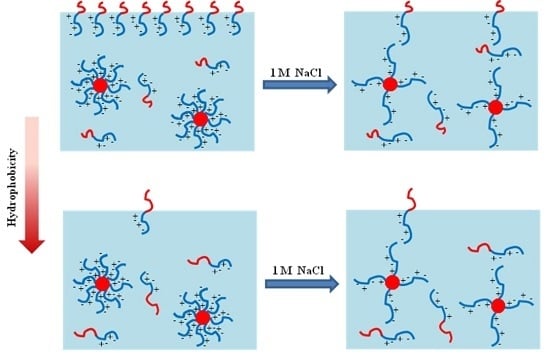
Surface Active to Non-Surface Active Transition and Micellization Behaviour of Zwitterionic Amphiphilic Diblock Copolymers: Hydrophobicity and Salt Dependency
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
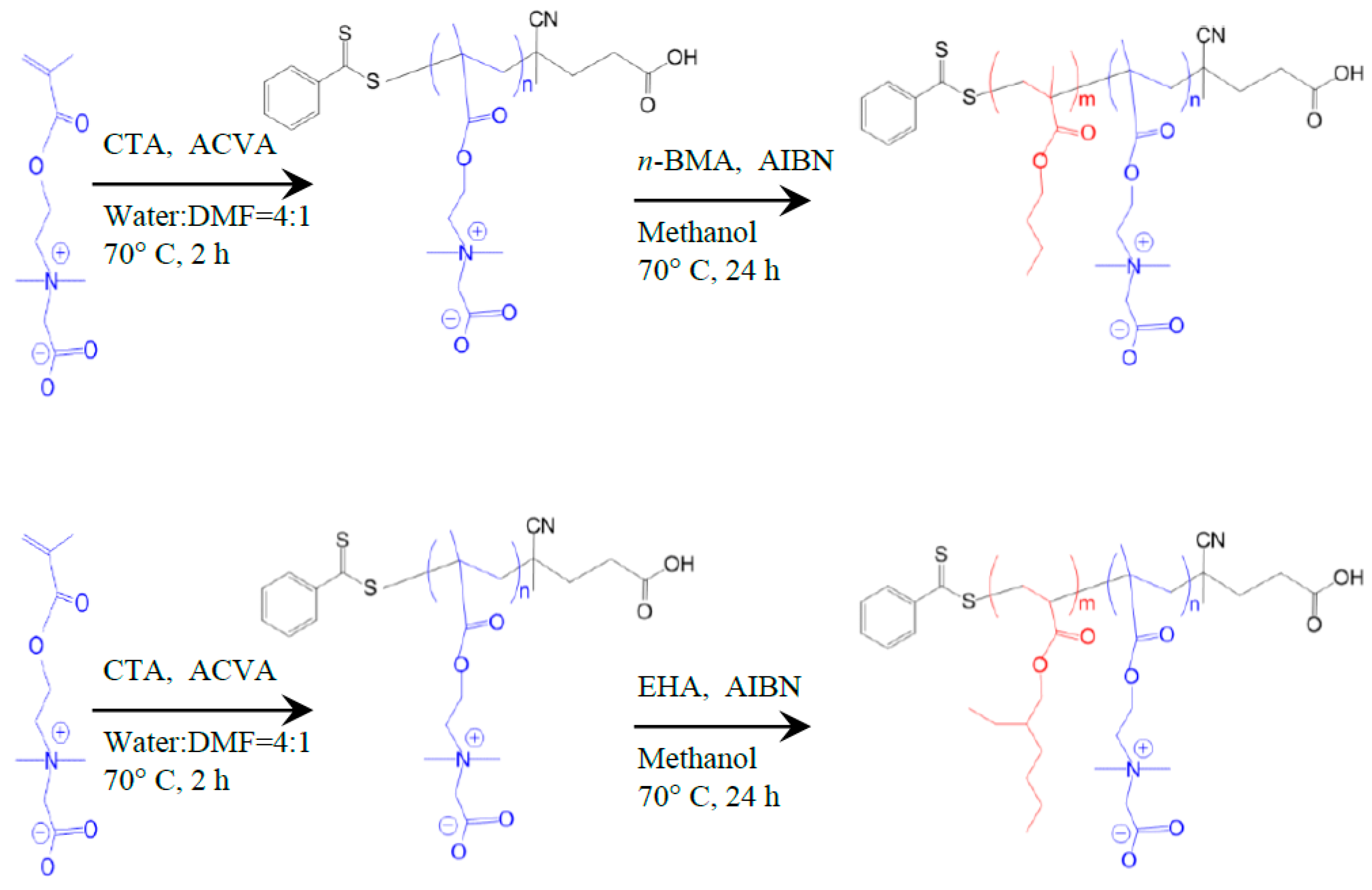
2.2. Synthesis
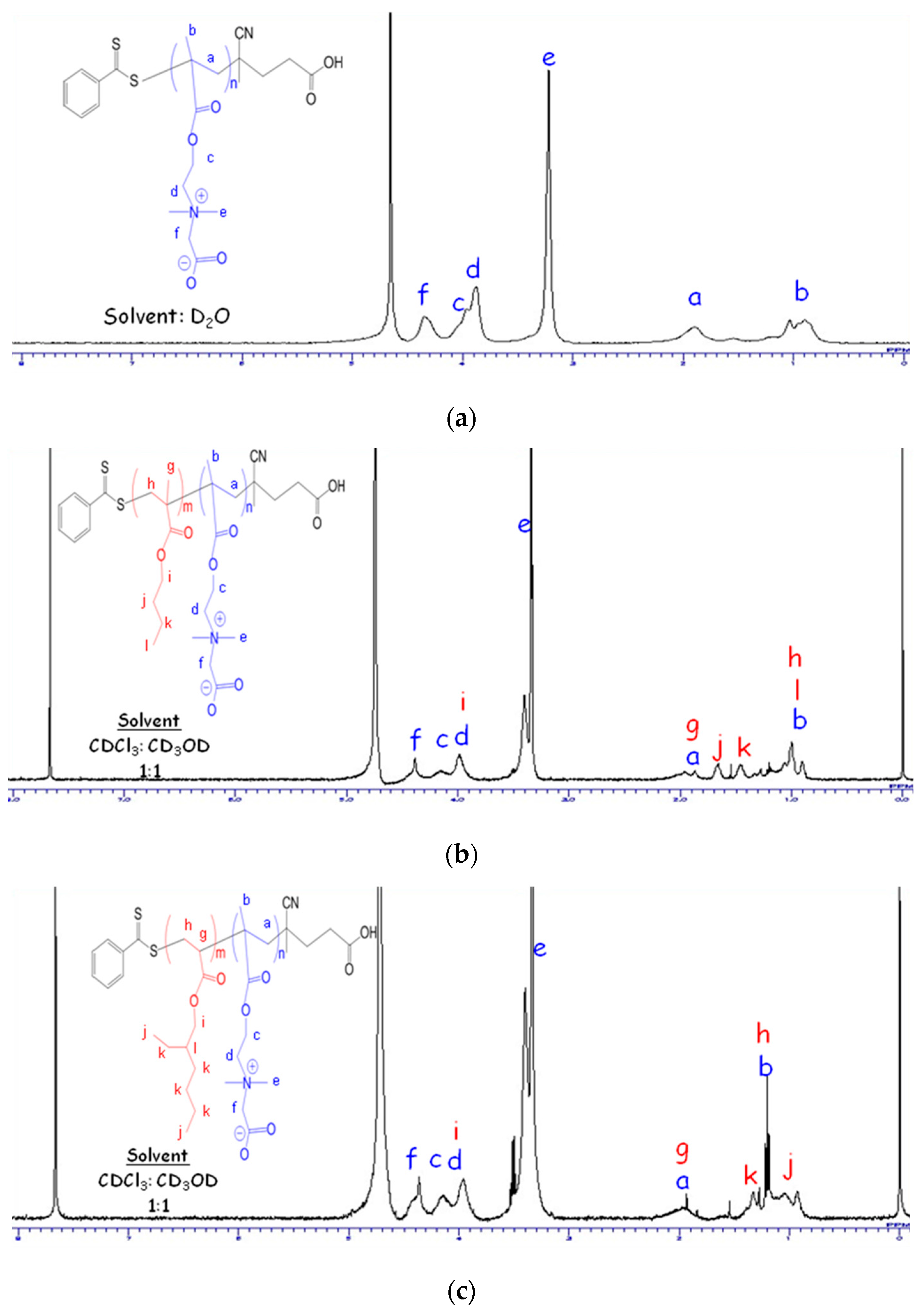
2.3. 1H Nuclear Magnetic Resonance (1H NMR)
2.4. Gel Permeation Chromatography (GPC)
2.5. Surface Tension Measurements
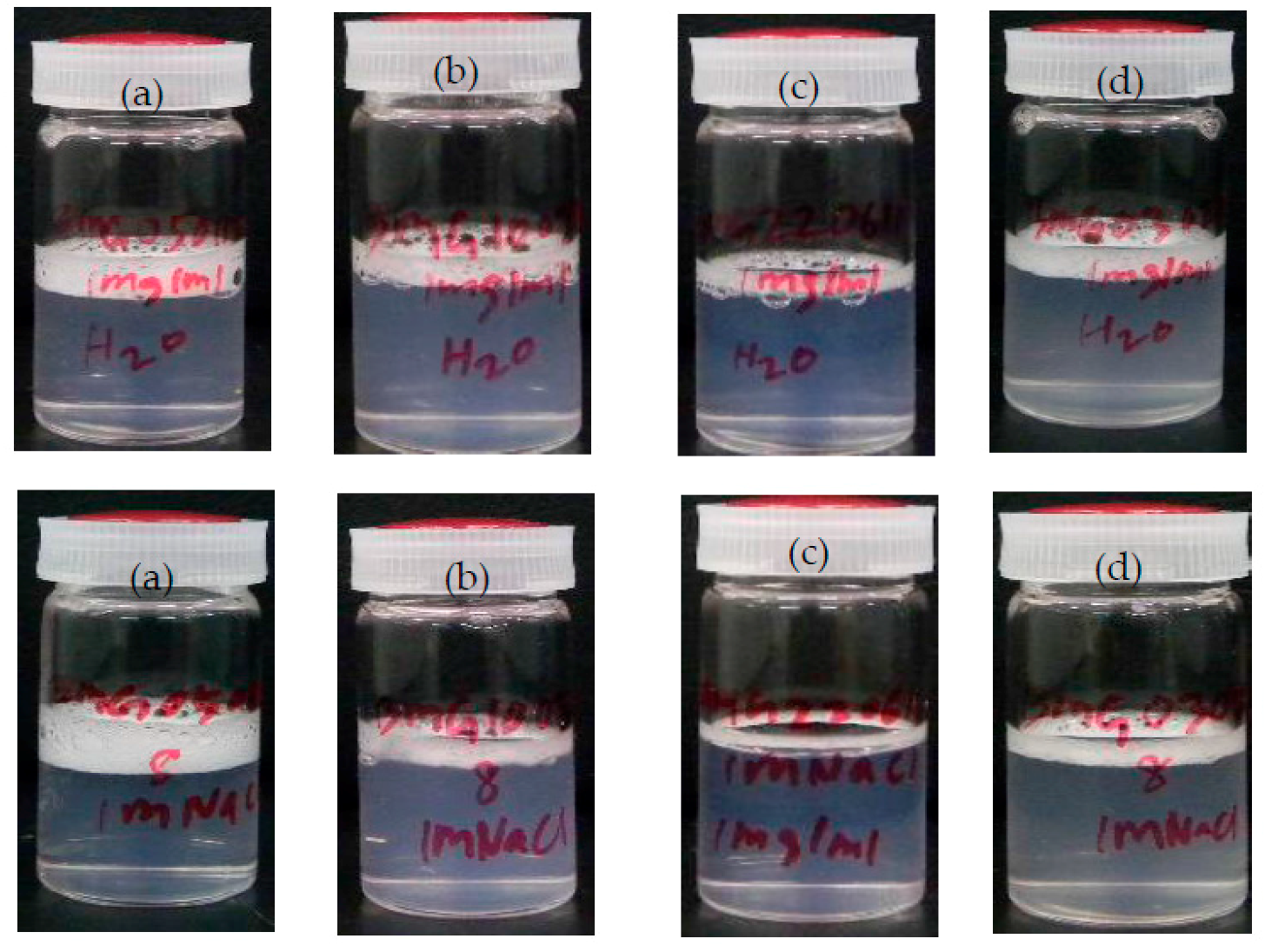
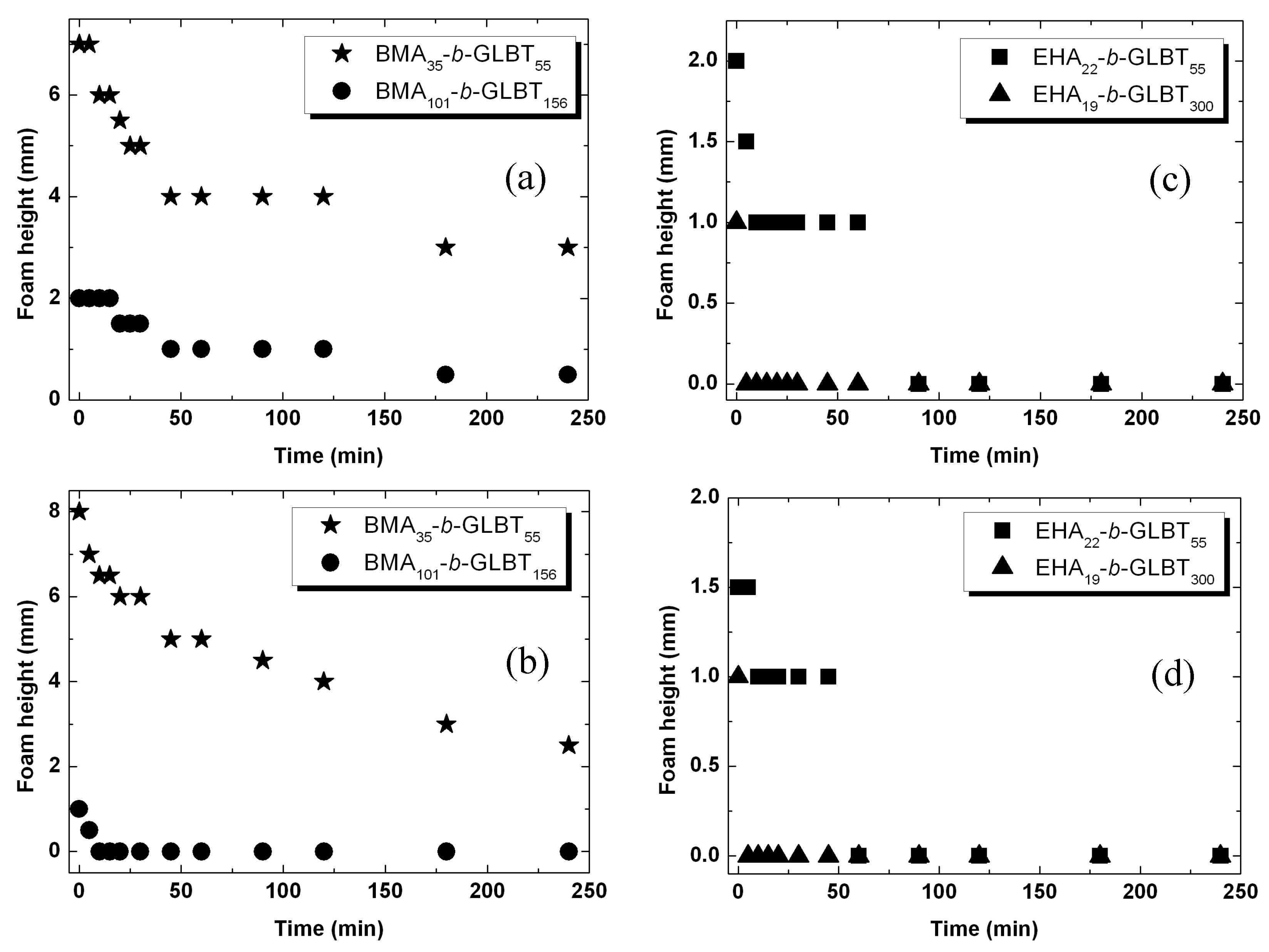
2.6. Foam Formation and Foam Height Measurements
2.7. Light Scattering Measurements
2.8. Specific Refractive Index Increment Measurements (dn/dcp)
3. Results and Discussion
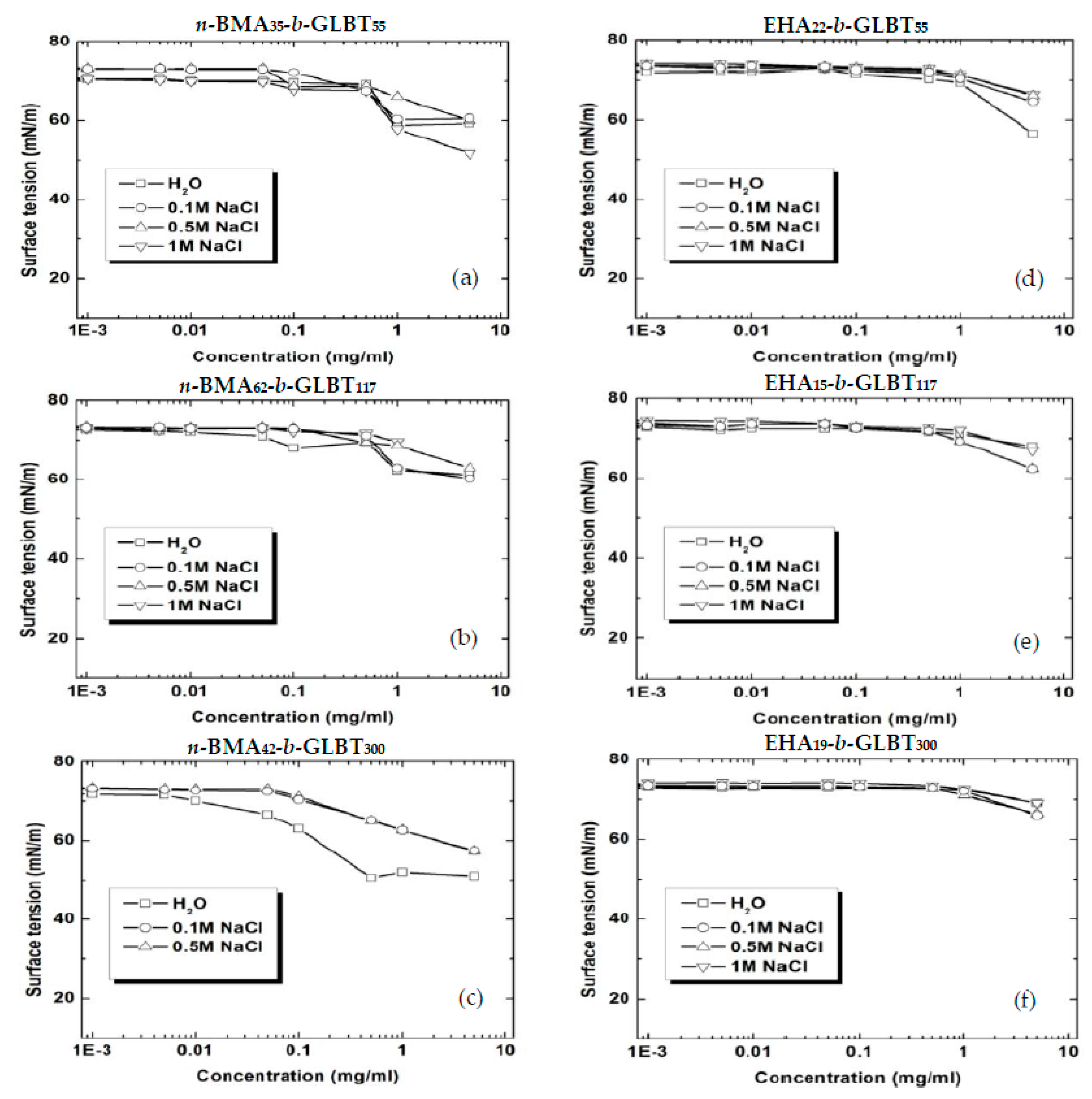
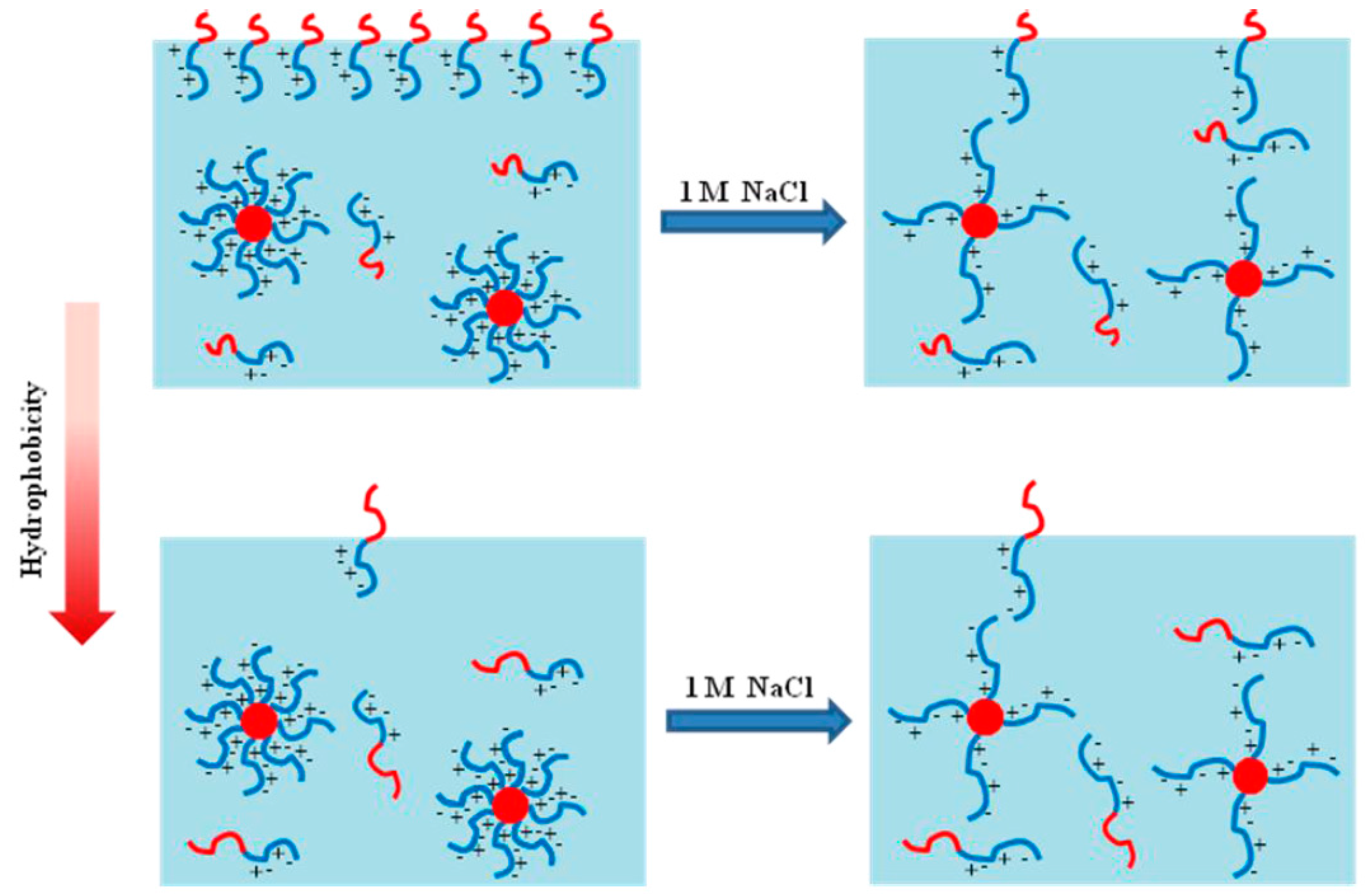
3.1. Hydrophobicity and Salt-Dependent Air–Water Interfacial Properties
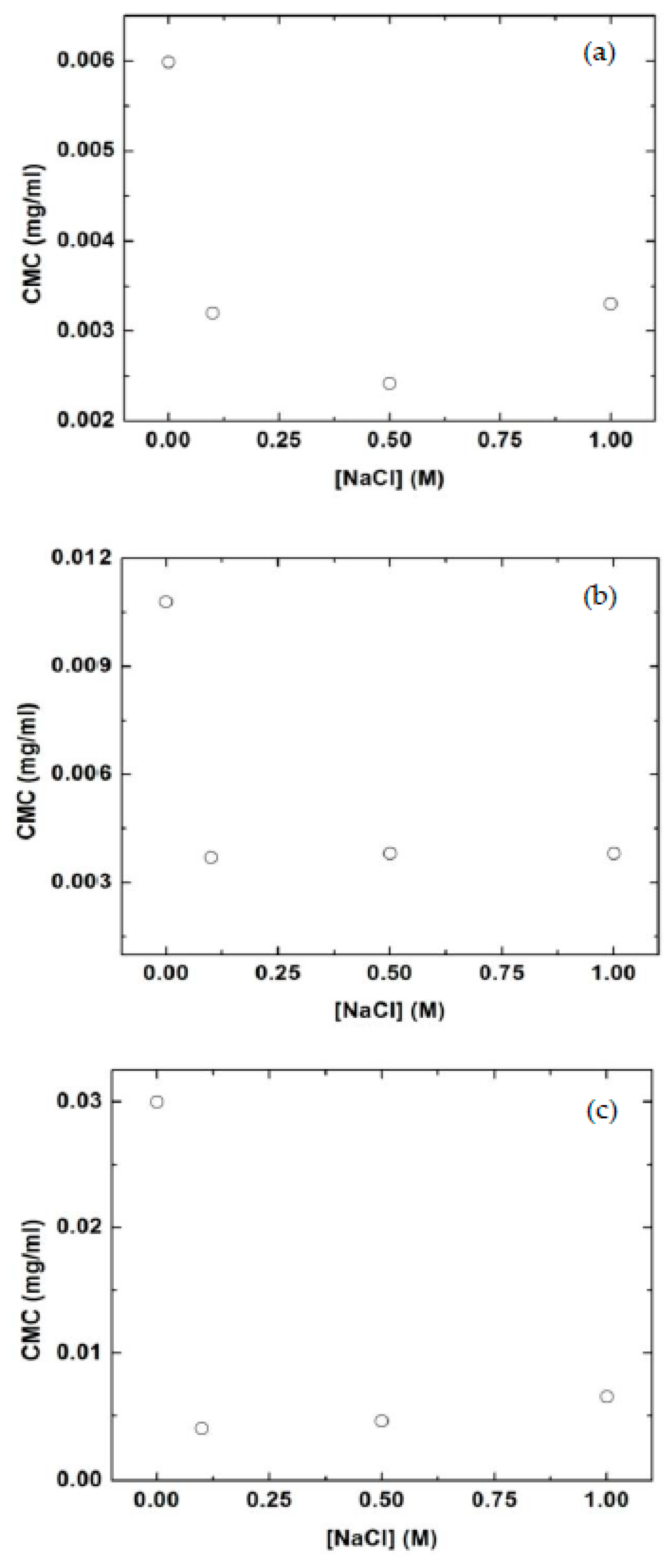
3.2. Effect of Salt on CMC
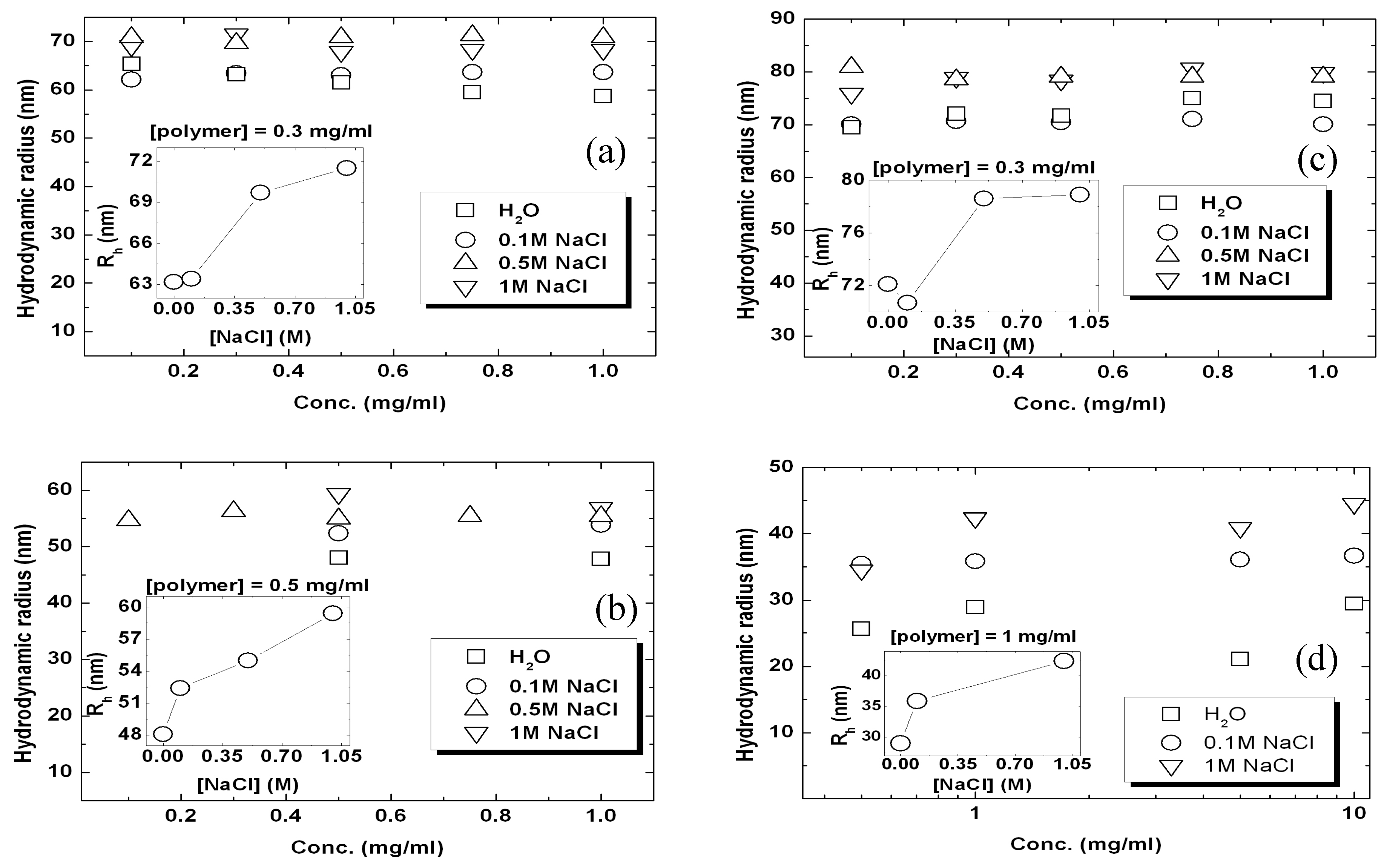
3.3. Influence of Salt on Hydrodynamic Radius
