The Effect of Moisture on Cellulose Nanocrystals Intended as a High Gas Barrier Coating on Flexible Packaging Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CNCs Extraction by Ammonium Persulfate Treatment and Coating Process
2.3. CNCs Morphological Characterization
2.4. Zeta-Potential and Conductivity of the CNCs
2.5. X-ray Powder Diffraction (XRPD)
2.6. Freezable Water Content (DSC)
2.7. Water Sorption Isotherm
2.8. Optical Properties of Coated Film
2.9. Water Contact Angles
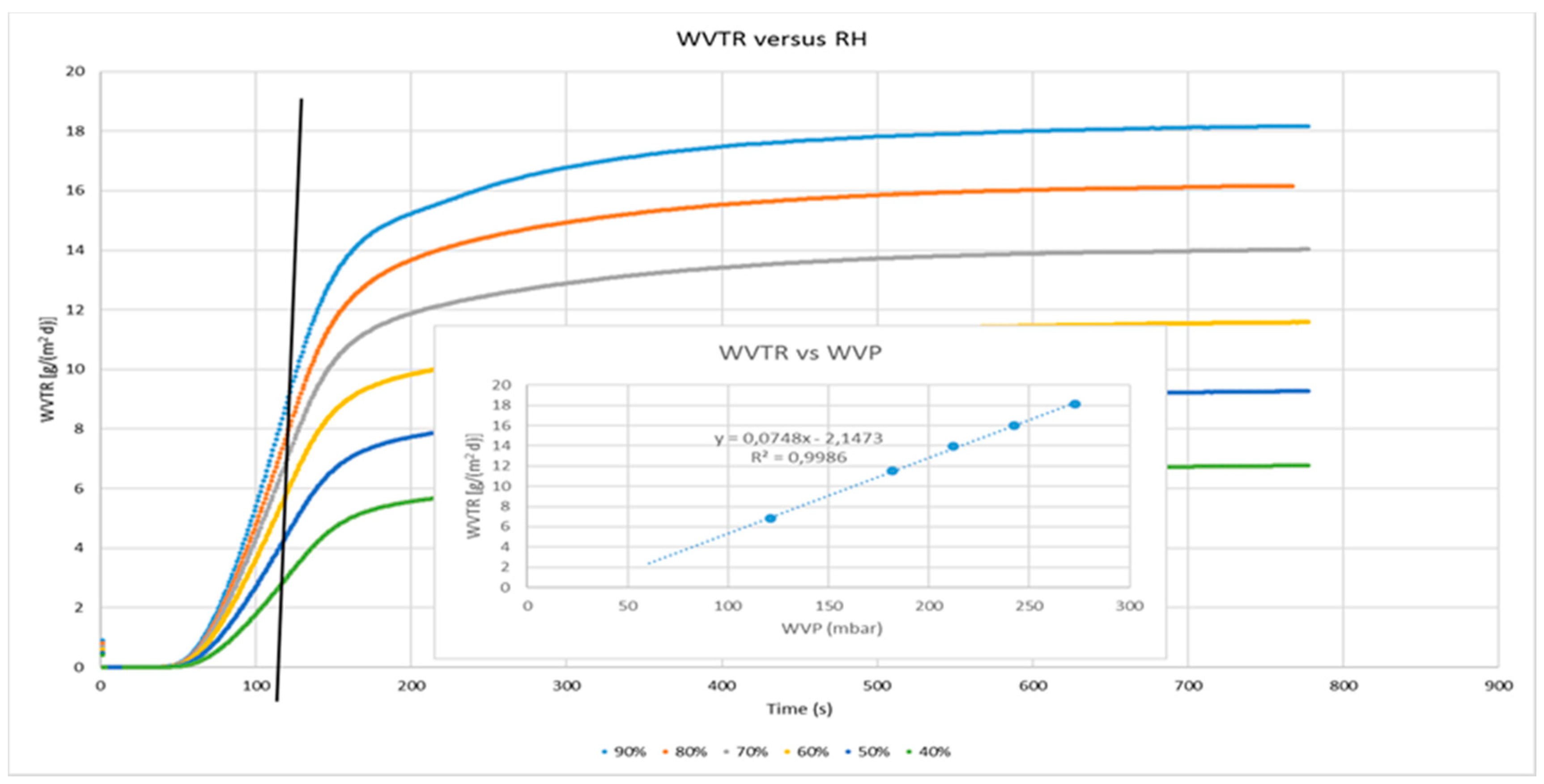
