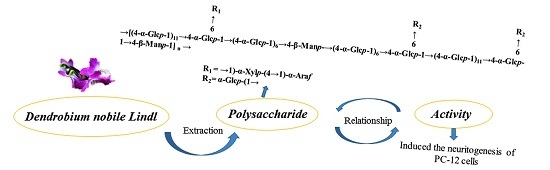
Structural Characterization of Mannoglucan from Dendrobium nobile Lindl and the Neuritogenesis-Induced Effect of Its Acetylated Derivative on PC-12 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of JCS1 and Its Acetylated Derivative
2.2.1. Preparation of JCS1
2.2.2. Preparation of Acetylated Derivative
2.3. Homogeneity and Molecular Weight
2.4. Monosaccharide Composition Analysis
2.5. Methylation Analysis
2.6. Fourier Transform Infrared (FTIR) and Nuclear Magnetic Resonance (NMR) Analysis
2.7. Partial Acid Hydrolysis
2.8. Bioactivity Test of Polysaccharide in PC-12 Cells
3. Results
3.1. Isolation, Purification, and Composition Analysis
3.2. IR and Specific Rotation Analysis
3.3. Linkage Type Analysis
3.4. Partial Acid Hydrolysis and Structure Characterization of Degraded Polysaccharide
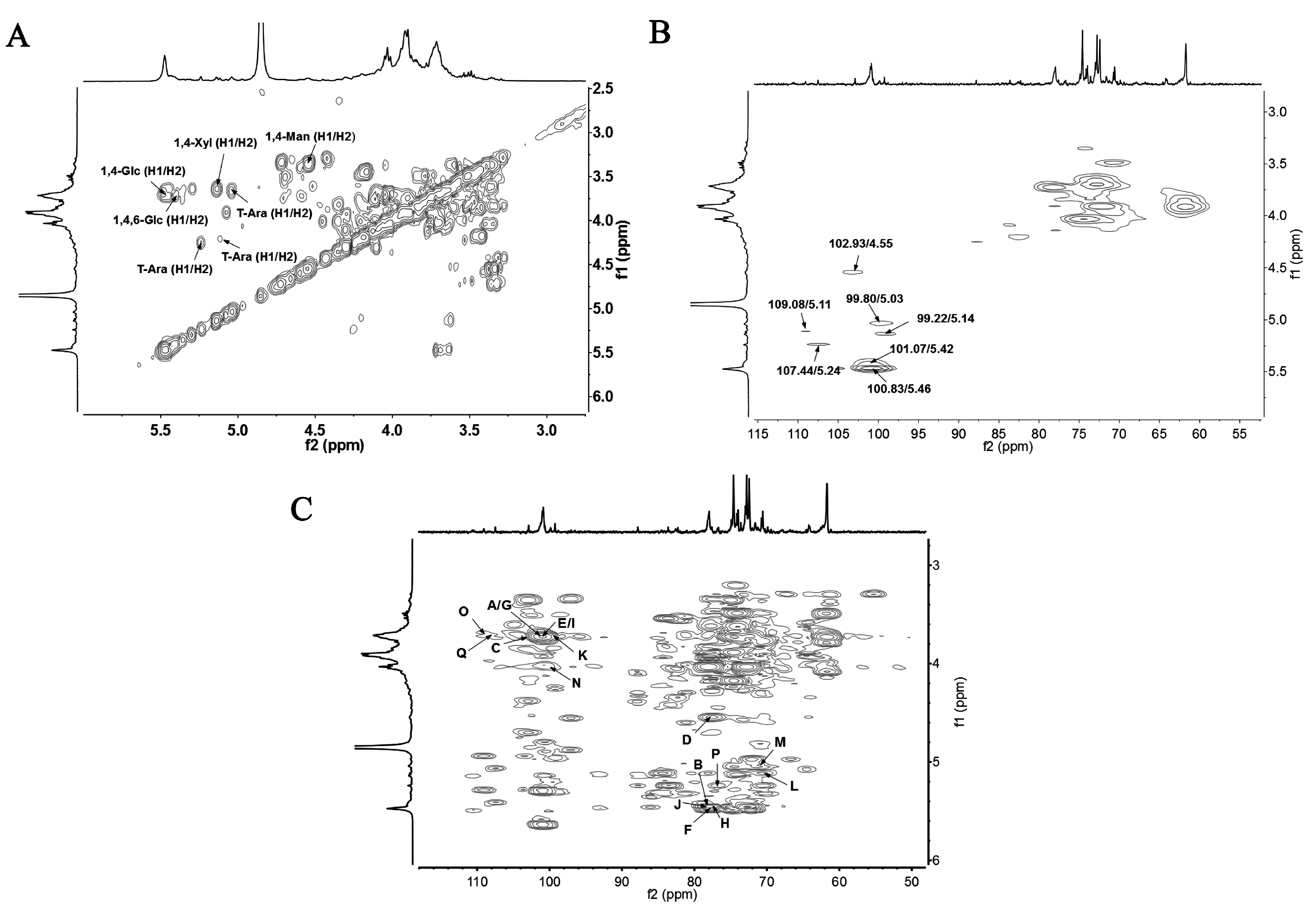
3.5. NMR Results
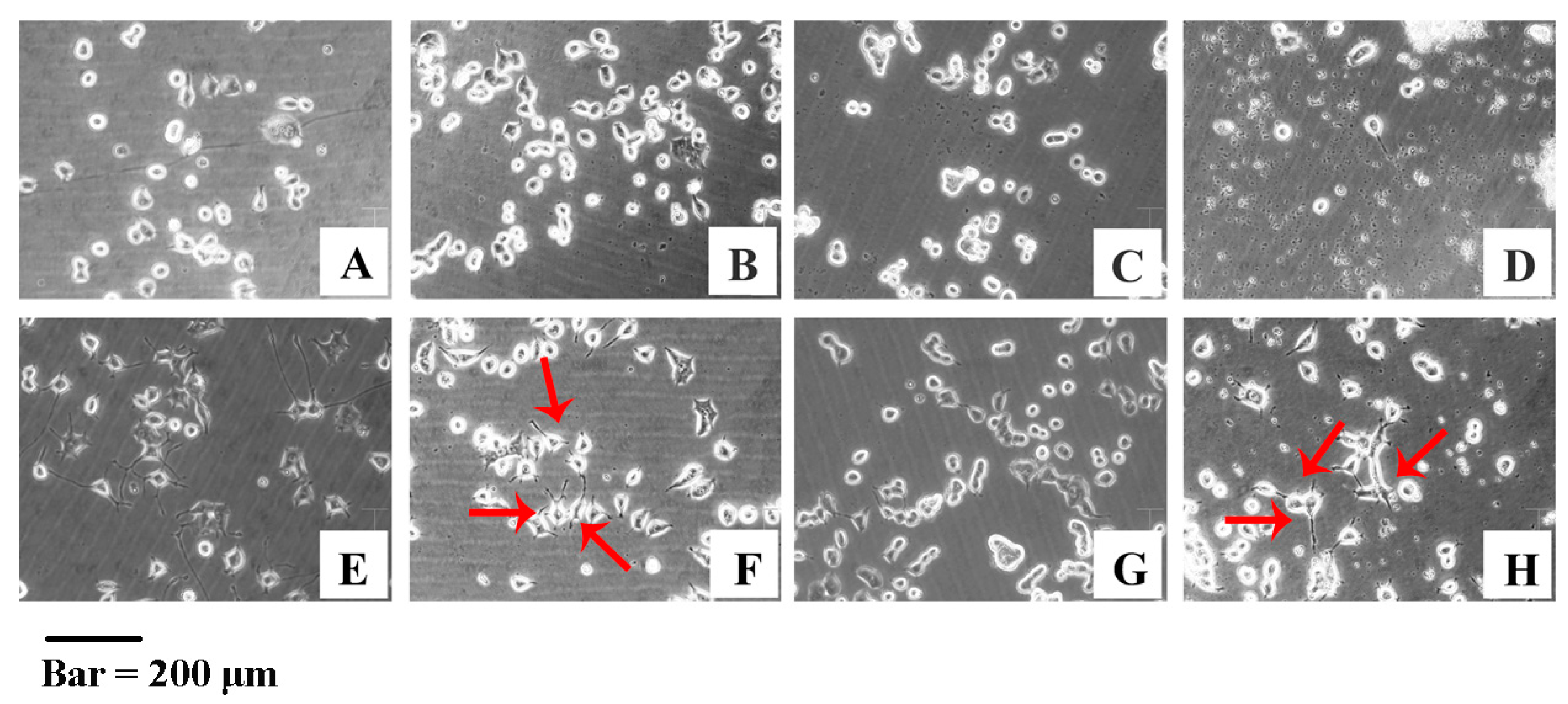
3.6. YJCS1 Induces Neurite Extension of PC-12 Cells
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, J.H.; Zha, X.Q.; Luo, J.P.; Yang, X.F. An acetylated galactomannoglucan from the stems of Dendrobium nobile Lindl. Carbohydr. Res. 2010, 345, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Luo, J.P.; Zha, X.Q. Structural features of a pectic polysaccharide from the stems of Dendrobium nobile Lindl. Carbohydr. Polym. 2010, 81, 1–7. [Google Scholar] [CrossRef]
- Wang, J.H.; Luo, J.P.; Yang, X.F.; Zha, X.Q. Structural analysis of a rhamnoarabinogalactan from the stems of Dendrobium nobile Lindl. Food Chem. 2010, 122, 572–576. [Google Scholar] [CrossRef]
- Luo, A.; He, X.; Zhou, S.; Fan, Y.; He, T.; Chun, Z. In vitro antioxidant activities of a water-soluble polysaccharide derived from Dendrobium nobile Lindl. Extracts. Int. J. Biol. Macromol. 2009, 45, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Luo, J.P.; Zha, X.Q.; Feng, B.J. Comparison of antitumor activities of different polysaccharide fractions from the stems of Dendrobium nobile Lindl. Carbohydr. Polym. 2010, 79, 114–118. [Google Scholar] [CrossRef]
- Pan, L.H.; Li, X.F.; Wang, M.N.; Zha, X.Q.; Yang, X.F.; Liu, Z.J.; Luo, Y.B.; Luo, J.P. Comparison of hypoglycemic and antioxidative effects of polysaccharides from four different Dendrobium species. Int. J. Biol. Macromol. 2014, 64, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, W.; Yao, W.; Pang, X.; Yin, D.; Gao, X. Carboxymethylation of a polysaccharide extracted from ganoderma lucidum enhances its antioxidant activities in vitro. Carbohydr. Polym. 2009, 78, 227–234. [Google Scholar] [CrossRef]