3.4. Hydrophobicity, Salt and Block Length-Dependent Aggregation Number and Second Virial Coefficient of Micelles
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bates, F.S.; Fredrickson, G.H. Block copolymers—Designer soft materials. Phys. Today 1999, 52, 32–38. [Google Scholar] [CrossRef]
- Hamley, I.W. The Physics of Block Copolymers; Oxford University Press: Oxford, UK, 1998; ISBN 9780198502180. [Google Scholar]
- Bahadur, P. Block copolymers—Their microdomain formation (in solid state) and surfactant behaviour (in solution). Curr. Sci. 2001, 80, 1002–1007. [Google Scholar]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Wittmer, J.; Joanny, J.F. Charged diblock copolymers at interfaces. Macromolecules 1993, 26, 2691–2697. [Google Scholar] [CrossRef]
- Netz, R.R.; Andelman, D. Neutral and charged polymers at interfaces. Phys. Rep. 2003, 380, 1–95. [Google Scholar] [CrossRef]
- Matsuoka, H.; Matsutani, M.; Mouri, E.; Matsumoto, K. Polymer micelle formation without Gibbs monolayer formation—Synthesis and characteristics of amphiphilic diblock copolymer having sulfonic acid groups. Macromolecules 2003, 36, 5321–5330. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ishizuka, T.; Harada, T.; Matsuoka, H. Association behavior of fluorine-containing and non-fluorine-containing methacrylate-based amphiphilic diblock copolymer in aqueous media. Langmuir 2004, 20, 7270–7282. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Maeda, S.; Kaewsaiha, P.; Matsumoto, K. Micellization of non-surface-active diblock copolymers in water, Special characteristics of poly(styrene)-block-poly(styrenesulfonate). Langmuir 2004, 20, 7412–7421. [Google Scholar] [CrossRef] [PubMed]
- Kaewsaiha, P.; Matsumoto, K.; Matsuoka, H. Non-surface activity and micellization of ionic amphiphilic diblock copolymers in water, Hydrophobic chain length dependence and salt effect on surface activity and the critical micelle concentration. Langmuir 2005, 21, 9938–9945. [Google Scholar] [CrossRef] [PubMed]