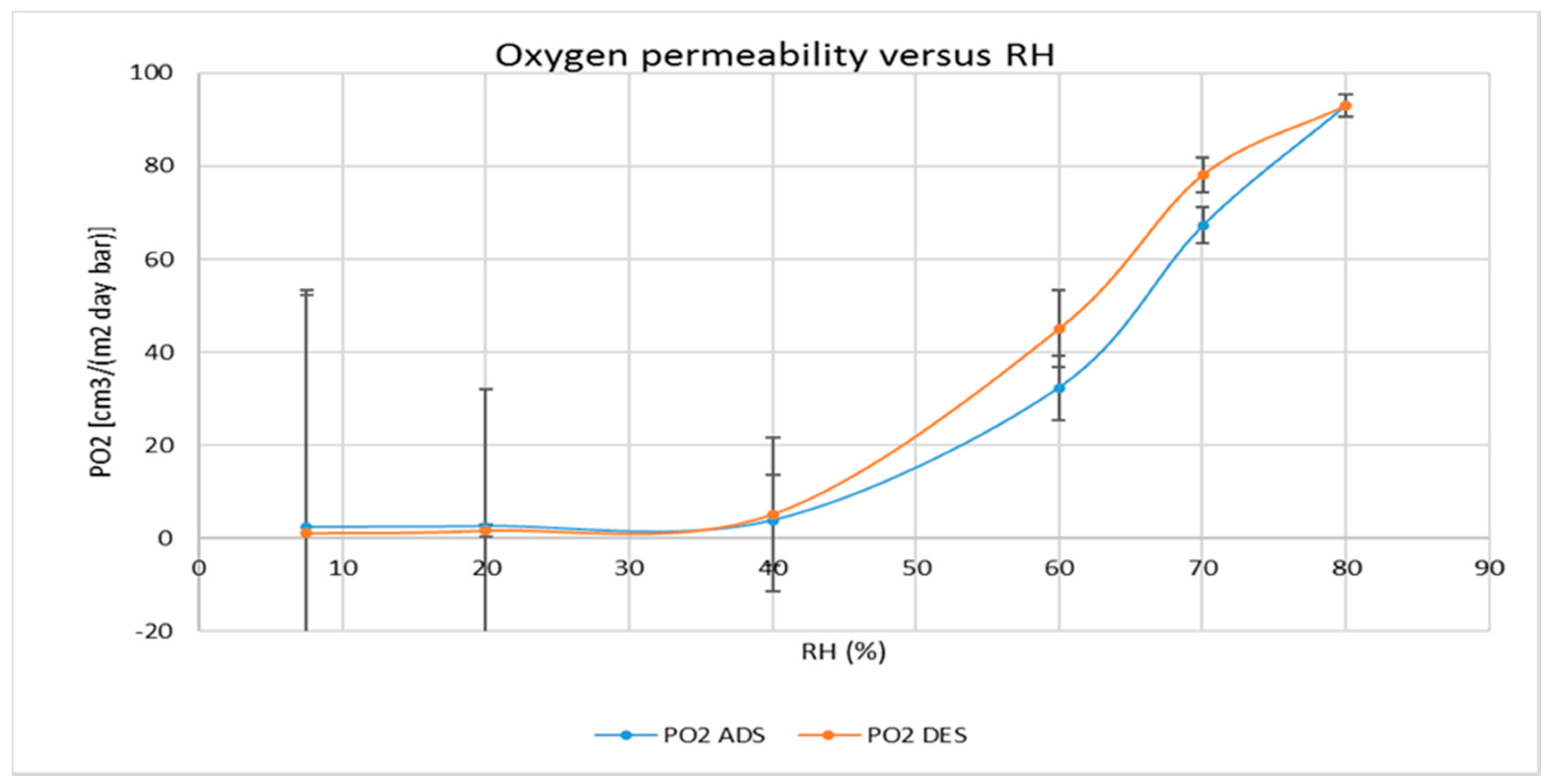
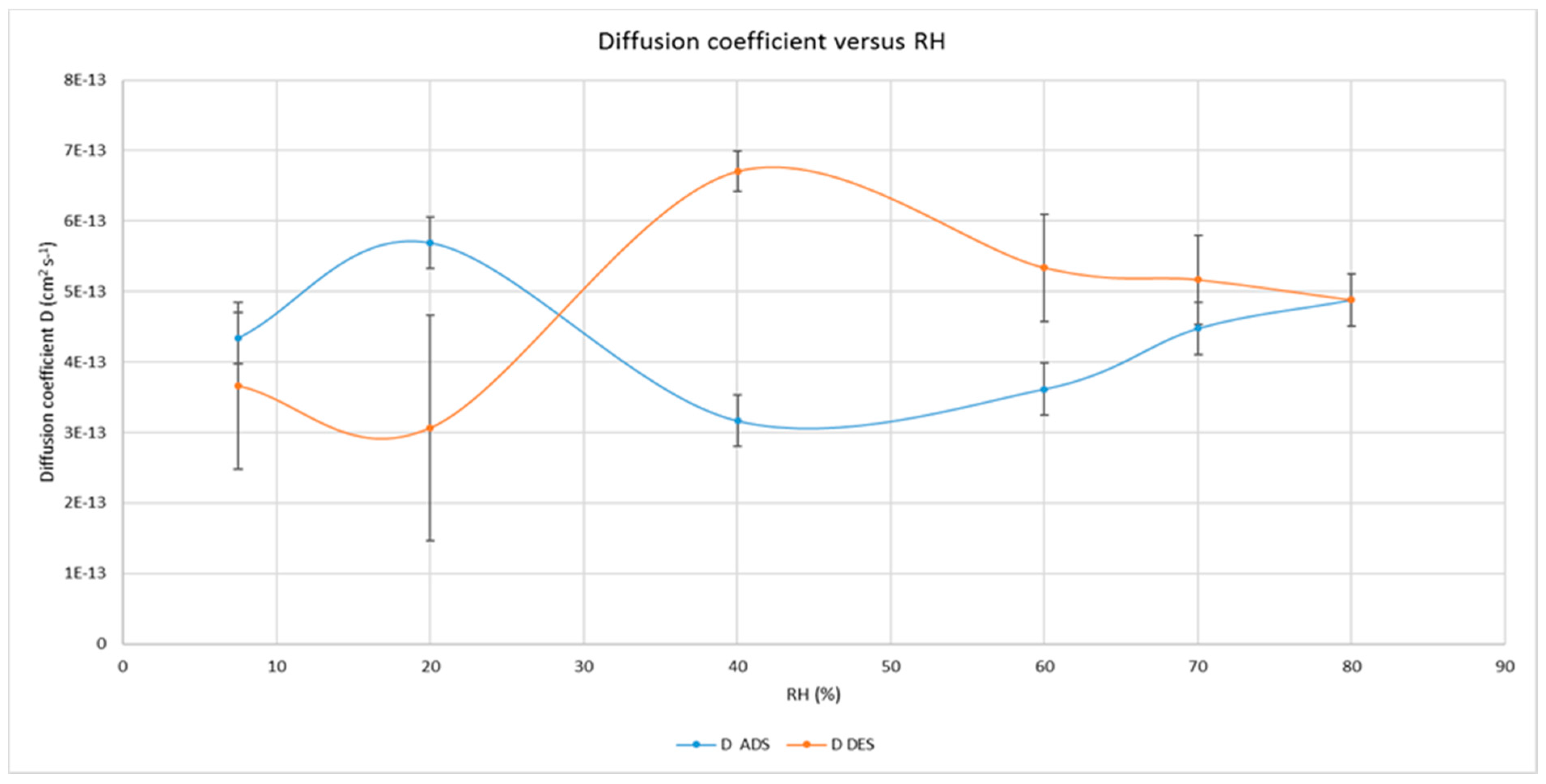
2.10. Gas and Water Vapour Permeability
3. Results
3.1. CNCs Production and Coating onto PET Film
