Preparation and Flame Retardance of Polyurethane Composites Containing Microencapsulated Melamine Polyphosphate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
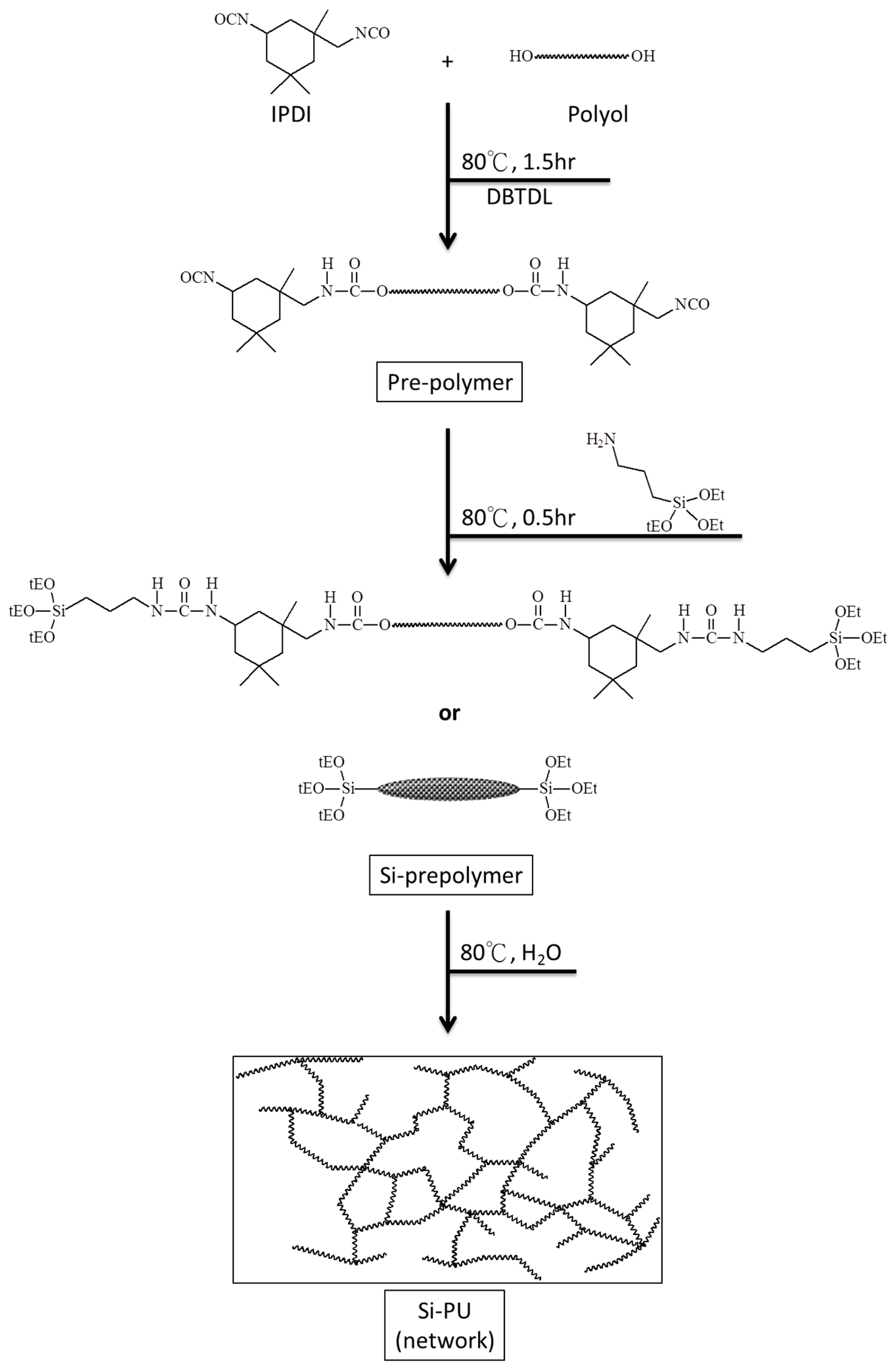
2.2. Preparation of Si-PU
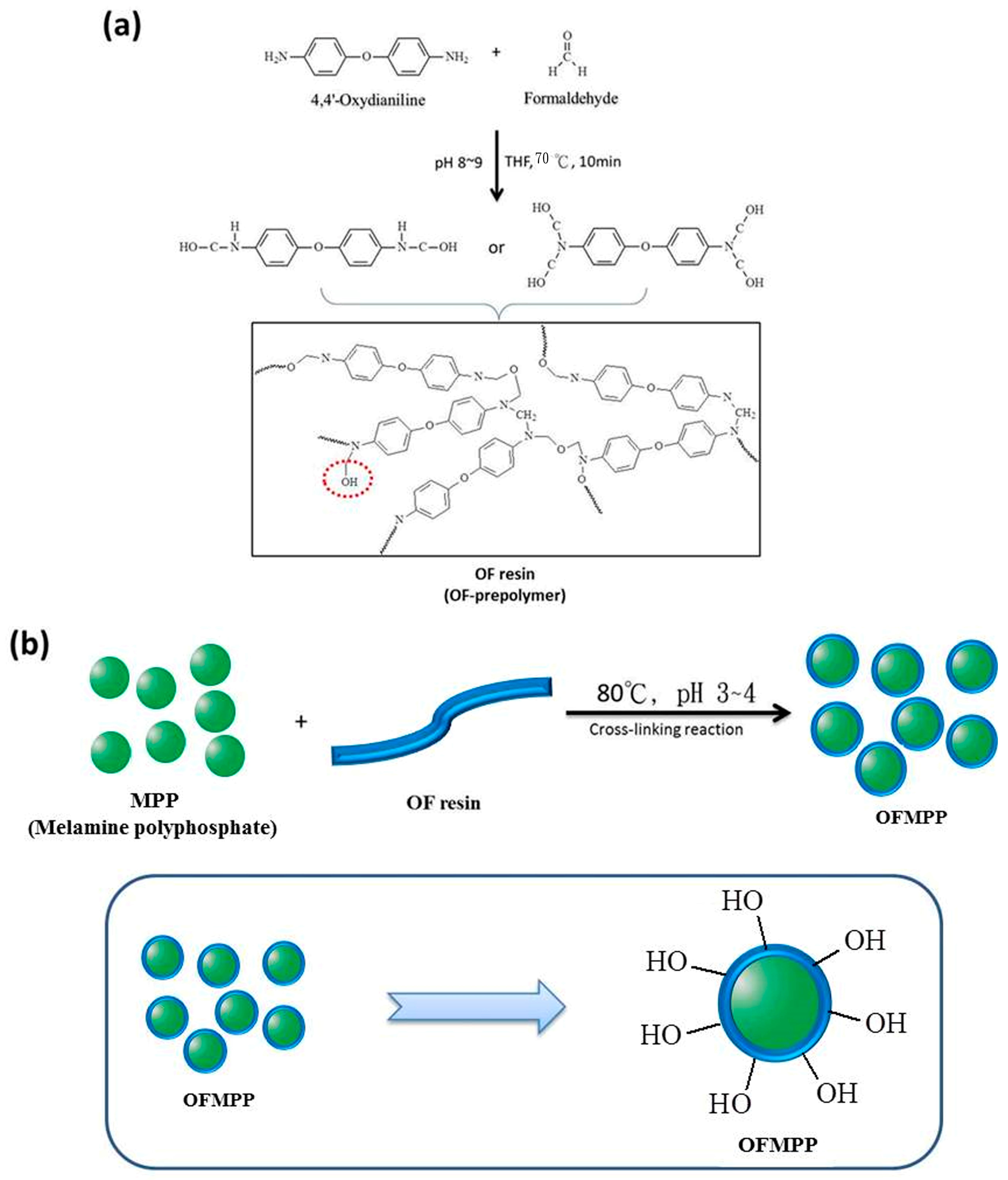
2.3. Preparation of OFMPP
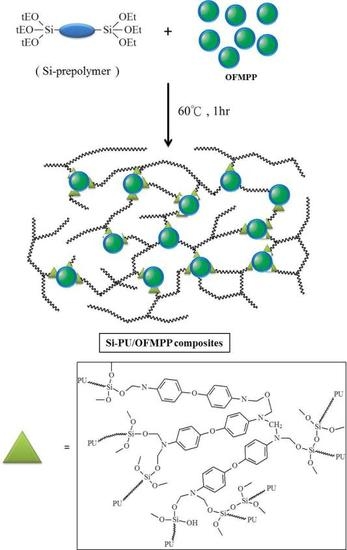
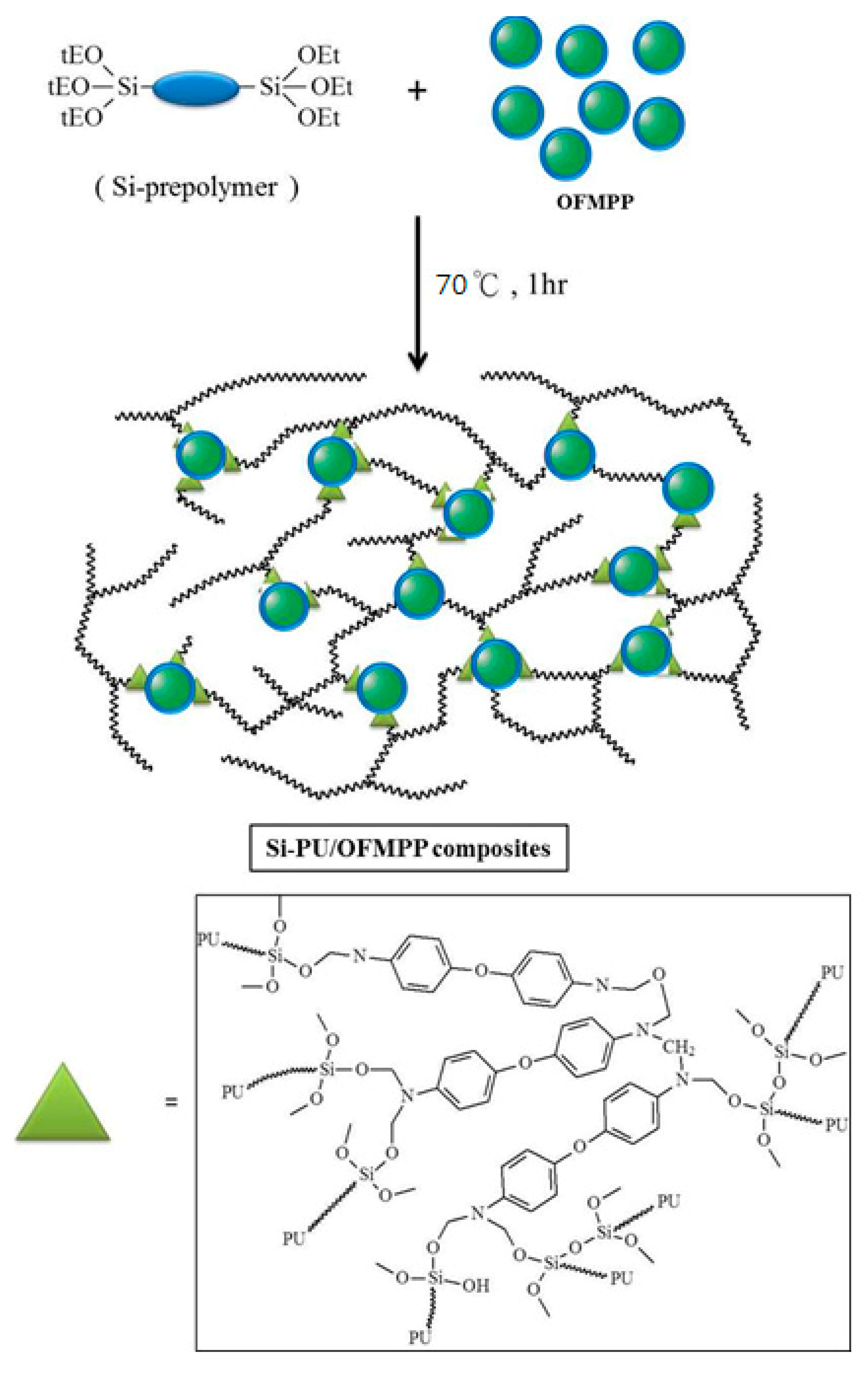
2.4. Preparation of Si-PU/OFMPP Composites
2.5. Measurements
3. Results and Discussion
3.1. 29Si-NMR of Si-PU
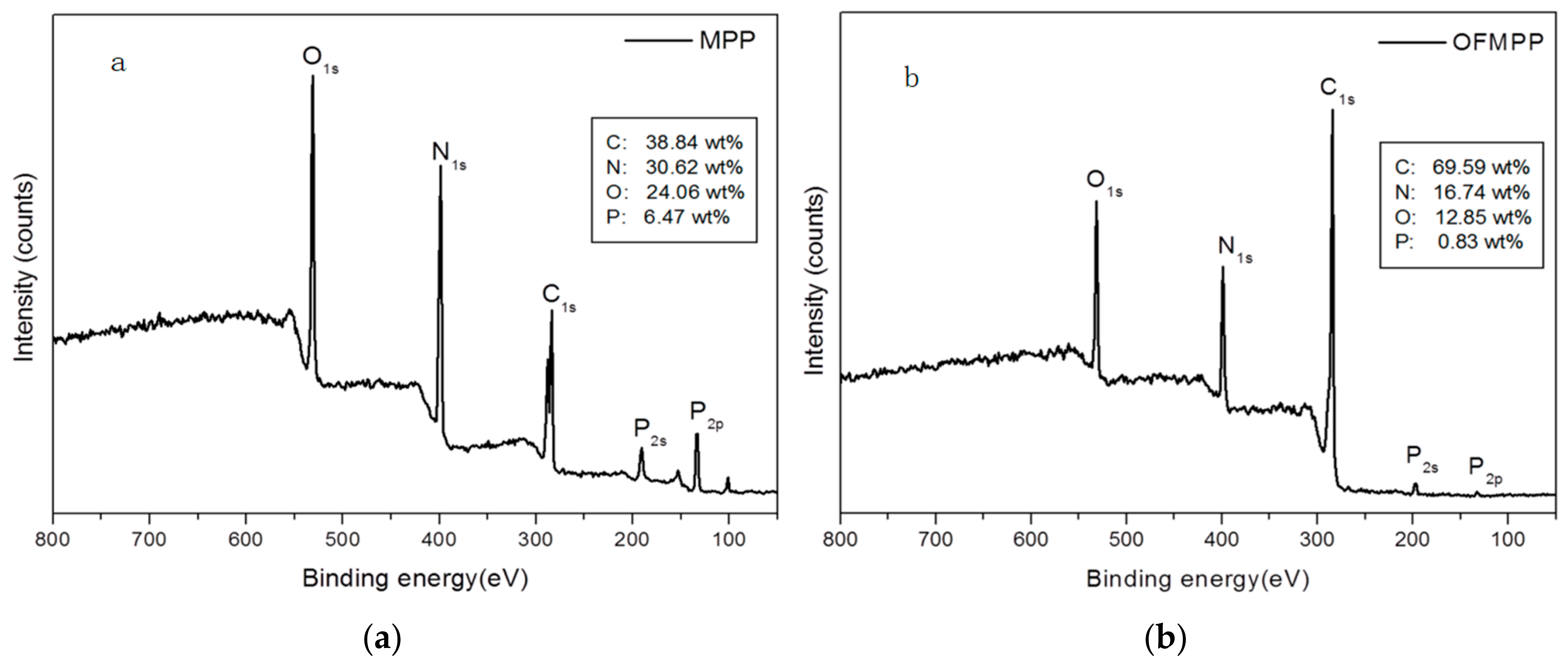
3.2. XPS of OFMPP
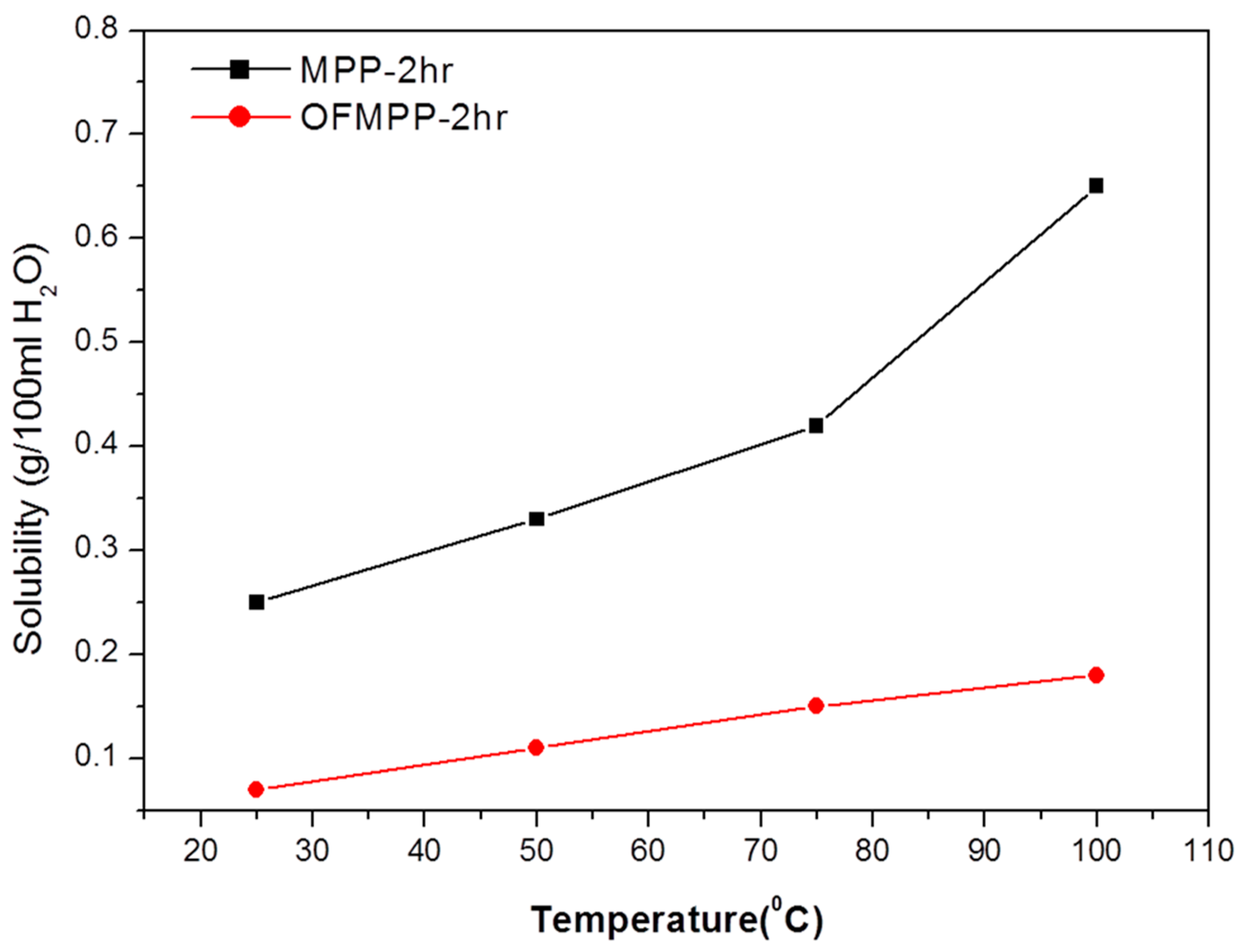
3.3. Water Solubility of OFMPP
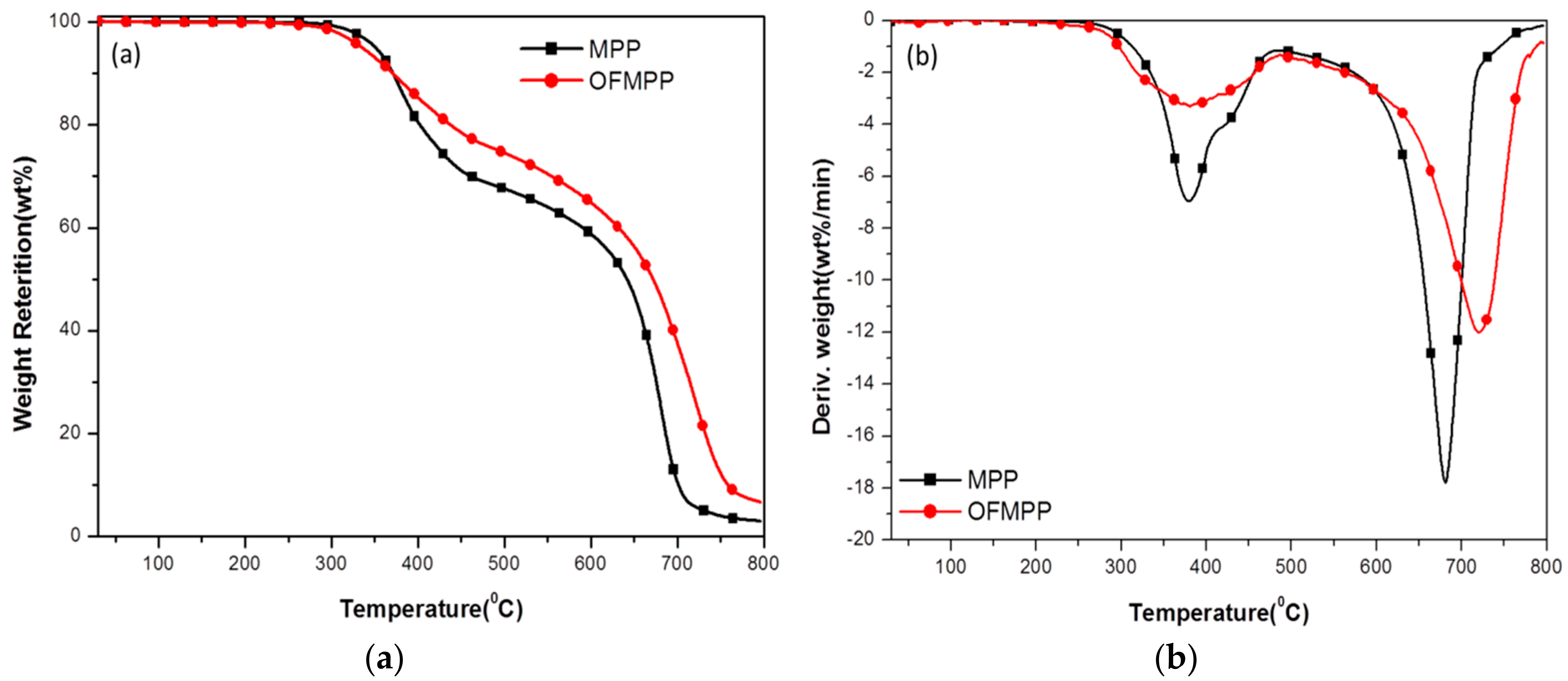
3.4. TGA of OFMPP
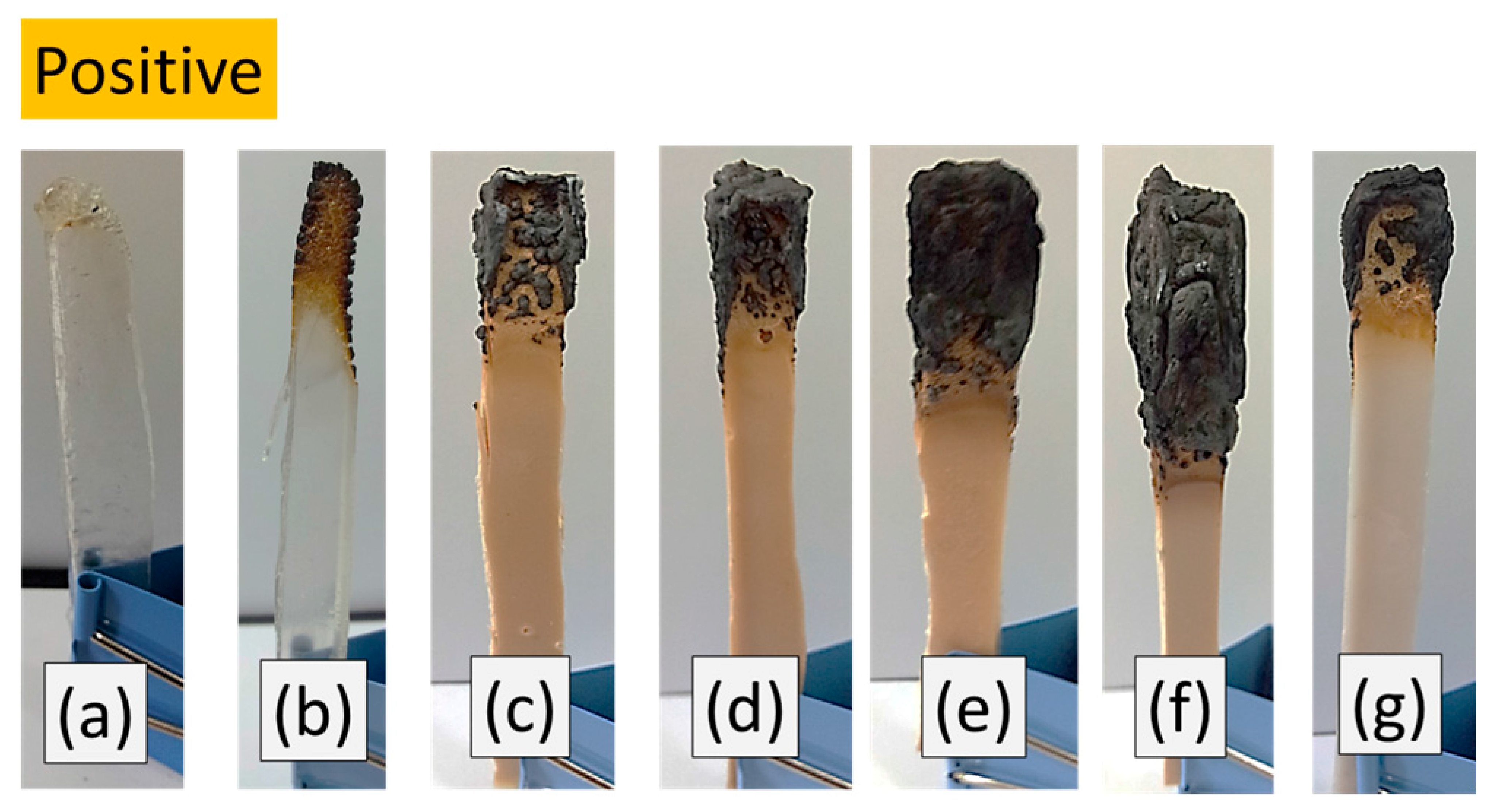
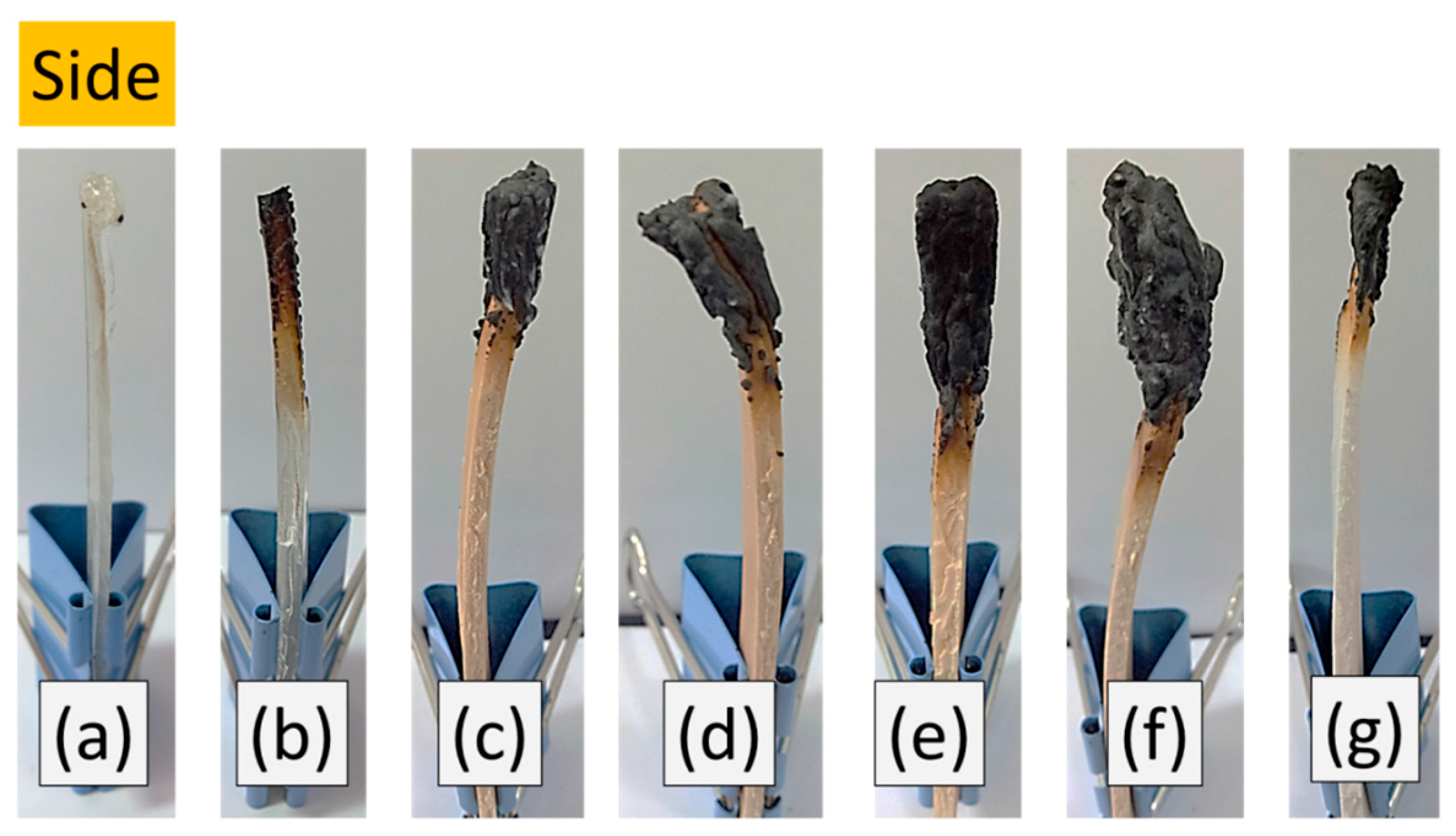
3.5. LOI and UL-94 of Si-PU/OFMPP Composites
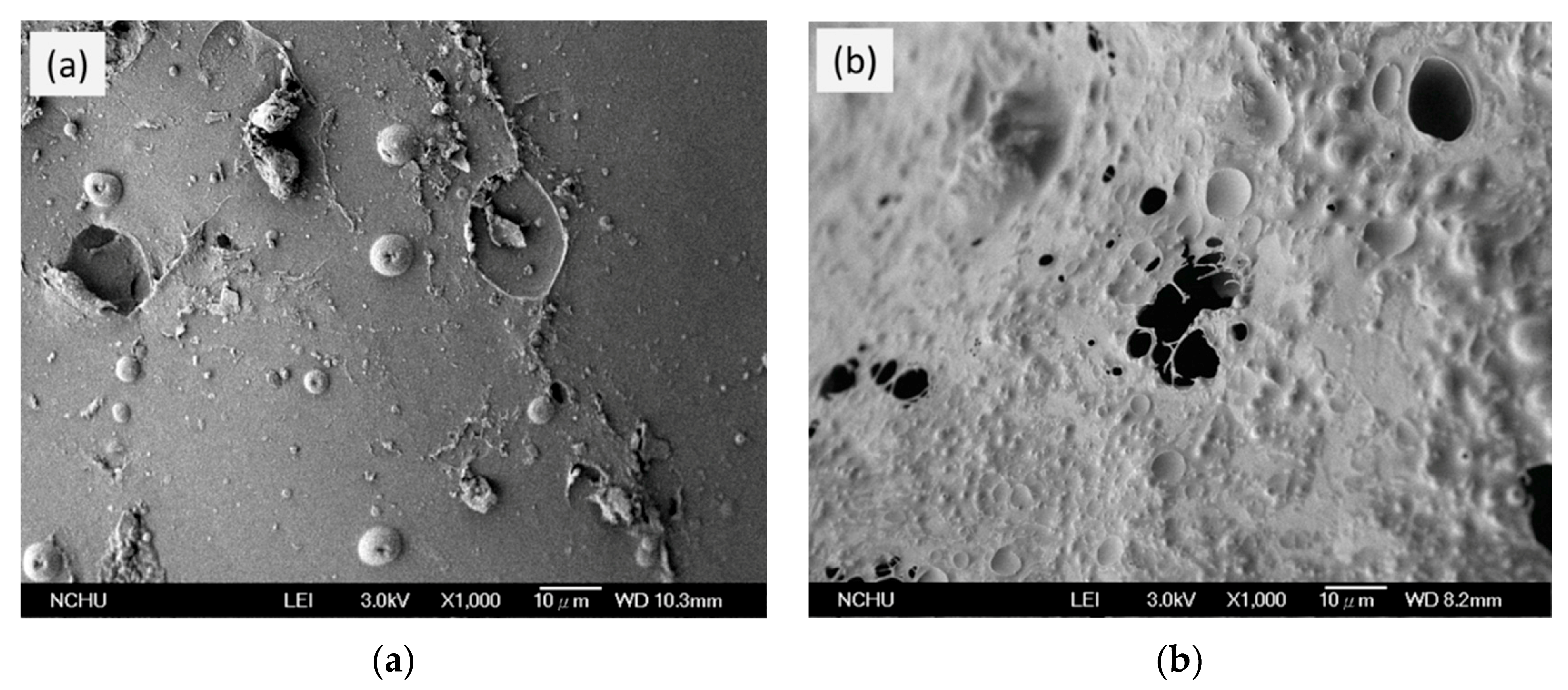
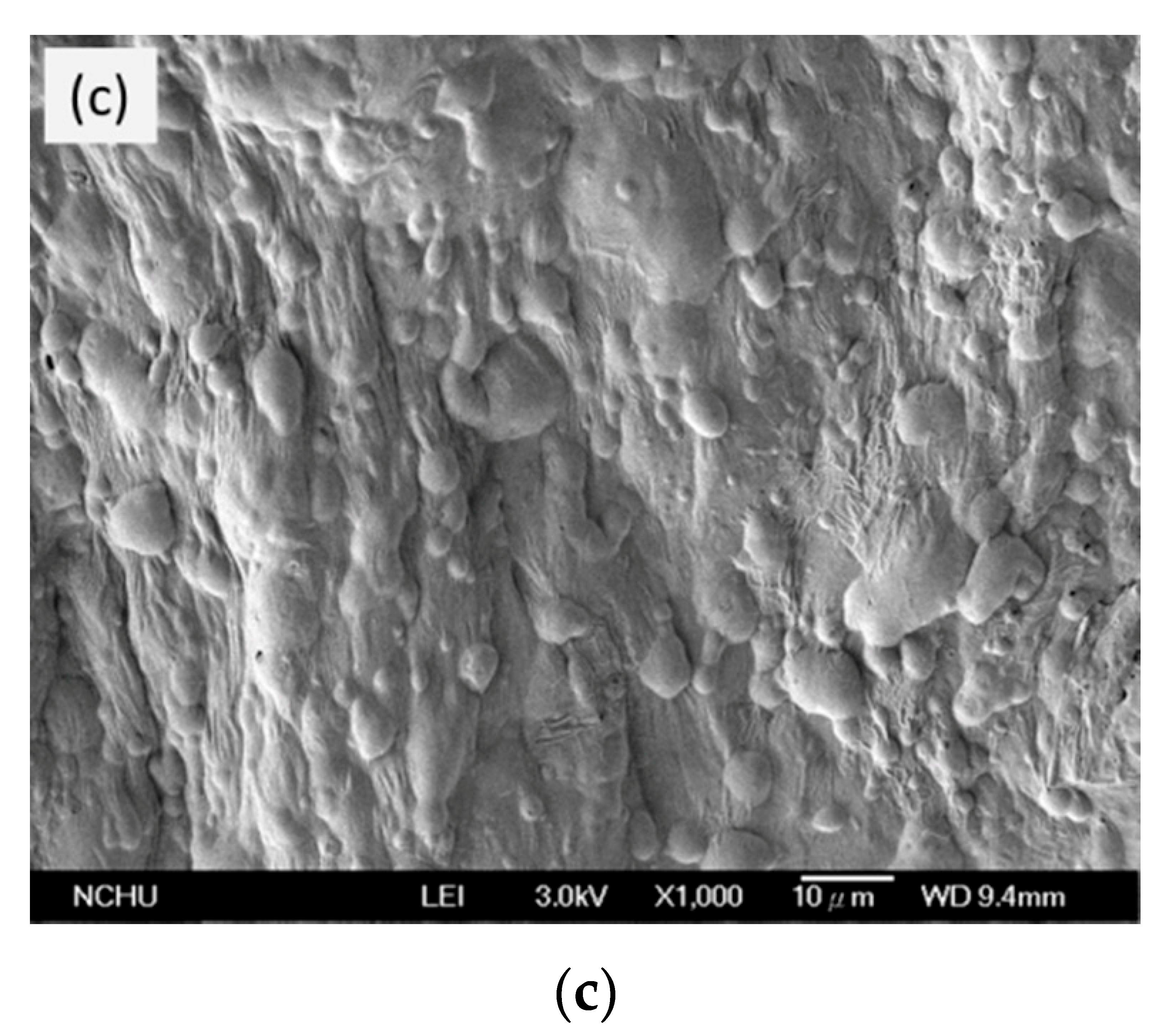
3.6. SEM of Si-PU/OFMPP Composites after Burning
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jeon, H.T.; Jang, M.K.; Kim, B.K.; Kim, K.H. Synthesis and characterizations of waterborne polyurethane-silica hybrids using sol-gel process. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 559–567. [Google Scholar] [CrossRef]