- Kaewsaiha, P.; Matsumoto, K.; Matsuoka, H. Sphere-to-rod transition of non-surface-active amphiphilic diblock copolymer micelles: A small-angle neutron scattering study. Langmuir 2007, 23, 9162–9169. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R.R.; Yamada, T.; Matsuoka, H. Non-surface activity of cationic amphiphilic diblock copolymers. IOP Conf. Ser. Mater. Sci. Eng. 2011, 24, 012024. [Google Scholar] [CrossRef]
- Ghosh, A.; Yusa, S.; Matsuoka, H.; Saruwatari, Y. Non-surface activity and micellization behavior of cationic amphiphilic block copolymer synthesized by reversible addition-fragmentation chain transfer process. Langmuir 2011, 27, 9237–9244. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Chen, H.; Matsumoto, K. Molecular weight dependence of non-surface activity for ionic amphiphilic diblock copolymers. Soft Matter 2012, 35, 9140–9146. [Google Scholar] [CrossRef]
- Ghosh, A.; Yusa, S.; Matsuoka, H.; Saruwatari, Y. Chain length dependence of non-surface activity and micellization behaviour of cationic amphiphilic diblock copolymers. Langmuir 2014, 30, 3319–3328. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Onishi, T.; Ghosh, A. pH-responsive non-surface-active/surface-active transition of weakly ionic amphiphilic diblock copolymers. Colloid Polym. Sci. 2014, 292, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Amiel, C.; Sikka, M.; Schneider, J.W.; Tsao, Y.H.; Tirrell, M.; Mays, J.W. Adsorption of hydrophilic-hydrophobic block copolymers on silica from aqueous solutions. Macromolecules 1995, 28, 3125–3134. [Google Scholar] [CrossRef]
- Theodoly, O.; Jacquin, M.; Muller, P.; Chhun, S. Adsorption kinetics of amphiphilic diblock copolymers: From kinetically frozen colloids to macrosurfactants. Langmuir 2009, 25, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Tuzar, Z.; Pospisil, H.; Plestil, J.; Lowe, A.B.; Baines, F.L.; Billingham, N.C.; Armes, S.P. Micelles of hydrophilic-hydrophobic poly(sulfobetaine)-based block copolymers. Macromolecules 1997, 30, 2509–2512. [Google Scholar] [CrossRef]
- Lowe, A.B.; Billingham, N.C.; Armes, S.P. Synthesis and properties of low-polydispersity poly(sulfopropylbetaine)s and their block copolymers. Macromolecules 1999, 32, 2141–2148. [Google Scholar] [CrossRef]