- Qin, T.; Chen, J.; Wang, D.; Hu, Y.; Zhang, J.; Wang, M.; Qiu, S.; Gao, Z.; Liu, R.; Yu, Y.; et al. Selenylation modification can enhance immune-enhancing activity of Chinese angelica polysaccharide. Carbohydr. Polym. 2013, 95, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chen, H.; Ma, L.; Zhang, Y. Physical modifications of polysaccharide from Inonotus obliquus and the antioxidant properties. Int. J. Biol. Macromol. 2013, 54, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhang, J.; Yao, J.; Song, S.; Yin, Z.; Gao, Q. Selenylation modification can enhance antioxidant activity of Potentilla anserina L. polysaccharide. Int. J. Biol. Macromol. 2013, 58, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, H.; Wang, Y.; Nie, S.; Li, C.; Xie, M. Acetylation and carboxymethylation of the polysaccharide from Ganoderma atrum and their antioxidant and immunomodulating activities. Food Chem. 2014, 156, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Zhang, F.; Wang, Z.J.; Shen, M.Y.; Nie, S.P.; Xie, M.Y. Preparation, characterization and antioxidant activities of acetylated polysaccharides from Cyclocarya paliurus leaves. Carbohydr. Polym. 2015, 133, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Wan, Z.J.; Shi, L.; Lu, X.X. Preparation and antiherpetic activities of chemically modified polysaccharides from polygonatum cyrtonema hua. Carbohydr. Polym. 2011, 83, 737–742. [Google Scholar] [CrossRef]
- Zeng, H. Investigation on Preparation of Acemannan Analogy by Molecular Modification of KGM and Its Bioactivity. Master’s Thesis, Jiangnan University, Wuxi, China, 2006. (In Chinese). [Google Scholar]
- Wang, H.J.; Shi, S.S.; Bao, B.; Li, X.J.; Wang, S.C. Structure characterization of an arabinogalactan from green tea and its anti-diabetic effect. Carbohydr. Polym. 2010, 81, 1–7. [Google Scholar] [CrossRef]
- Needs, P.W.; Selvendran, R.R. Avoiding oxidative-degradation duringsodium-hydroxide methyl iodide-mediated carbohydrate methylation indimethyl-sulfoxide. Carbohydr. Res. 1993, 245, 1–10. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, L.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Blumenkrantz, N.; Asboehansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Wang, J.; Song, H.; Zhang, H.; Niu, X. Chemical modification and influence of function groups on the in vitro-antioxidant activities of porphyran from porphyra haitanensis. Carbohydr. Polym. 2010, 79, 290–295. [Google Scholar] [CrossRef]
