Polyol Structure and Ionic Moieties Influence the Hydrolytic Stability and Enzymatic Hydrolysis of Bio-Based 2,5-Furandicarboxylic Acid (FDCA) Copolyesters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Enzyme
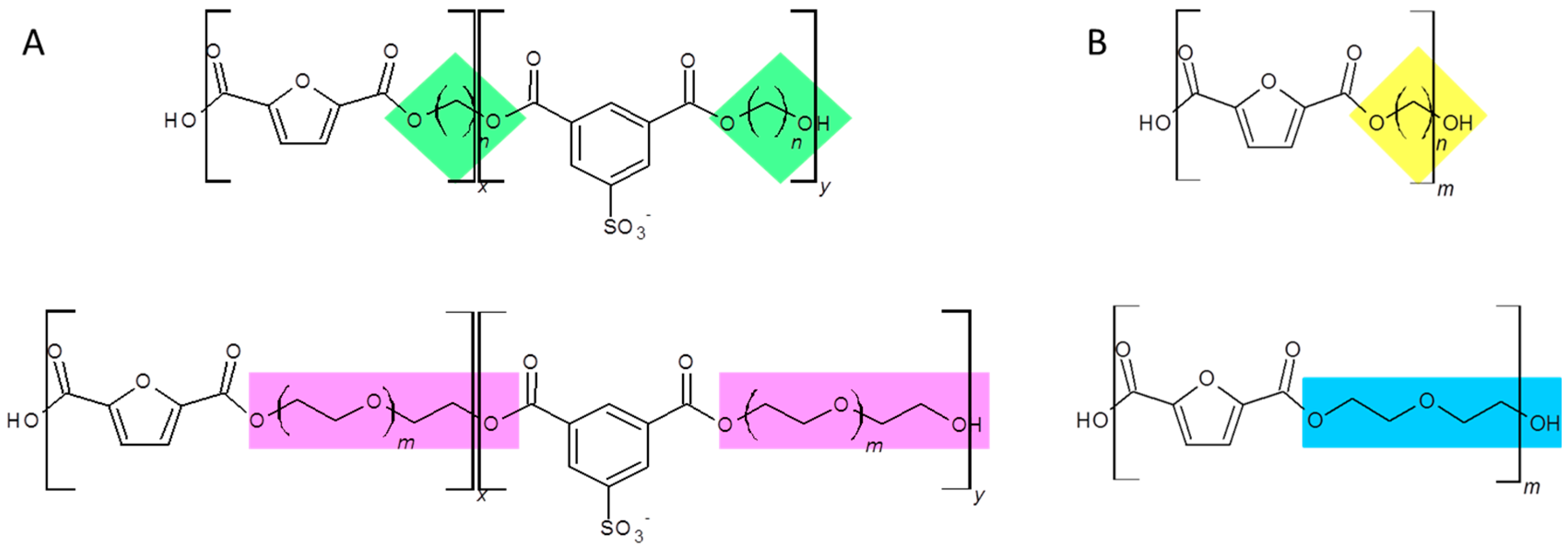
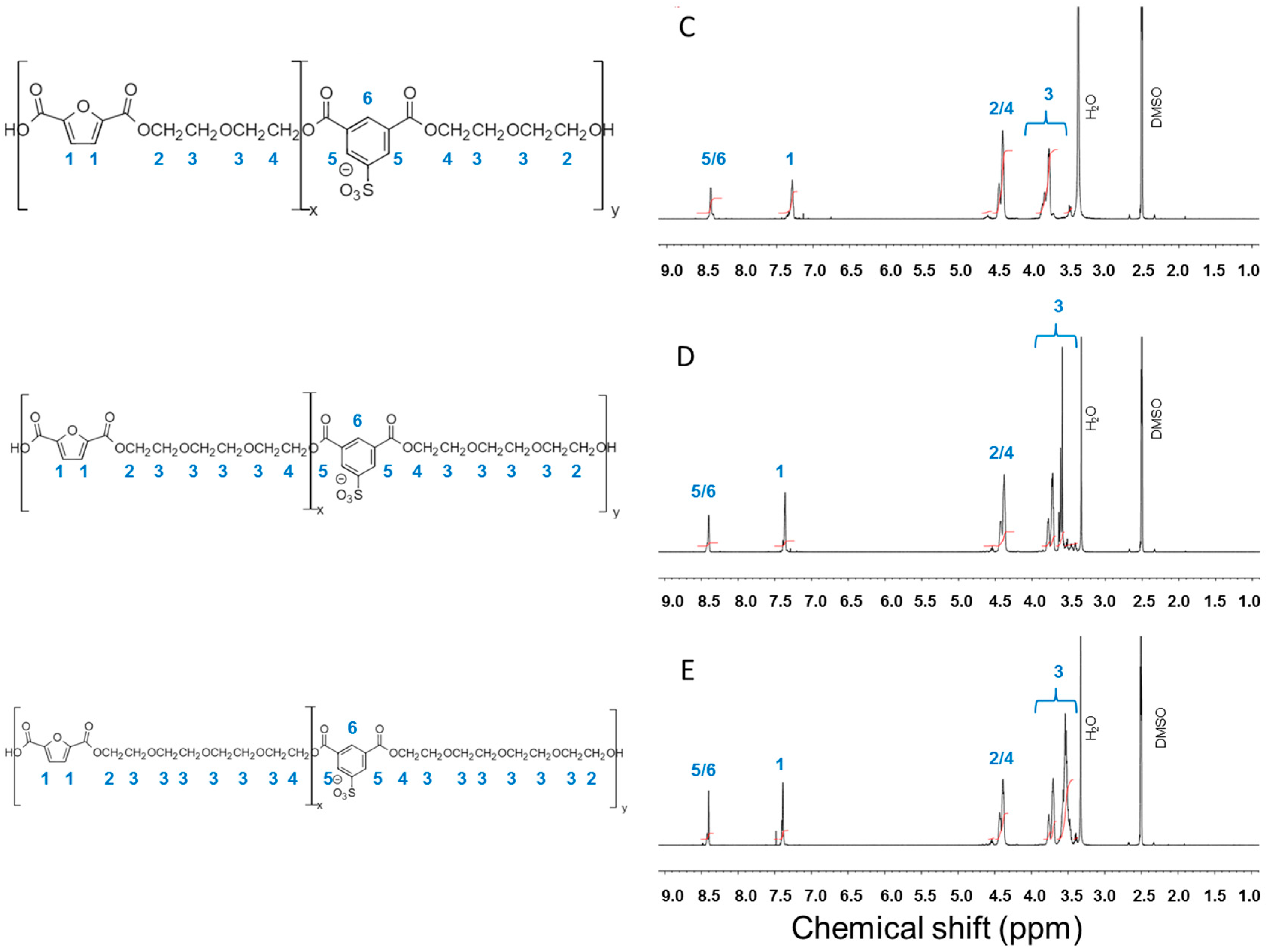
2.2. Synthesis and Characterization of Furanic-Sulfonated Isophthalic Copolyesters
2.3. Protein Quantification and Enzyme Activity
2.4. Hydrolytic Stability and Enzymatic Hydrolysis
2.5. Determination of Released Acids
3. Results and Discussion
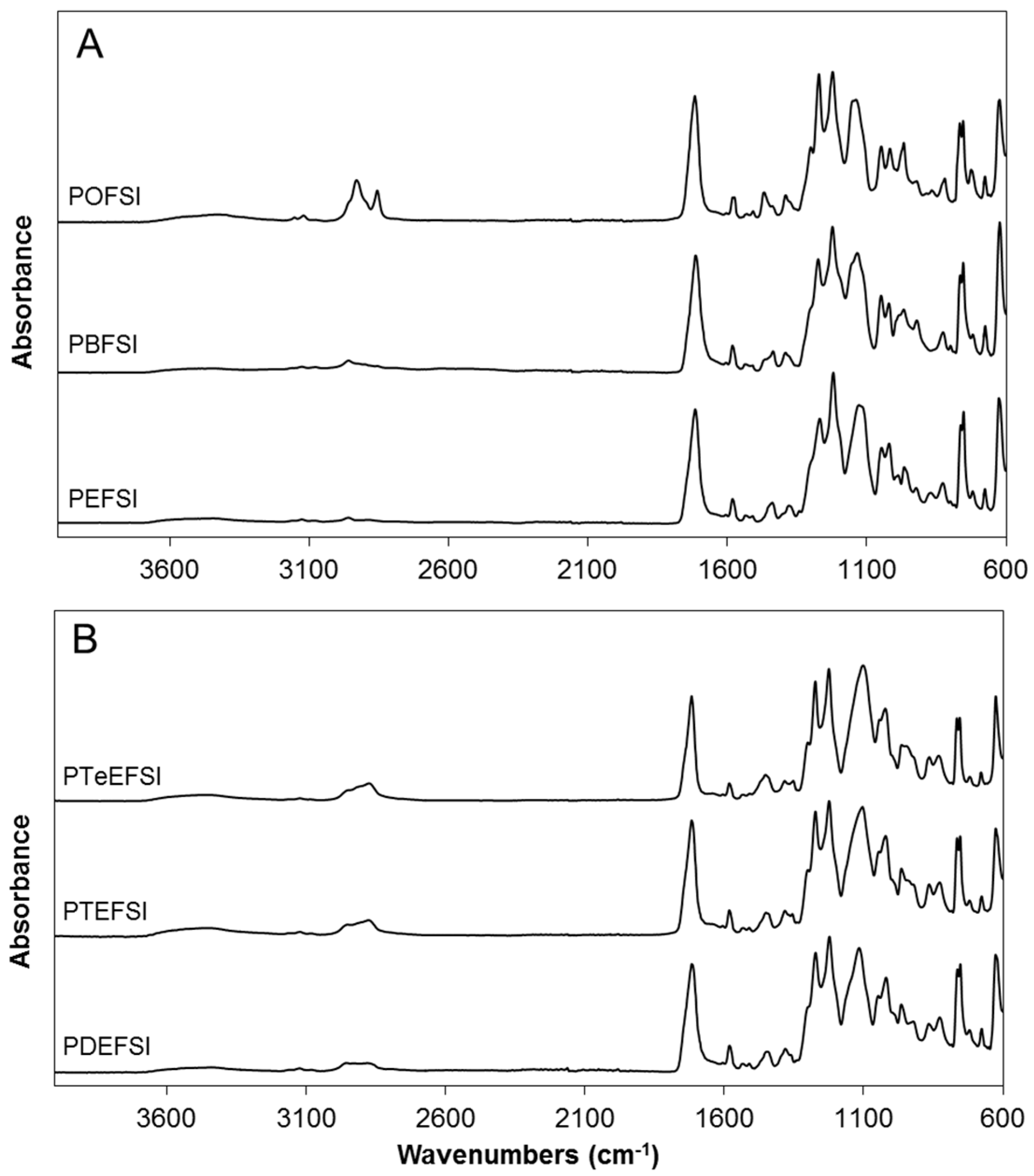
3.1. Furanic-Sulfonated Isophthalic Copolyesters