- Yusa, S.; Fukuda, K.; Yamamoto, T.; Ishihara, K.; Morishima, Y. Synthesis of well-defined amphiphilic block copolymers having phospholipid polymer sequences as a novel biocompatible polymer micelle reagent. Biomacromolecules 2005, 6, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Sivanantham, M.; Matsuoka, H. Salt-dependent surface activity and micellization behaviour of zwitterionic amphiphilic diblock copolymers having carboxybetaine. Colloid Polym. Sci. 2015, 293, 1317–1328. [Google Scholar] [Green Version]
- Onsager, L.; Samaras, N.N.T. The surface tension of Debye-Hückel electrolytes. J. Chem. Phys. 1934, 2, 528–536. [Google Scholar] [CrossRef]
- Leonard, E.C. Vinyl and Diene Monomers, High Polymers Series; Wiley-Interscience: New York, NY, USA, 1970; Volume XXIV. [Google Scholar]
- Vijayendran, B.R. Polymer polarity and surfactant adsorption. J. Appl. Polym. Sci. 1979, 23, 733–742. [Google Scholar] [CrossRef]
- Dumitriu, S.; Popa, V. Polymeric Biomaterials; CRC Press: Boca Raton, FL, USA, 2002; ISBN 9781420094725. [Google Scholar]