- Zheng, C.; Dong, Q.; Chen, H.; Cong, Q.; Ding, K. Structural characterization of a polysaccharide from chrysanthemum morifolium flowers and its antioxidant activity. Carbohydr. Polym. 2015, 130, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liao, W.; Fang, J.; Liu, Q.; Yao, J.; Hu, M.; Ding, K. A glucan isolated from flowers of Lonicera japonica Thunb. Inhibits aggregation and neurotoxicity of abeta42. Carbohydr. Polym. 2014, 110, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cao, D.; Zhou, L.; Jin, H.; Dong, Q.; Yao, J.; Ding, K. Structure of a polysaccharide from Gastrodia elata Bl., and oligosaccharides prepared thereof with anti-pancreatic cancer cell growth activities. Carbohydr. Polym. 2011, 86, 1300–1305. [Google Scholar] [CrossRef]
- Zha, X.Q.; Luo, J.P.; Luo, S.Z.; Jiang, S.T. Structure identification of a new immunostimulating polysaccharide from the stems of Dendrobium huoshanense. Carbohydr. Polym. 2007, 69, 86–93. [Google Scholar] [CrossRef]
- Dey, B.; Bhunia, S.K.; Maity, K.K.; Patra, S.; Mandal, S.; Maiti, S.; Maiti, T.K.; Sikdar, S.R.; Islam, S.S. Chemical analysis of an immunoenhancing water-soluble polysaccharide of an edible mushroom, pleurotus florida blue variant. Carbohydr. Res. 2010, 345, 2736–2741. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Yao, J.; Fang, J.N.; Ding, K. Structural characterization and immunological activity of two cold-water extractable polysaccharides from cistanche deserticola Y. C. Ma. Carbohydr. Res. 2007, 342, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; Ge, Z.; Fan, Y.; Luo, A.; Chun, Z.; He, X. In vitro and in vivo antioxidant activity of a water-soluble polysaccharide from Dendrobium denneanum. Molecules 2011, 16, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.H.; Feng, B.J.; Wang, J.H.; Zha, X.Q.; Luo, J.P. Structural characterization and anti-glycation activity in vitro of a water-soluble polysaccharide from Dendrobium huoshanense. Int. J. Biol. Macromol. 2013, 37, 313–321. [Google Scholar]
- Song, Y.; Yang, Y.; Zhang, Y.; Duan, L.; Zhou, C.; Ni, Y.; Liao, X.; Li, Q.; Hu, X. Effect of acetylation on antioxidant and cytoprotective activity of polysaccharides isolated from pumpkin (Cucurbita pepo, lady godiva). Carbohydr. Polym. 2013, 98, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, H.; Zhang, Y.; Zhang, N.; Fu, L. Chemical modification and antioxidant activities of polysaccharide from mushroom Inonotus obliquus. Carbohydr. Polym. 