- Jin, J.; Dong, Q.X.; Shu, Z.J.; Wang, W.J.; He, K. Flame retardant Properties of Polyurethane/expandable Praphite Composites. Procedia. Eng. 2014, 71, 304–309. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Nando, G.B.; Naik, Y.P.; Singha, N.K. Halogen-free flame retardant PUF: Effect of melamine compounds on mechanical, thermal and flame retardant properties. Polym. Degrad. Stabil. 2010, 95, 1138–1145. [Google Scholar] [CrossRef]
- Liu, X.Q.; Wang, D.Y.; Wang, X.L.; Chen, L.; Wang, Y.Z. Synthesis of functionalized a-zirconium phosphate modified with intumescent flame retardant and its application in poly(lactic acid). Polym. Degrad. Stabil. 2013, 98, 1731–1737. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Lv, P.; Hu, Y.; Hu, K. Thermal degradation study of intumescent flame retardants by TG and FTIR: Melamine phosphate and its mixture with pentaerythritol. J. Anal. Appl. Pyrolysis 2009, 86, 207–214. [Google Scholar] [CrossRef]
- Xia, Y.; Jin, F.F.; Mao, Z.W.; Guan, Y.; Zheng, A. Effects of ammonium polyphosphate to pentaerythritol ratio on composition and properties of carbonaceous foam deriving from intumescent flame-retardant polypropylene. Polym. Degrad. Stabil. 2014, 107, 64–73. [Google Scholar] [CrossRef]