- Mitsukami, Y.; Donovan, M.S.; Lowe, A.B.; McCormick, C.L. Water-soluble polymers. 81. Direct synthesis of hydrophilic styrenic-based homopolymers and block copolymers in aqueous solution via raft. Macromolecules 2001, 34, 2248–2256. [Google Scholar] [CrossRef]
- Oae, S.; Yagihara, T.; Okabe, T. Reduction of semipolar sulphur linkages with carbodithioic acids and addition of carbodithioic acids to olefins. Tetrahedron 1972, 28, 3203–3216. [Google Scholar] [CrossRef]
- Berne, B.; Pecora, R. Dynamic Light Scattering; John Wiley: New York, NY, USA, 1976; p. 174. ISBN 0486411559. [Google Scholar]
- Zimm, B.H. Apparatus and methods for measurement and interpretation of the angular variation of light scattering; Preliminary results on polystyrene solutions. J. Chem. Phys. 1948, 16, 1099–1116. [Google Scholar] [CrossRef]
- Huglin, M.B. Light Scattering from Polymer Solutions; Academic Press: New York, NY, USA, 1972. [Google Scholar]
- Inayama, R.; Nakamura, S.; Tatsumi, N.; Otsuka Electronics Co., Osaka, Japan. Private communication, 2013.