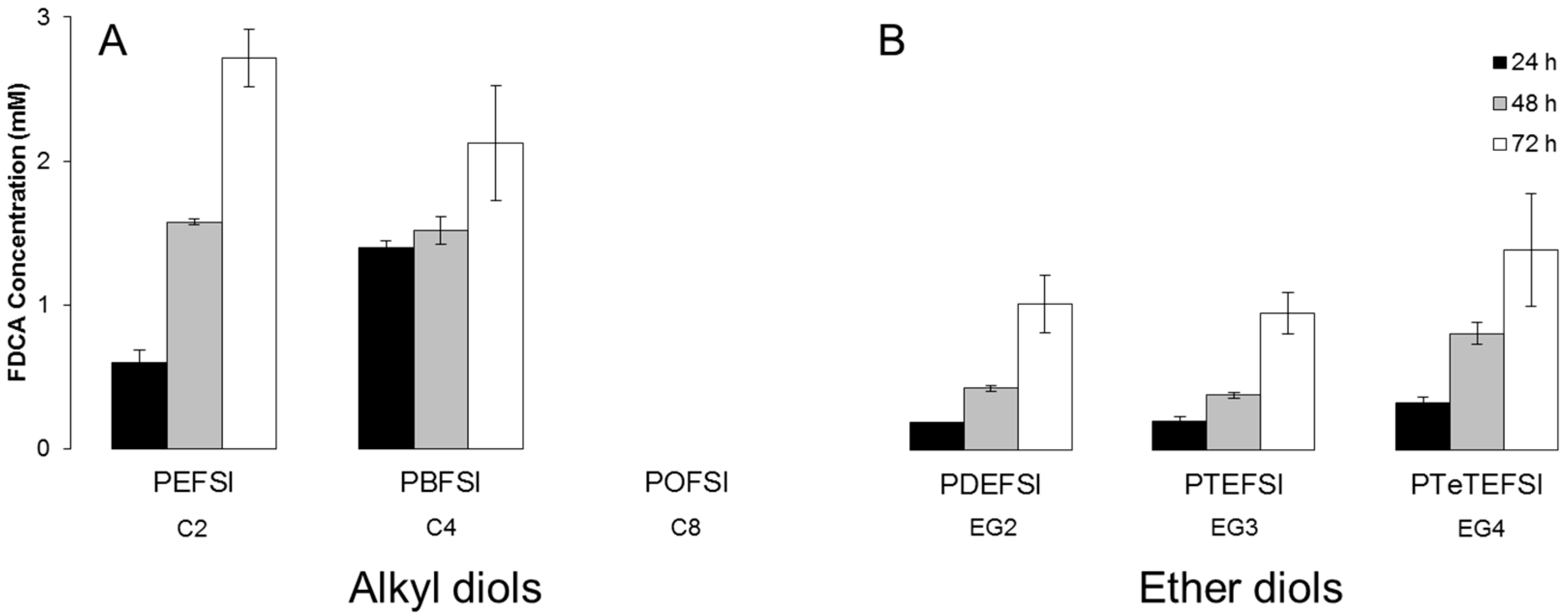
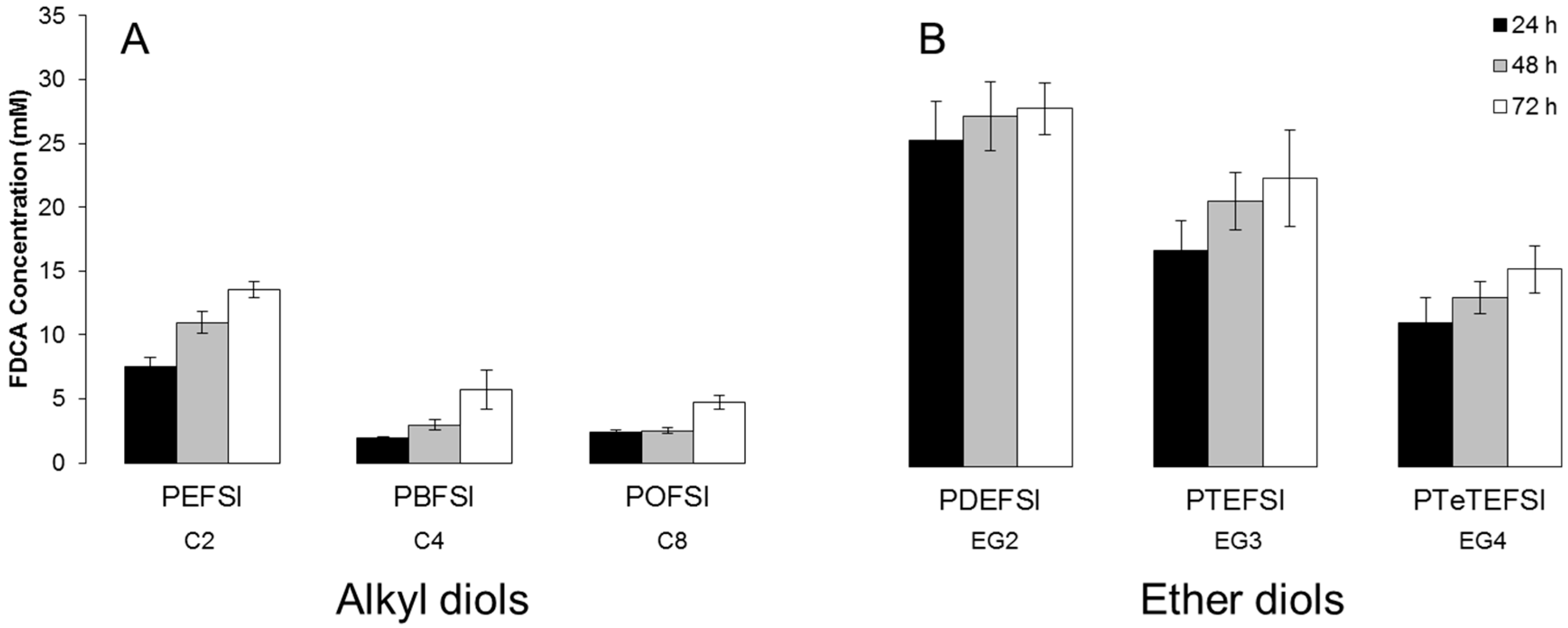
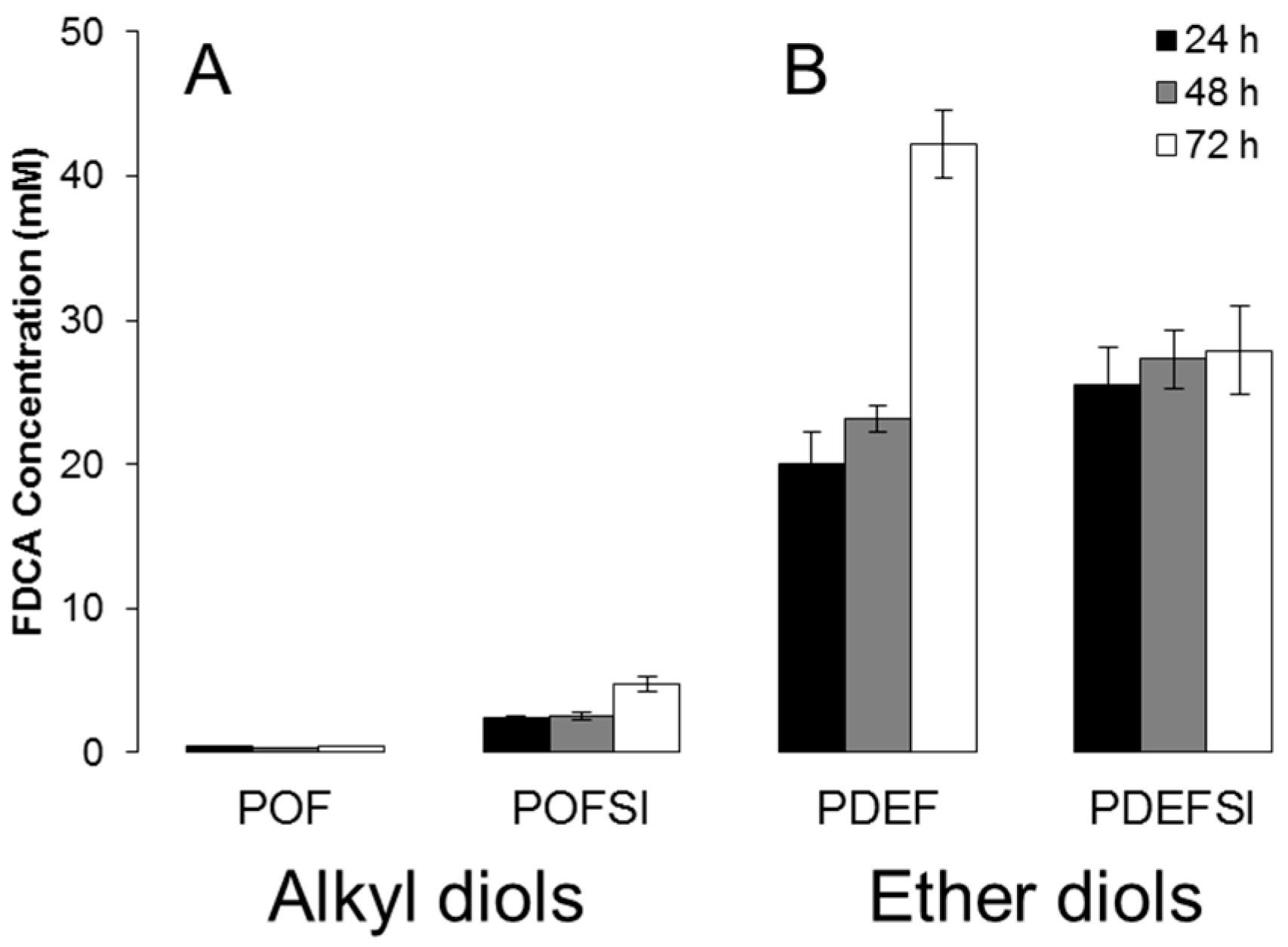
3.2. Hydrolytic Stability
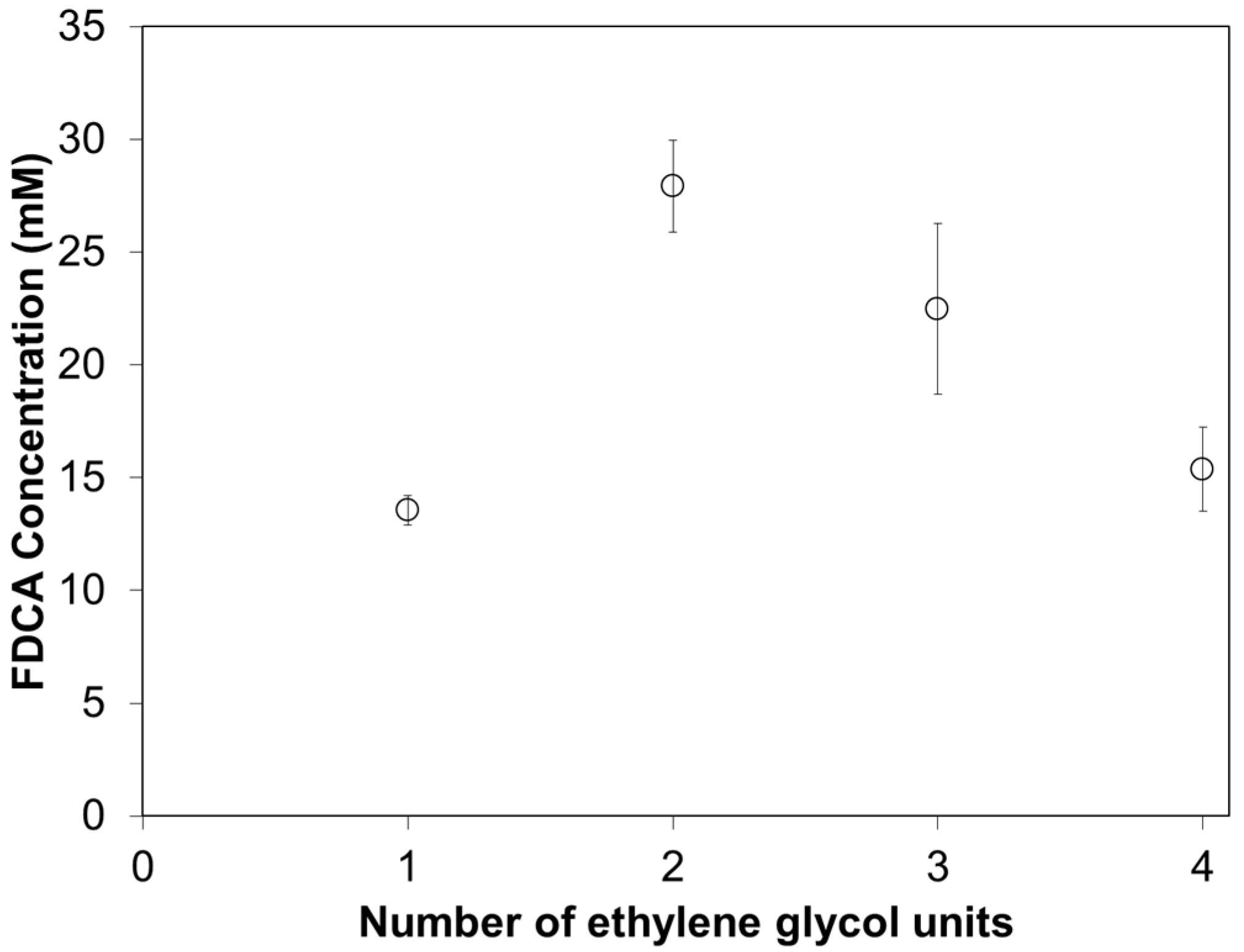
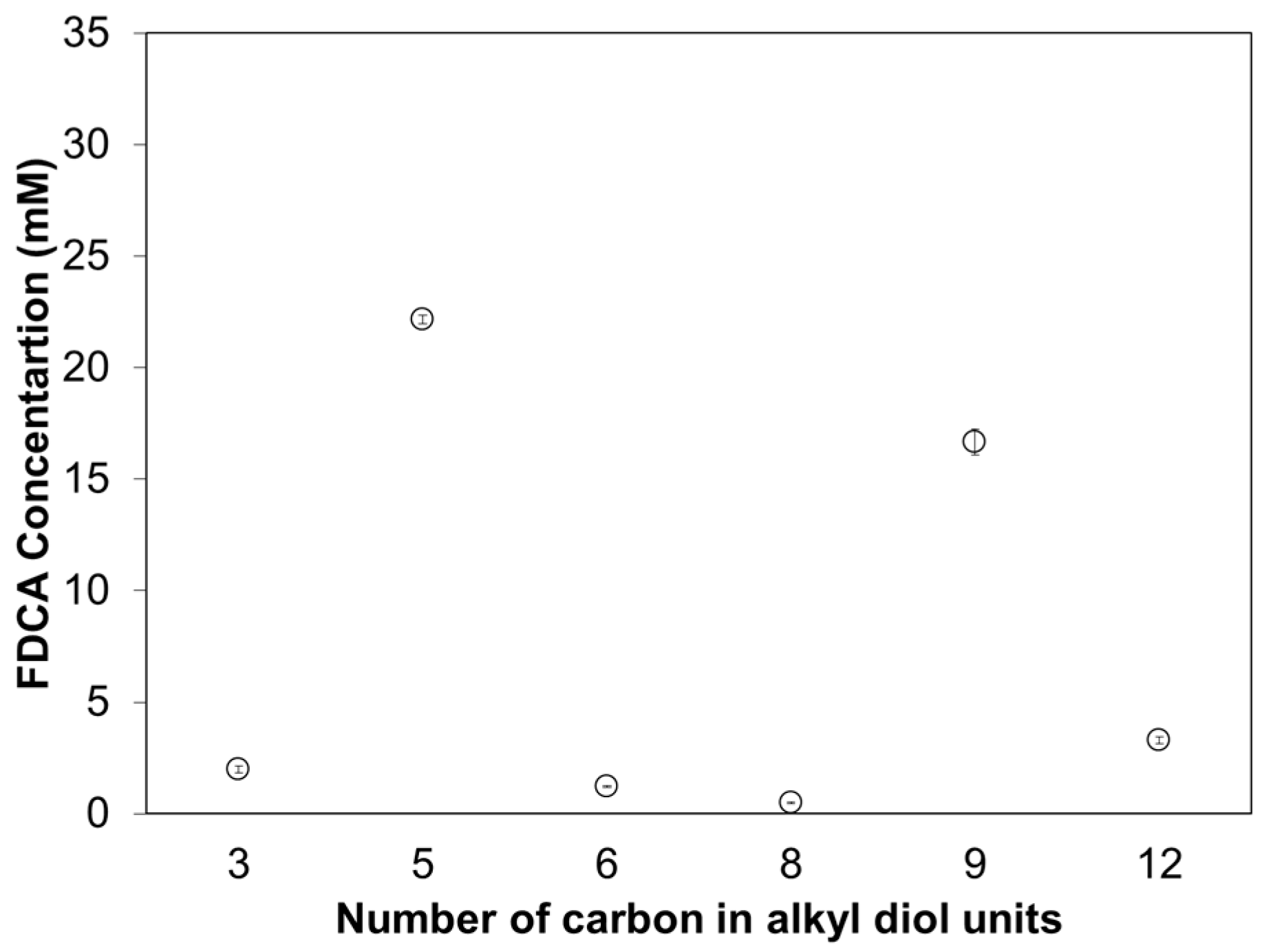
3.3. Enzymatic Hydrolysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Papageorgiou, G.Z.; Tsanaktsis, V.; Bikiaris, D.N. Synthesis of poly(ethylene furandicarboxylate) polyester using monomers derived from renewable resources: Thermal behavior comparison with PET and PEN. Phys. Chem. Chem. Phys. 2014, 16, 7946–7958. [Google Scholar] [CrossRef] [PubMed]