- Tai, Q.; Yuen, K.K.; Yang, W.; Qiao, Z.; Song, L.; Hu, Y. Iron-montmorillonite and zinc borate as synergistic agents in flame-retardant glass fiber reinforced polyamide 6 composites in combination with melamine polyphosphate. Compos. Part A Appl. Sci. Manuf. 2012, 43, 415–422. [Google Scholar] [CrossRef]
- Yang, H.Y.; Song, L.; Tai, Q.; Wang, X.; Yu, B.; Yuan, Y.; Hu, Y.; Yuen, K.K. Comparative study on the flame retarded efficiency of melamine phosphate, melamine phosphite and melamine hypophosphite on poly(butylene succinate) composites. Polym. Degrad. Stabil. 2014, 105, 248–256. [Google Scholar] [CrossRef]
- Naik, A.D.; Fontaine, G.; Samyn, F.; Delva, X.; Bourgeois, Y.; Bourbigot, S. Melamine integrated metal phosphates as non-halogenated flame retardants: Synergism with aluminium phosphinate for flame retardancy in glass fiber reinforced polyamide 66. Polym. Degrad. Stabil. 2013, 98, 2653–2662. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Wu, K.; Hu, Y. Study on flame retardance of co-microencapsulated ammonium polyphosphate and dipentaerythritol in polypropylene. Polym. Eng. Sci. 2008, 48, 2426–2431. [Google Scholar] [CrossRef]
- Wu, K.; Wang, Z.Z.; Liang, H. Microencapsulation of ammonium polyphosphate: Preparation, characterization, and its flame retardance in polypropylene. Polym. Compos. 2008, 29, 854–860. [Google Scholar] [CrossRef]