3.2. Water and Gas Permeability of Coated PET Film
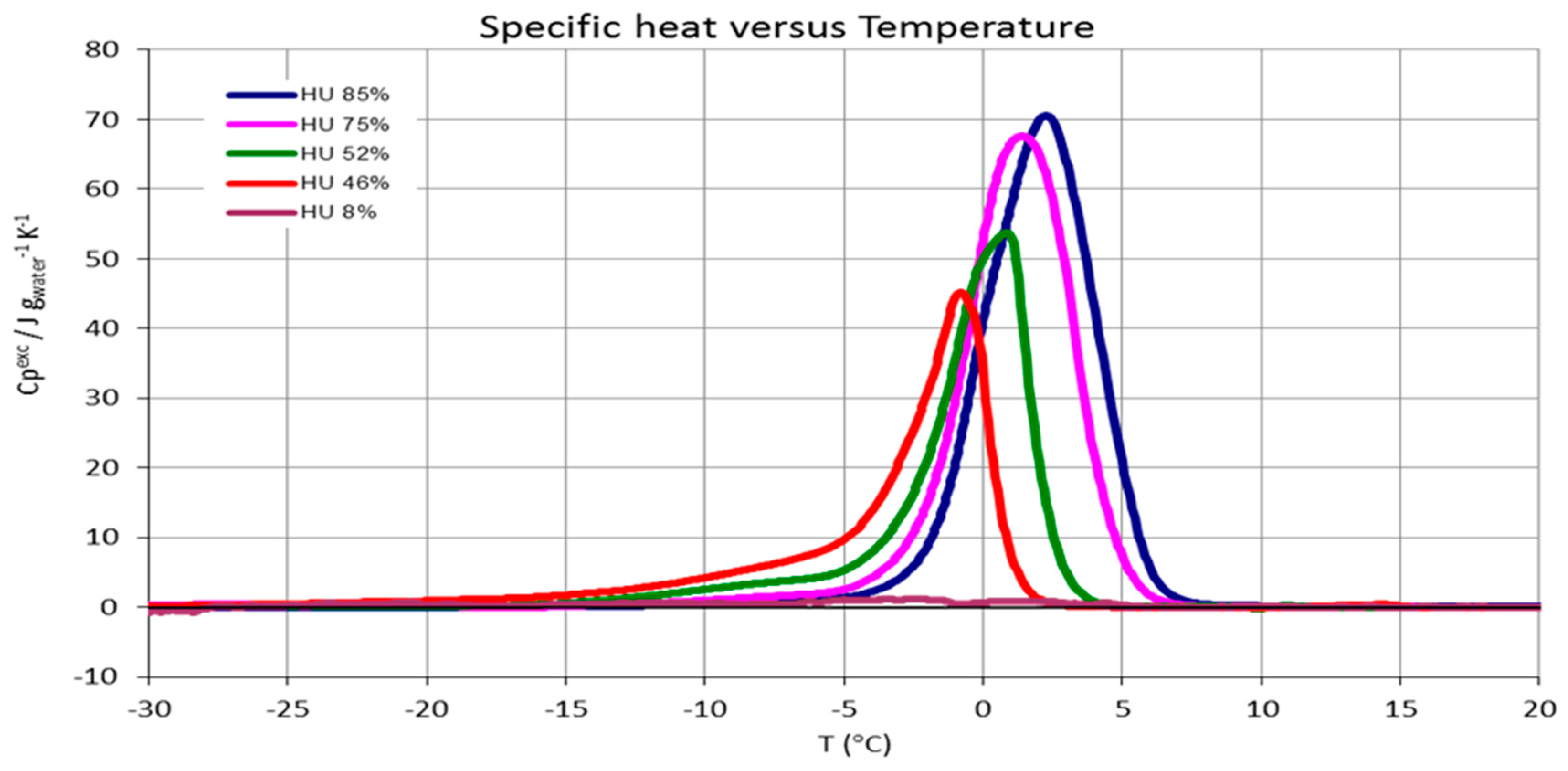
3.3. CNCs Freezable Water Content
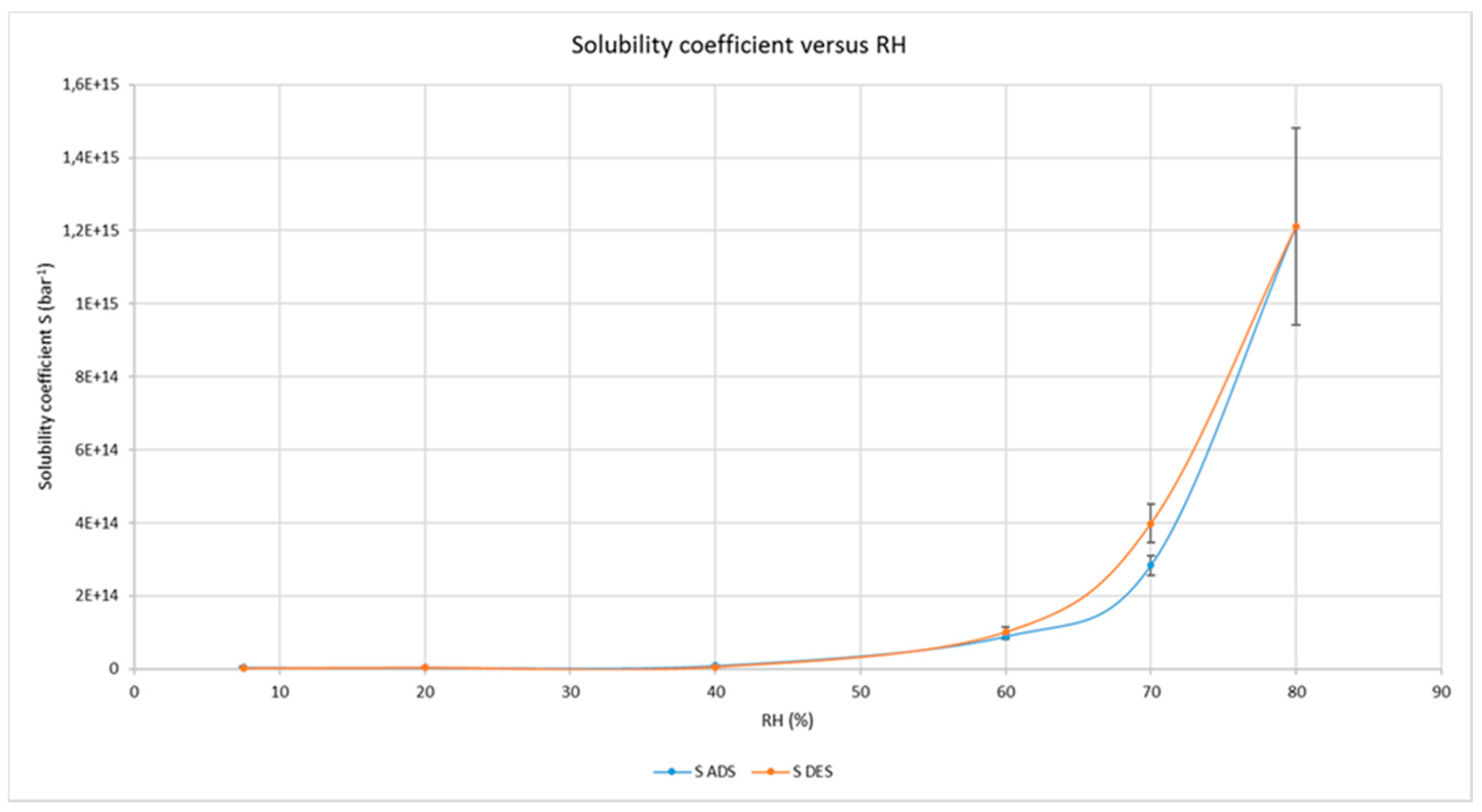