- Tsanaktsis, V.; Papageorgiou, G.Z.; Bikiaris, D.N. A facile method to synthesize high-molecular-weight biobased polyesters from 2,5-furandicarboxylic acid and long-chain diols. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2617–2632. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Tsanaktsis, V.; Papageorgiou, D.G.; Exarhopoulos, S.; Papageorgiou, M.; Bikiaris, D.N. Evaluation of polyesters from renewable resources as alternatives to the current fossil-based polymers. Phase transitions of poly(butylene 2,5-furan-dicarboxylate). Polymer 2014, 55, 3846–3858. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Terzopoulou, Z.; Bikiaris, D.N. Production of bio-based 2,5-furan dicarboxylate polyesters: Recent progress and critical aspects in their synthesis and thermal properties. Eur. Polym. J. 2016, 83, 202–229. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; National Renewable Energy Lab: Golden, CO, USA, 2004.
- Gandini, A. Furans as offspring of sugars and polysaccharides and progenitors of a family of remarkable polymers: A review of recent progress. Polym. Chem. 2010, 1, 245–251. [Google Scholar] [CrossRef]
- Morales-Huerta, J.C.; Martínez de Ilarduya, A.; Muñoz-Guerra, S. Poly(alkylene 2,5-furandicarboxylate)s (PEF and PBF) by ring opening polymerization. Polymer 2016, 87, 148–158. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Terzopoulou, Z.; Nerantzaki, M.; Papageorgiou, G.Z.; Bikiaris, D.N. New poly(pentylene furanoate) and poly(heptylene furanoate) sustainable polyesters from diols with odd methylene groups. Mater. Lett. 2016, 178, 64–67. [Google Scholar] [CrossRef]
- Mou, Z.H.; Feng, S.; Chen, E.Y.X. Bio-based difuranic polyol monomers and their derived linear and cross-linked polyurethanes. Polym. Chem. 2016, 7, 1593–1602. [Google Scholar] [CrossRef]
- Available online: http://avantium.com/yxy/YXY-technology.html (accessed on 31 August 2017).