- Luo, J.; Wang, X.; Li, J.; Zhao, X.; Wang, F. Conductive hybrid film from polyaniline and polyurethaneesilica. Polymer 2007, 48, 4368–4374. [Google Scholar] [CrossRef]
- Bocz, K.; Szolnoki, B.; Marosi, A.; Tábi, T.; Wladyka, P.M.; Marosi, G. Flax fibre reinforced PLA/TPS biocomposites flame retarded with multifunctional additive system. Polym. Degrad. Stabil. 2014, 106, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Huo, L.; Jiao, C.; Li, S. TG-FTIR characterization of volatile compounds from flame retardant polyurethane foams materials. J. Anal. Appl. Pyrolysis 2013, 100, 186–191. [Google Scholar] [CrossRef]
- Han, Y.H.; Taylor, A.; Mantle, M.; Knowles, K. Sol-gel-derived organic-inorganic hybrid materials. J. Non-Cryst. Solids 2007, 353, 313–320. [Google Scholar] [CrossRef]
- Lee, T.M.; Ma, C.C.; Hsu, C.W.; Wu, H.L. Effect of molecular structures and mobility on the thermal and dynamical mechanical properties of thermally cured epoxy-bridged polyorganosiloxanes. Polymer 2005, 46, 8286–8296. [Google Scholar] [CrossRef]
