PES fibre mats for water treatments. RSC Adv. 2016, 6, 42778–42785. [Google Scholar] [CrossRef]
- Mohammad, H.; Ragab, A.Z.; Enas, H.; Linn, B.; Yvonne, A.; Kristiina, O. Membranes based on cellulose nanofibers and activated carbon for removal of escherichia coli bacteria from water. Polymers 2017, 9, 335–348. [Google Scholar]
- Ong, Y.K.; Shi, G.M.; Le, N.L.; Tang, Y.P.; Zuo, J.; Nunes, S.P.; Chung, T.S. Recent membrane development for pervaporation processes. Prog. Polym. Sci. 2016, 57, 1–31. [Google Scholar] [CrossRef]
- Zuo, Z.C.; Fu, Y.Z.; Manthiram, A. Novel blend membranes based on acid-base interactions for fuel cells. Polymer 2012, 4, 1627–1644. [Google Scholar] [CrossRef]
- Gianluca, C.; Ignazio, B.; Alberta, L.; Giulia, O.; Francesco, A.B.; Maria, E.F. PES/POSS soluble veils as advanced modifiers for multifunctional fiber reinforced composites. Polymers 2017, 9, 281–296. [Google Scholar]
- Fei, Z.D.; Wan, L.S.; Wang, W.M.; Zhong, M.Q.; Xu, Z.K. Thermo-responsive polyacrylonitrile membranes prepared with poly(acrylonitrile-g-isopropylacrylamide) as an additive. J. Membr. Sci. 2013, 432, 42–49. [Google Scholar] [CrossRef]
- Schulze, A.; Breite, D.; Kim, Y. Bio-inspired polymer membrane surface cleaning. Polymers 2017, 9, 97. [Google Scholar] [CrossRef]
- Peng, Y.L.; Han, H.; Fan, H.W. Characterization and performance of PVA/Psf composite hollow fiber UF membrane prepared with interfacial polymerization. Polym. Eng. Sci. 2012, 52, 557–565. [Google Scholar] [CrossRef]
- Morgado, P.I.; Ricardo, A.; Correia, I.J. Asymmetric membranes as ideal wound dressings: An overview on production methods, structure, properties and performance relationship. J. Membr. Sci. 2015, 490, 139–151. [Google Scholar] [CrossRef]
- Park, S.J.; Choi, W.; Nam, S.E.; Hong, S.; Lee, J.S.; Lee, J.H. Fabrication of polyamide thin film composite reverse osmosis membranes via support-free interfacial polymerization. J. Membr. Sci. 2017, 526, 52–59. [Google Scholar] [CrossRef]
- Zhang, J.L.; Liu, H.D.; Liu, H.X.; Hu, J.; Tan, S.Z.; Wu, T. Using diethylamine as crosslinking agent for getting polyepichlorohydrin-based composite membrane with high tensile strength and good chemical stability. Polym. Bull. 2017, 74, 625–639. [Google Scholar] [CrossRef]
- Reinelt, S.; Tabatabai, M.; Fischer, U.K.; Moszner, N.; Utterodt, A. Investigations of thiol-modified phenol derivatives for the use in thiol-ene photopolymerizations. Beilstein J. Org. Chem. 2014, 10, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.B. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis: A first update. Polym. Chem. 2014, 5, 4820–4870. [Google Scholar] [CrossRef]
- Cigil, A.B.; Cankurtaran, H.; Kahraman, M.V. Photo-crosslinked thiol-ene based hybrid polymeric sensor for humidity detection. React. Funct. Polym. 2017, 114, 75–85. [Google Scholar] [CrossRef]
- Lange, S.C.; Andel, E.V.; Smulders, M.M.J.; Han, Z. Efficient and tunable three-dimensional functionalization of fully zwitterionic antifouling surface coatings. Langmuir 2016, 32, 10199–10205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.G.; Wan, L.S.; Xu, Z.K. Surface engineerings of polyacrylonitrile-based asymmetric membranes towards biomedical applications: An overview. J. Membr. Sci. 2007, 304, 8–23. [Google Scholar] [CrossRef]
- Matsumoto, A.; Asai, S.; Shimizu, S.; Aota, H. Free-radical cross-linking polymerization of unsymmetrical divinyl compounds 2. Steric effect on gelation in the copolymerizations of allyl methacrylate with several alkyl methacrylates. Eur. Polym. J. 2002, 38, 863–868. [Google Scholar] [CrossRef]
- Heatley, F.; Lovell, P.A.; McDonald, J. NMR-studies of free-radical polymerization and copolymerization of monomers and polymers containing allyl groups. Eur. Polym. J. 1993, 29, 255–268. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, R.S.; Huglin, M.B.; Holmes, P.A. Reactivity ratios in copolymerizations involving allyl methacrylate. Polymer 1996, 37, 1437–1441. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Baggerman, J.; Paulusse, J.M.J.; Rijn, C.J.M.; Zuilhof, H. Stable protein-repellent zwitterionic polymer brushes grafted from silicon nitride. Langmuir 2011, 27, 2587–2594. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Zheng, J.; Li, L.Y.; Jiang, S.Y. Strong resistance of phosphorylcholine self-assembled monolayers to protein adsorption: Insights into nonfouling properties of zwitterionic materials. J. Am. Chem. Soc. 2005, 125, 14473–14478. [Google Scholar] [CrossRef] [PubMed]

| Membrane | Tensile Strength (MPa) | Young’s Modulus (MPa) | RP (MPa) | ΔS (%) | GF (%) |
|---|---|---|---|---|---|
| CM-0 | 0.36 | 18.9 | 0.01 | / | 0 |
| CM-0.4 | 0.96 | 19.1 | 0.05 | 34.3 | 81.8 |
| CM-0.8 | 1.25 | 24.4 | 0.08 | 25.2 | 91.3 |
| CM-1.2 | 1.64 | 33.4 | 0.11 | 1.5 | 94.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fei, Z.; Wang, T.; Fan, P.; Chen, F.; Zhong, M. Facile Preparation of Crosslinked PAN Membranes Based on Thiol-Ene Photopolymerization. Polymers 2017, 9, 390. https://doi.org/10.3390/polym9090390
Fei Z, Wang T, Fan P, Chen F, Zhong M. Facile Preparation of Crosslinked PAN Membranes Based on Thiol-Ene Photopolymerization. Polymers. 2017; 9(9):390. https://doi.org/10.3390/polym9090390
Chicago/Turabian StyleFei, Zhengdong, Tao Wang, Ping Fan, Feng Chen, and Mingqiang Zhong. 2017. "Facile Preparation of Crosslinked PAN Membranes Based on Thiol-Ene Photopolymerization" Polymers 9, no. 9: 390. https://doi.org/10.3390/polym9090390