- Jiang, Y.; Maniar, D.; Woortman, A.J.J.; Loos, K. Enzymatic synthesis of 2,5-furandicarboxylic acid-based semi-aromatic polyamides: enzymatic polymerization kinetics, effect of diamine chain length and thermal properties. RSC Adv. 2016, 6, 67941–67953. [Google Scholar] [CrossRef]
- Jiang, Y.; Maniar, D.; Woortman, A.J.J.; Alberda van Ekenstein, G.O.R.; Loos, K. Enzymatic polymerization of furan-2,5-dicarboxylic acid-based furanic-aliphatic polyamides as sustainable alternatives to polyphthalamides. Biomacromolecules 2015, 16, 3674–3685. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Woortman, A.J.J.; Alberda van Ekenstein, G.O.R.; Loos, K. A biocatalytic approach towards sustainable furanic-aliphatic polyesters. Polym Chem. 2015, 6, 5198–5211. [Google Scholar] [CrossRef]
- Gandini, A.; Coelho, D.; Gomes, M.; Reis, B.; Silvestre, A. Materials from renewable resources based on furan monomers and furan chemistry: work in progress. J. Mater. Chem. 2009, 19, 8656–8664. [Google Scholar] [CrossRef] [Green Version]
- De Jong, E.; Dam, M.A.; Sipos, L.; Gruter, G.J.M. Furandicarboxylic Acid (FDCA), A Versatile Building Block for a Very Interesting Class of Polyesters. In Biobased Monomers, Polymers, and Materials; American Chemical Society: Washington, DC, USA, 2012; pp. 1–13. [Google Scholar]
- Zhang, L.; Brostowitz, N.R.; Cavicchi, K.A.; Weiss, R.A. Perspective: Ionomer research and applications. Macromol. React. Eng. 2014, 8, 81–99. [Google Scholar]
- Chisholm, B.J.; Sisti, L.; Soloveichik, S.; Gillette, G. Hydrolytic stability of sulfonated poly(butylene terephthalate). Polymer 2003, 44, 1903–1910. [Google Scholar] [CrossRef]
- Gaona, O.; Kint, D.P.R.; Martínez de Ilarduya, A.; Alla, A.; Bou, J.; Muñoz-Guerra, S. Preparation and hydrolytic degradation of sulfonated poly(ethylene terephthalate) copolymers. Polymer 2003, 44, 7281–7289. [Google Scholar] [CrossRef]
- Bautista, M.; Martínez de Ilarduya, A.; Alla, A.; Muñoz-Guerra, S. Sulfonated poly(hexamethylene terephthalate) copolyesters: Enhanced thermal and mechanical properties. J. Appl. Polym. Sci. 2013, 129, 3527–3535. [Google Scholar] [CrossRef]
- Penn, L.S.; Wang, H. Chemical modification of polymer surfaces: A review. Polym. Adv. Technol. 1994, 5, 809–817. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Desmet, T.; Dubruel, P.; Leys, C. Plasma surface modification of biodegradable polymers: A review. Plasma Process. Polym. 2011, 8, 171–190. [Google Scholar] [CrossRef]
- Pellis, A.; Herrero Acero, E.; Ferrario, V.; Ribitsch, D.; Guebitz, G.M.; Gardossi, L. The closure of the cycle: enzymatic synthesis and functionalization of bio-based polyesters. Trends Biotechnol. 2016, 34, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, W.; Billig, S. Enzymes for the biofunctionalization of poly(ethylene terephthalate). In S.G. Nyanhongo; Steiner, W., Gübitz, G., Eds.; Springer: Berlin, Germany, 2011; pp. 97–120. [Google Scholar]
- Gamerith, C.; Herrero Acero, E.; Pellis, A.; Vielnasche, R.; Luschnig, D.; Zartl, B.; Haernall, K.; Zitzenbacher, S.; Strohmeier, G.; Hoff, O.; et al. Improving enzymatic polyurethane hydrolysis by tuning enzyme sorption. Polym. Degrad. Stab. 2016, 132, 69–77. [Google Scholar] [CrossRef]
- Pellis, A.; Herrero Acero, E.; Weber, H.; Obersriebnig, M.; Srebotnik, E.; Guebitz, G.M. Biocatalyzed approach for the surface functionalization of poly(l-lactic acid) films using hydrolytic enzyme. Biotechnol. J. 2015, 10, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Biundo, A.; Hromic, A.; Pavkov-Keller, T.; Gruber, K.; Quartinello, F.; Haernvall, K.; Perz, V.; Arrell, M.S.; Zinn, M.; Ribitsch, D.; et al. Characterization of a poly(butylene adipate-co-terephthalate)-hydrolyzing lipase from Pelosinus fermentans. Appl. Microbiol. Biotechnol. 2015, 100, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Herrero Acero, E.; Ribitsch, D.; Diaz Rodriguez, R.; Dellacher, A.; Zitzenbacher, S.; Marold, A.; Greimel, K.J.; Schroeder, M.; Kandelbauer, A.; Heumann, S.; et al. Two-step enzymatic functionalisation of polyamide with phenolics. J. Mol. Catal. B Enzym. 2012, 79, 54–60. [Google Scholar] [CrossRef]
- Hsieh, Y.-L.; Cram, L.A. Enzymatic hydrolysis to improve wetting and absorbency of polyester fabrics. Text. Res. J. 1998, 68, 311–319. [Google Scholar] [CrossRef]