- Qian, X.; Song, L.; Bihe, Y.; Yu, B.; Yongqian, S.; Hu, Y.; Yuen, K.K. Organic/inorganic flame retardants containing phosphorus, nitrogen and silicon: Preparation and their performance on the flame retardancy of epoxy resins as a novel intumescent flame retardant system. Mater. Chem. Phys. 2014, 143, 1243–1252. [Google Scholar] [CrossRef]
- Wang, X.; Pang, H.; Chen, W.; Lin, Y.; Ning, G. Nanoengineering core/shell structured brucite@polyphosphate@amine hybrid system for enhanced flame retardant properties. Polym. Degrad. Stabil. 2013, 98, 2609–2616. [Google Scholar] [CrossRef]
- Hua, X.; Guo, Y.; Chen, L.; Wang, X.; Li, L.; Wang, Y. A novel polymeric intumescent flame retardant: Synthesis, thermal degradation mechanism and application in ABS copolymer. Polym. Degrad. Stabil. 2012, 97, 1772–1778. [Google Scholar] [CrossRef]
- Salauün, F.; Huang, Z.; Zhang, Y. Preparation of CMC-modified melamine resin spherical nano-phase change energy storage materials. Carbohydr. Polym. 2014, 101, 83–88. [Google Scholar]
- Poljansek, I.; Krajnc, M. Characterization of phenol-formaldehyde prepolymer resins by in line FT-IR spectroscopy. Acta Chim. Slov. 2005, 52, 238–244. [Google Scholar]
- Salaün, F.; Vroman, I. Influence of core materials on thermal properties of melamine-formaldehyde microcapsules. Eur. Polym. J. 2008, 44, 849–860. [Google Scholar] [CrossRef]