- Jiang, W.; Fischer, G.; Girmay, Y.; Irgum, K. Zwitterionic stationary phase with covalently bonded phosphorylcholine type polymer grafts and its applicability to separation of peptides in the hydrophilic interaction liquid chromatography mode. J. Chromatogr. A 2006, 1127, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, K.; Hashizume, M.; Kikuchi, J.; Taketani, Y.; Murakami, M. Creation of asymmetric bilayer membrane on monodispersed colloidal silica particles. Colloids Surf. B 2004, 38, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.P.; Reynolds, P.A.; Lewis, A.L.; Kirkwood, L.; Hughes, L.G. Investigation into potential mechanisms promoting biocompatibility of polymeric biomaterials containing the phosphorylcholine moiety: A physicochemical and biological study. Colloids Surf. B 2005, 46, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Matsuoka, H. Photo-responsive block copolymer: Synthesis, characterization and surface activity control. Langmuir 2014, 30, 3957–3966. [Google Scholar] [CrossRef] [PubMed]
- Corrin, M.L.; Harkins, W.D. The effect of salts on the critical concentration for the formation of micelles in colloidal electrolytes. J. Am. Chem. Soc. 1947, 69, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Khougaz, K.; Astafieva, I.; Eisenberg, A. Micellization in block polyelectrolyte solutions. 3. Static light scattering characterization. Macromolecules 1995, 28, 7135–7147. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 2nd ed.; Academic Press: London, UK; New York, NY, USA, 1991. [Google Scholar]
- Nagarajan, R. Molecular packing parameter and surfactant self-assembly: The neglected role of the surfactant tail. Langmuir 2002, 18, 31–38. [Google Scholar] [CrossRef]

| Diblock Copolymer | Zd a | PDI b | ||
|---|---|---|---|---|
| Water | 1 MNaCl | Water | 1 MNaCl | |
| n-BMA35-b-GLBT55 | 1.6 | 1.5 | 0.16 | 0.13 |
| n-BMA62-b-GLBT117 | 2.1 | 2 | 0.13 | 0.13 |
| n-BMA101-b-GLBT156 | 1.5 | 1.5 | 0.11 | 0.08 |
| n-BMA42-b-GLBT300 | 2.7 | 2.6 | 0.2 | 0.19 |
| EHA22-b-GLBT55 | 3.5 | 3.2 | 0.29 | 0.28 |
| EHA15-b-GLBT117 | 4.4 | 4.5 | 0.29 | 0.29 |
| EHA20-b-GLBT156 | 3.9 | 3.9 | 0.3 | 0.32 |
| EHA19-b-GLBT300 | 3.5 | 3.7 | 0.19 | 0.21 |
| Diblock Copolymer | Nagg a | A2 × 105 (cm3·mol·g−2) b | Rg (nm) c | β d | ||||
|---|---|---|---|---|---|---|---|---|
| Water | 1 MNaCl | Water | 1 MNaCl | Water | 1 MNaCl | Water | 1 MNaCl | |
| n-BMA35-b-GLBT55 | 555 | 146 | 4.7 | 0.25 | 65.9 | 58.1 | 0.031 | 0.02 |
| n-BMA101-b-GLBT156 | 117 | 32 | 2.7 | 1.0 | 58.8 | 55.8 | 0.032 | 0.01 |
| EHA22-b-GLBT55 | 891 | 152 | −3.5 | −17.6 | 203.3 | 172.8 | 0.1 | 0.03 |
| EHA20-b-GLBT156 | 91 | 13 | −3.1 | −88.1 | 218.7 | 139.8 | 0.032 | 0.01 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murugaboopathy, S.; Matsuoka, H. Surface Active to Non-Surface Active Transition and Micellization Behaviour of Zwitterionic Amphiphilic Diblock Copolymers: Hydrophobicity and Salt Dependency. Polymers 2017, 9, 412. https://doi.org/10.3390/polym9090412
Murugaboopathy S, Matsuoka H. Surface Active to Non-Surface Active Transition and Micellization Behaviour of Zwitterionic Amphiphilic Diblock Copolymers: Hydrophobicity and Salt Dependency. Polymers. 2017; 9(9):412. https://doi.org/10.3390/polym9090412
Chicago/Turabian StyleMurugaboopathy, Sivanantham, and Hideki Matsuoka. 2017. "Surface Active to Non-Surface Active Transition and Micellization Behaviour of Zwitterionic Amphiphilic Diblock Copolymers: Hydrophobicity and Salt Dependency" Polymers 9, no. 9: 412. https://doi.org/10.3390/polym9090412