- Pellis, A.; Haernvall, K.; Pichler, C.M.; Ghazaryan, G.; Breinbauer, R.; Guebitz, G.M. Enzymatic hydrolysis of poly(ethylene furanoate). J Biotechnol. 2016, 235, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Haernvall, K.; Zitzenbacher, S.; Amer, H.; Zumstein, M.T.; Sander, M.; McNeill, K.; Yamamoto, M.; Schick, M.B.; Ribitsch, D.; Guebitz, G.M. Polyol structure influences enzymatic hydrolysis of bio-based 2,5-furandicarboxylic acid (FDCA) polyesters. Biotechnol. J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, X.; Yang, B.; Xu, Y.; Zhang, W.; Zhang, Y.; Ji, J. Synthesis, physical properties and enzymatic degradation of bio-based poly(butylene adipate-co-butylene furandicarboxylate) copolyesters. Polym. Degrad. Stab. 2013, 98, 2177–2183. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Guigo, N.; Tsanaktsis, V.; Papageorgiou, D.G.; Exarhopoulos, S.; Sbirrazzuoli, N.; Bikiaris, D.N. On the bio-based furanic polyesters: Synthesis and thermal behavior study of poly(octylene furanoate) using fast and temperature modulated scanning calorimetry. Eur. Polym. J. 2015, 68, 115–127. [Google Scholar] [CrossRef]
- Papaspyrides, C.D.; Vouyiouka, S.; Georgousopoulou, I.-N.; Marinkovic, S.; Estrine, B.; Joly, C.; Dole, P. Feasibility of solid-State postpolymerization on fossil- and bio-based poly(butylene succinate) Including Polymer Upcycling Routes. Ind. Eng. Chem. Res. 2016, 55, 5832–5842. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Guigo, N.; Tsanaktsis, V.; Exarhopoulos, S.; Bikiaris, D.N.; Sbirrazzuoli, N.; Papageorgiou, G.Z. Fast crystallization and melting behavior of a long-spaced aliphatic furandicarboxylate biobased polyester, poly(dodecylene 2,5-furanoate). Ind. Eng. Chem. Res. 2016, 55, 5315–5326. [Google Scholar] [CrossRef]
- Bougarech, A.; Abid, M.; DaCruz-Boisson, F.; Abid, S.; El Gharbi, R.; Fleury, E. Modulation of furanic-sulfonated isophthalic copolyesters properties through diols units control. Eur. Polym. J. 2014, 58, 207–217. [Google Scholar] [CrossRef]
- Donelli, I.; Freddi, G.; Nierstrasz, V.A.; Taddei, P. Surface structure and properties of poly-(ethylene terephthalate) hydrolyzed by alkali and cutinase. Polym. Degrad. Stab. 2010, 95, 1542–1550. [Google Scholar] [CrossRef] [Green Version]
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Chain mobility, thermal, and mechanical properties of poly(ethylene furanoate) compared to poly(ethylene terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, Q.; Zhang, Q.; Ye, C.; Zhou, G. A series of furan-aromatic polyesters synthesized via direct esterification method based on renewable resources. J. Appl. Polym. Sci. A. 2011, 50, 1026–1036. [Google Scholar] [CrossRef]
- Gomes, M.; Gandini, A.; Silvestre, A.J.D.; Reis, B. Synthesis and characterization of poly(2,5-furan dicarboxylate)s based on a variety of diols. J. Appl. Polym. Sci. A 2011, 49, 3759–3768. [Google Scholar] [CrossRef]
- Eisenberg, A.; Hird, B.; Moore, R.B. A new multiplet-cluster model for the morphology of random ionomers. Macromolecules 1990, 23, 4098–4107. [Google Scholar] [CrossRef]
- Herrero Acero, E.; Ribitsch, D.; Steinkellner, G.; Gruber, K.; Greimel, K.; Eiteljoerg, I.; Trotscha, E.; Wei, R.; Zimmermann, W.; Zinn, M. Enzymatic surface hydrolysis of PET: Effect of structural diversity on kinetic properties of cutinases from thermobifida. Macromolecules 2011, 44, 4632–4640. [Google Scholar] [CrossRef] [Green Version]
- Okada, M.; Tachikawa, K.; Aoi, K. Biodegradable polymers based on renewable resources. II. Synthesis and biodegradability of polyesters containing furan rings. J. Polym. Sci. A 1997, 35, 2729–2737. [Google Scholar] [CrossRef]
- Ejlertsson, J.; Alnervik, M.; Jonsson, S.; Svensson, B.H. Influence of water solubility, side-chain degradability, and side-chain structure on the degradation of phthalic acid esters under methanogenic conditions. Environ. Sci. Technol. 1997, 31, 2761–2764. [Google Scholar] [CrossRef]
- O'Grady, D.P.; Howard, P.H.; Werner, A.F. Activated sludge biodegradation of 12 commercial phthalate esters. Appl. Environ. Microbiol. 1985, 49, 443–445. [Google Scholar] [PubMed]
- Haernvall, K.; Zitzenbacher, S.; Wallig, K.; Yamamoto, M.; Schick, M.B.; Ribitsch, D.; Guebitz, G.M. Hydrolysis of ionic phthalic acid based polyesters by wastewater microorganisms and their enzymes. Environ. Sci. Technol. 2017, 51, 4596–4605. [Google Scholar] [CrossRef] [PubMed]
- Haernvall, K.; Zitzenbacher, S.; Yamamoto, M.; Schick, M.B.; Ribitsch, D.; Guebitz, G.M. A new arylesterase from pseudomonas pseudoalcaligenes can hydrolyze ionic phthalic polyesters. J. Biotechnol. 2017, 257, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Marten, E.; Mueller, R.-J.; Deckwer, W.-D. Studies on the enzymatic hydrolysis of polyesters. II. Aliphatic–aromatic copolyesters. Polym. Degrad. Stab. 2005, 88, 371–381. [Google Scholar] [CrossRef]
- Ronkvist, Å.M.; Xie, W.; Lu, W.; Gross, R.A. Cutinase-catalyzed hydrolysis of poly(ethylene terephthalate). Macromolecules 2009, 42, 5128–5138. [Google Scholar] [CrossRef]
- Brueckner, T.; Eberl, A.; Heumann, S.; Rabe, M.; Guebitz, G.M. Enzymatic and chemical hydrolysis of poly(ethylene terephthalate) fabrics. J. Polym. Sci. A 2008, 46, 6435–6443. [Google Scholar] [CrossRef]