- Zheng, Z.; Yan, J.; Sun, H.; Cheng, Z.; Li, W.; Wang, H.; Cui, X. Microencapsulated ammonium polyphosphate and its synergistic flame-retarded polyurethane rigid foams with expandable graphite. Polym. Int. 2011, 63, 84–92. [Google Scholar] [CrossRef]
- Wang, B.; Tang, Q.; Hong, N.; Song, L.; Wang, L.; Shi, Y.; Hu, Y. Effect of cellulose acetate butyrate microencapsulated ammonium polyphosphate on the flame retardancy, mechanical, electrical, and thermal properties of intumescent flame-retardant ethylene_vinyl acetate copolymer/microencapsulated ammonium polyphosphate/polyamide-6 blends. Appl. Mater. Interfaces 2011, 3, 3754–3761. [Google Scholar]
- Wang, G.; Yang, J. Thermal degradation study of fire resistive coating containing melamine polyphosphate and dipentaerythritol. Prog. Org. Coat. 2011, 72, 605–611. [Google Scholar] [CrossRef]
- Kang, C.; Huang, J.; He, W.; Zhang, F. Periodic mesoporous silica-immobilized palladium(II) complex as an effective and reusable catalyst for water-medium carbon-carbon coupling reactions. J. Organomet. Chem. 2010, 695, 120–127. [Google Scholar] [CrossRef]
- Chiu, Y.C.; Ma, C.C.; Liu, F.Y.; Chiang, C.L.; Riang, L.; Yang, J.C. Effect of P/Si polymeric silsesquioxane and the monomer compound on thermalproperties of epoxy nanocomposite. Eur. Polym. J. 2008, 44, 1003–1011. [Google Scholar] [CrossRef]