2012, 89, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.F.; Zhang, M.; Fu, C.X.; Chen, Z.H.; Chan, G.Y. Structural characterization of a 2-O-acetylglucomannan from Dendrobium officinale stem. Carbohydr. Res. 2004, 339, 2219–2224. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.S.; Chien, C.; Liao, S.K.; Liao, S.F.; Hung, W.T.; Yang, W.B.; Lin, C.C.; Cheng, T.J.; Chang, C.C.; Fang, J.M.; et al. Structure and bioactivity of the polysaccharides in medicinal plant Dendrobium huoshanense. Bioorg. Med. Chem. 2008, 16, 6054–6068. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xie, Y.; Su, J.; Ye, Q.; Jia, Z. Isolation and structural characterization of a neutral polysaccharide from the stems of Dendrobium densiflorum. Int. J. Biol. Macromol. 2012, 50, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.Y.; Liu, Q.L.; Li, D.; Chen, A.Z.; Huang, B.H.; Cheng, S.J. Effects of Dendrobium officinale polysaccharides on the activities of T lymphocytes and macrophages. J. Sun Yat-Sen Univ. Med. Sci. 1989, 10, 66–67. [Google Scholar]
- He, T.G.; Yang, L.T.; Li, Y.R.; Wang, C.Q.; Hu, J.S. Effects of the polysaccharides DCPP1a-1 from suspension-cultured protocorms of Dendrobium candidum on oxygenradical and lipid peroxidation. Nat. Prod. Res. Dev. 2007, 19, 410–414. [Google Scholar]
- Luo, A.X.; Fan, Y.J. Immune stimulating activity of water-soluble polysaccharide fractions from Dendrobium nobile Lindl. Afr. J. Pharm. Pharmacol. 2011, 5, 625–631. [Google Scholar] [CrossRef]
- Fan, Y. Evaluation of anti-tumor activity of water-soluble polysaccharides from Dendrobium denneanum. Afr. J. Pharm. Pharmacol. 2011, 5, 415–420. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Son, Y.O.; Kim, S.S.; Jang, Y.S.; Lee, J.C. Antioxidant and anti-hyperglycemic activity of polysaccharide isolated from Dendrobium chrysotoxum Lindl. J. Biochem. Mol. Biol. 2007, 40, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Guo, W.; Zou, C.H.; Fu, T.T.; Li, X.Y.; Zhu, M.; Qi, J.H.; Song, J.; Dong, C.H.; Li, Z.; et al. Acemannan accelerates cell proliferation and skin wound healing through akt/mtor signaling pathway. J. Dermatol. Sci. 2015, 79, 101–109. [Google Scholar] [CrossRef]