- Eberl, A.; Heumann, S.; Brueckner, T.; Araujo, R.; Cavaco-Paulo, A.; Kaufmann, F.; Kroutil, W.; Guebitz, G.M. Enzymatic surface hydrolysis of poly(ethylene terephthalate) and bis(benzoyloxyethyl) terephthalate by lipase and cutinase in the presence of surface active molecules. J. Biotechnol. 2009, 143, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Vertommen, M.A.M.E.; Nierstrasz, V.A.; van der Veer, M.; Warmoeskerken, M.M.C.G. Enzymatic surface modification of poly(ethylene terephthalate). J. Biotechnol. 2005, 120, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Herzog, K.; Mueller, R.J.; Deckwer, W.D. Mechanism and kinetics of the enzymatic hydrolysis of polyester nanoparticles by lipases. Polym. Degrad. Stab. 2006, 91, 2486–2498. [Google Scholar] [CrossRef]
- Marten, E.; Mueller, R.-J.; Deckwer, W.-D. Studies on the enzymatic hydrolysis of polyesters I. Low molecular mass model esters and aliphatic polyesters. Polym. Degrad. Stab. 2003, 80, 485–501. [Google Scholar] [CrossRef]
- Gigli, M.; Negroni, A.; Soccio, M.; Zanaroli, G.; Lotti, N.; Fava, F.; Munari, A. Influence of chemical and architectural modifications on the enzymatic hydrolysis of poly(butylene succinate). Green Chem. 2012, 14, 2885–2893. [Google Scholar] [CrossRef]
- Pepic, D.; Zagar, E.; Zigon, M.; Krzan, A.; Kunaver, M.; Djonlagic, J. Synthesis and characterization of biodegradable aliphatic copolyesters with poly(ethylene oxide) soft segments. Eur. Polym. J. 2008, 44, 904–917. [Google Scholar] [CrossRef]
- Nagata, M.; Kiyotsukuri, T.; Minami, S.; Tsutsumi, N.; Sakai, W. Enzymatic degradation of poly(ethylene terephthalate) copolymers with aliphatic dicarboxylic acids and/or poly(ethylene glycol). Eur. Polym. J. 1997, 33, 1701–1705. [Google Scholar] [CrossRef]
- Deschamps, A.A.; van Apeldoorn, A.A.; Hayen, H.; de Bruijn, J.D.; Karst, U.; Grijpma, D.W.; Feijen, J. In vivo and in vitro degradation of poly(ether ester) block copolymers based on poly(ethylene glycol) and poly(butylene terephthalate). Biomaterials 2004, 25, 247–258. [Google Scholar] [CrossRef]

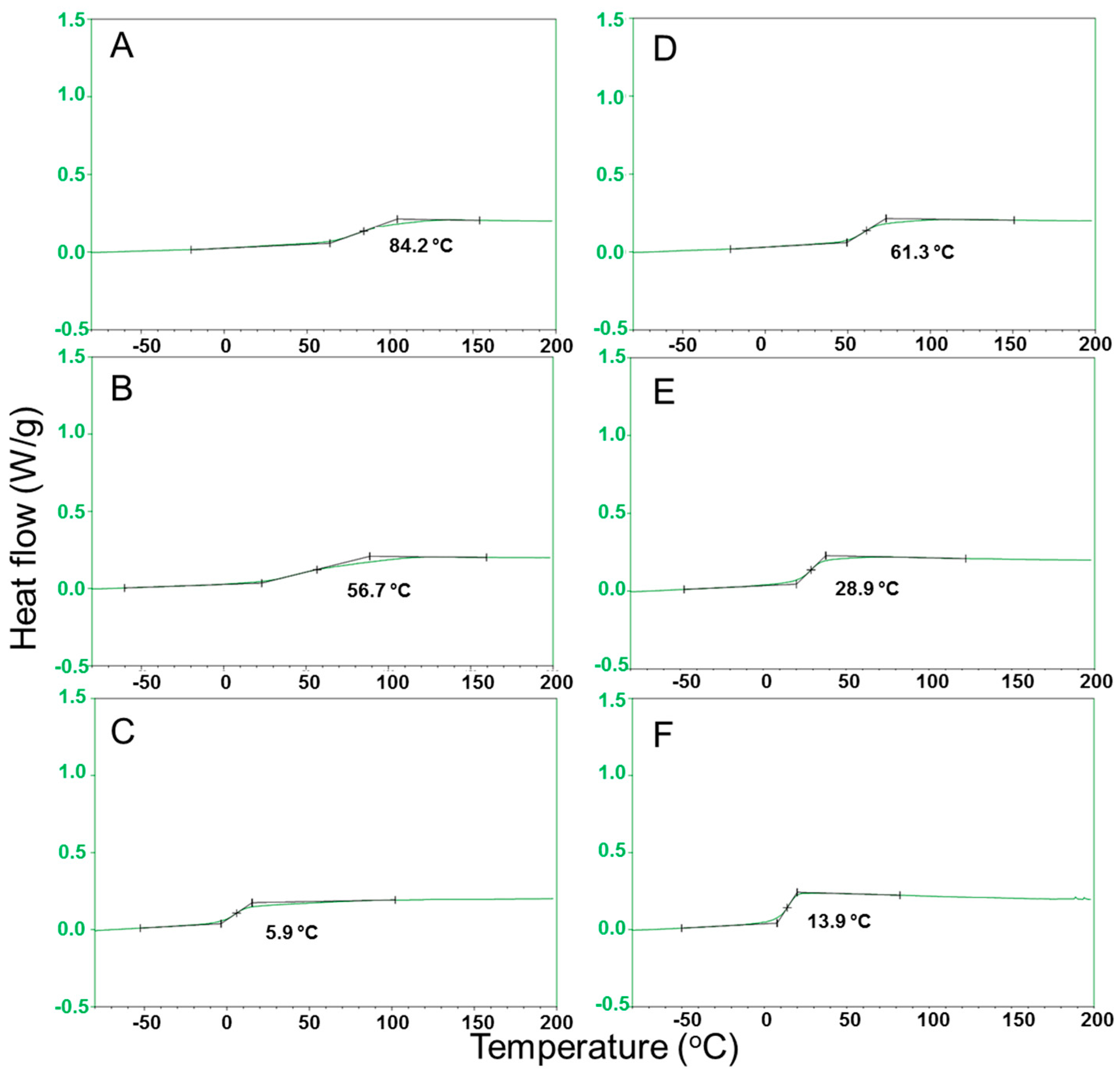
| Group | Polyol | Code | Composition a | GPC b | DSC e | ||
|---|---|---|---|---|---|---|---|
| Feed | Copolyester | ||||||
| (F):(SI) (mol %) | (F):(SI) (mol %) | Mn c × 1000 (g mol−1) | PDI d | Tg f (°C) | |||
| Alkyl diol | 1,2-Ethanediol | PEFSI | 70:30 | - | 3.28 | 1.4 | 84 |
| 1,4-Butanediol | PBFSI | 70:30 | 60:40 | 2.63 | 1.3 | 57 | |
| 1,8-Octanediol | POFSI | 70:30 | 62:38 | 5.79 | 2.3 | 6 | |
| Ether diol | Diethylene glycol | PDEFSI | 70:30 | 69:31 | 6.83 | 1.9 | 61 |
| Triethylene glycol | PTEFSI | 70:30 | 70:30 | 8.03 | 2.1 | 29 | |
| Tetraethylene glycol | PTeEFSI | 70:30 | 69:31 | 6.96 | 2.0 | 14 | |
| Polyol | Code | Assignment (cm−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| =CH (Furan) | C–H (CH2) | C=O (ester) | C=C (Furan) | C–O (ester) | Furan Ring Breathing | 2,5-Disubstituted Furan Ring | SO2 Asymmetric Stretching Vibrations | SO2 Symmetric Stretching Vibrations | S–O | ||
| 1,2-Ethanediol | PEFSI | 3127 | 2960 | 1716 | 1582 | 1269 | 1020 | 966,827,763 | 1047 | 1127 | 753 |
| 1,4-Butanediol | PBFSI | 3128 | 2958 | 1713 | 1582 | 1274 | 1021 | 967,827,765 | 1049 | 1134 | 754 |
| 1,8-Octanediol | POFSI | 3121 | 2976 | 1717 | 1578 | 1272 | 1017 | 967,820,766 | 1048 | 1139 | 754 |
| Diethylene glycol | PDEFSI | 3121 | 2957 | 1717 | 1581 | 1272 | 1019 | 965,827,763 | 1047 | 1117 | 753 |
| Triethylene glycol | PTEFSI | 3122 | 2875 | 1717 | 1582 | 1272 | 1021 | 964,827,764 | 1047 | 1104 | 753 |
| Tetraethylene glycol | PTeEFSI | 3121 | 2874 | 1717 | 1582 | 1273 | 1022 | 963,831,765 | 1047 | 1102 | 755 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haernvall, K.; Zitzenbacher, S.; Yamamoto, M.; Schick, M.B.; Ribitsch, D.; Guebitz, G.M. Polyol Structure and Ionic Moieties Influence the Hydrolytic Stability and Enzymatic Hydrolysis of Bio-Based 2,5-Furandicarboxylic Acid (FDCA) Copolyesters. Polymers 2017, 9, 403. https://doi.org/10.3390/polym9090403
Haernvall K, Zitzenbacher S, Yamamoto M, Schick MB, Ribitsch D, Guebitz GM. Polyol Structure and Ionic Moieties Influence the Hydrolytic Stability and Enzymatic Hydrolysis of Bio-Based 2,5-Furandicarboxylic Acid (FDCA) Copolyesters. Polymers. 2017; 9(9):403. https://doi.org/10.3390/polym9090403
Chicago/Turabian StyleHaernvall, Karolina, Sabine Zitzenbacher, Motonori Yamamoto, Michael Bernhard Schick, Doris Ribitsch, and Georg M. Guebitz. 2017. "Polyol Structure and Ionic Moieties Influence the Hydrolytic Stability and Enzymatic Hydrolysis of Bio-Based 2,5-Furandicarboxylic Acid (FDCA) Copolyesters" Polymers 9, no. 9: 403. https://doi.org/10.3390/polym9090403