- Wu, C.S.; Liu, Y.L.; Chiu, Y.S. Epoxy resins possessing flame retardant elements from silicon incroporaed epoxy compouds cured with phosphorus or nitrogen containing curing agents. Polymer 2002, 43, 4277–4284. [Google Scholar] [CrossRef]
- Liu, Y.L.; Hsu, C.Y.; Wei, W.L.; Jeng, R.J. Preparation and thermal properties of epoxy-silica nanocomposites from nanoscale colloidal silica. Polymer 2003, 44, 5159–5167. [Google Scholar] [CrossRef]
- Wu, C.S.; Liu, Y.L.; Chiu, Y.C.; Chiu, Y.S. Thermal stability of epoxy resins containing flame retardant components: An evaluation with thermogravimetric analysis. Polym. Degrad. Stabil. 2002, 78, 41–48. [Google Scholar] [CrossRef]
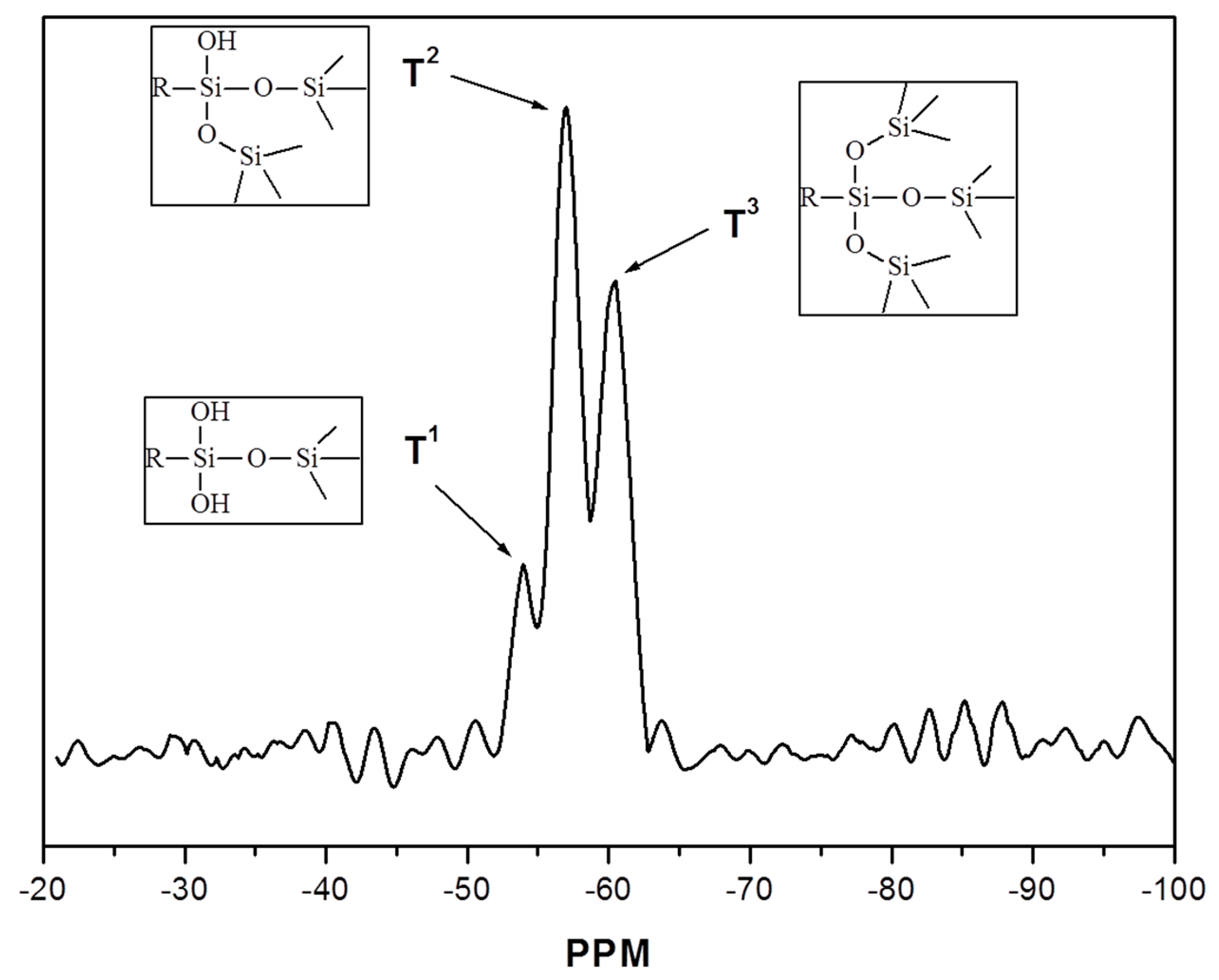
| Sample No. | Area (%) | Degree of condensation (%) | ||
|---|---|---|---|---|
| T1 | T2 | T3 | ||
| Si-PU composites | 8 | 55 | 37 | 76.33 |
| Sample code | 25 °C (g/100 mL H2O) | 50 °C (g/100 mL H2O) | 75 °C (g/100 mL H2O) | 100 °C (g/100 mL H2O) |
|---|---|---|---|---|
| MPP | 0.25 | 0.33 | 0.42 | 0.65 |
| OFMPP | 0.07 | 0.11 | 0.15 | 0.18 |
| Sample No. | LOI (%) | UL-94 | ||||
|---|---|---|---|---|---|---|
| Before soakage | After soakage | Before soakage | After soakage | |||