- Jettanacheawchankit, S.; Sasithanasate, S.; Sangvanich, P.; Banlunara, W.; Thunyakitpisal, P. Acemannan stimulates gingival fibroblast proliferation; expressions of keratinocyte growth factor-1, vascular endothelial growth factor, and type i collagen; and wound healing. J. Pharmacol. Sci. 2009, 109, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.Y.; Zhu, J.F. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Chokboribal, J.; Tachaboonyakiat, W.; Sangvanich, P.; Ruangpornvisuti, V.; Jettanacheawchankit, S.; Thunyakitpisal, P. Deacetylation affects the physical properties and bioactivity of acemannan, an extracted polysaccharide from aloe vera. Carbohydr. Polym. 2015, 133, 556–566. [Google Scholar] [CrossRef] [PubMed]


| Methylated Sugars | Linkages | Molar Ratio % | |
|---|---|---|---|
| JCS1 | JCS10.1N * | ||
| 2,3,6-Me3-Glc | 1,4-Glcp | 34.2 | 17.8 |
| 2,3,4,6-Me4-Glc | T-Glcp | 2.6 | 2.6 |
| 2,3-Me2-Glc | 1,4,6-Glcp | 3.2 | 3.0 |
| 2,3,6-Me3-Man | 1,4-Manp | 2.2 | 2.3 |
| 2,3-Me2-Xyl | 1,4-Xylp | 1.6 | 0.6 |
| 2,3,5-Me3-Ara | T-Araf | 1.0 | _ |
| Residues | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|
| 1,4-α-Glcp | H | 5.46 | 3.71 | 4.02 | 3.72 | 3.73 | 3.90 |
| C | 100.83 | 72.75 | 74.53 | 78.02 | 72.45 | 61.57 | |
| T-α-Glcp | H | 5.03 | 3.68 | 3.83 | 3.49 | 3.79 | 3.90 |
| C | 99.80 | 71.94 | 74.06 | 69.84 | 72.76 | 61.65 | |
| 1,4,6-α-Glcp | H | 5.42 | 3.69 | 4.00 | 3.72 | 3.99 | 3.72/4.02 |
| C | 101.07 | 71.46 | 73.50 | 78.02 | 74.93 | 70.40 | |
| 1,4-β-Manp | H | 4.55 | 3.36 | 3.85 | 3.72 | 3.84 | 3.75 |
| C | 102.93 | 73.95 | 73.61 | 77.58 | 71.51 | 64.09 | |
| 1,4-α-Xylp | H | 5.14 | 3.68 | 3.71 | 3.72 | 3.72 | - |
| C | 99.22 | 73.61 | 73.02 | 76.70 | 61.25 | - | |
| T-α-Araf | H | 5.11 | 4.20 | 4.03 | 4.12 | 3.46 | - |
| C | 109.08 | 82.57 | 76.66 | 83.56 | 64.07 | - | |
| T-α-Araf | H | 5.24 | 4.26 | nd | nd | nd | - |
| C | 107.44 | nd | nd | nd | nd | - | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, C.; Du, Z.; Lin, L.; Zhou, L.; Li, S.; Liu, Q.; Ding, K. Structural Characterization of Mannoglucan from Dendrobium nobile Lindl and the Neuritogenesis-Induced Effect of Its Acetylated Derivative on PC-12 Cells. Polymers 2017, 9, 399. https://doi.org/10.3390/polym9090399
Jin C, Du Z, Lin L, Zhou L, Li S, Liu Q, Ding K. Structural Characterization of Mannoglucan from Dendrobium nobile Lindl and the Neuritogenesis-Induced Effect of Its Acetylated Derivative on PC-12 Cells. Polymers. 2017; 9(9):399. https://doi.org/10.3390/polym9090399
Chicago/Turabian StyleJin, Can, Zhenyun Du, Liyan Lin, Lishuang Zhou, Saijuan Li, Qin Liu, and Kan Ding. 2017. "Structural Characterization of Mannoglucan from Dendrobium nobile Lindl and the Neuritogenesis-Induced Effect of Its Acetylated Derivative on PC-12 Cells" Polymers 9, no. 9: 399. https://doi.org/10.3390/polym9090399