| Ranking | Dripping | Ranking | Dripping | |||
| Pristine PU | 17 | 17 | Fail | YES | Fail | YES |
| Si-PU | 18 | 18 | Fail | NO | Fail | NO |
| Si-PU/OFMPP 10% | 19 | 19 | Fail | NO | Fail | NO |
| Si-PU/OFMPP 20% | 25 | 24 | Fail | NO | Fail | NO |
| Si-PU/OFMPP 30% | 32 | 30 | V-0 | NO | V-1 | NO |
| Si-PU/OFMPP 40% | 38 | 36 | V-0 | NO | V-0 | NO |
| Si-PU/MPP 30% | 27 | 24 | Fail | NO | Fail | NO |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-H.; Kuan, C.-F.; Kuan, H.-C.; Shen, M.-Y.; Yang, J.-M.; Chiang, C.-L. Preparation and Flame Retardance of Polyurethane Composites Containing Microencapsulated Melamine Polyphosphate. Polymers 2017, 9, 407. https://doi.org/10.3390/polym9090407
Liu S-H, Kuan C-F, Kuan H-C, Shen M-Y, Yang J-M, Chiang C-L. Preparation and Flame Retardance of Polyurethane Composites Containing Microencapsulated Melamine Polyphosphate. Polymers. 2017; 9(9):407. https://doi.org/10.3390/polym9090407
Chicago/Turabian StyleLiu, Shang-Hao, Chen-Feng Kuan, Hsu-Chiang Kuan, Ming-Yuan Shen, Jia-Ming Yang, and Chin-Lung Chiang. 2017. "Preparation and Flame Retardance of Polyurethane Composites Containing Microencapsulated Melamine Polyphosphate" Polymers 9, no. 9: 407. https://doi.org/10.3390/polym9090407