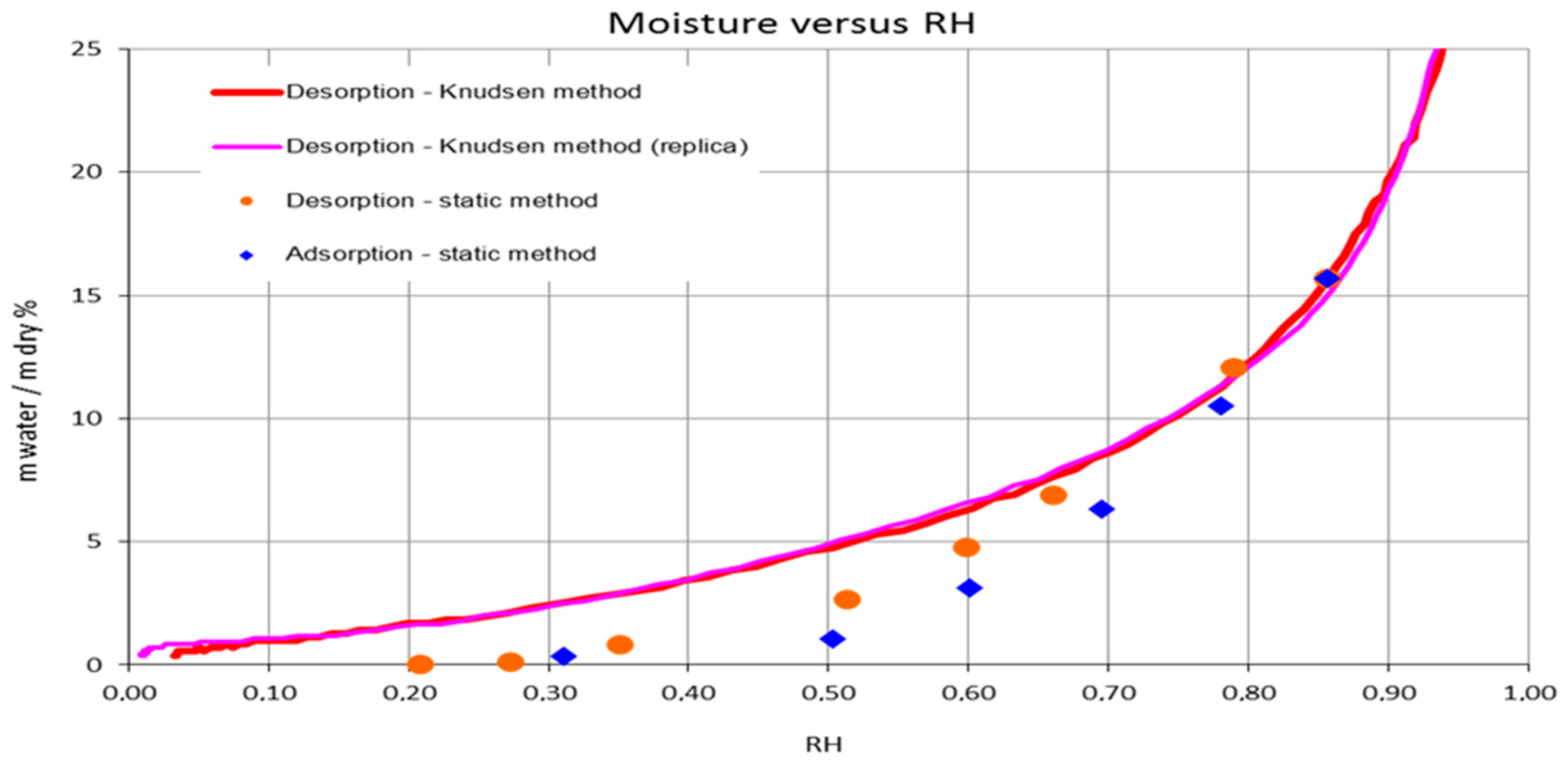
3.4. CNCs Water Sorption Isotherms
3.5. X-ray Diffraction Patterns
4. Discussion
4.1. Permeability of CNCs Coated Film
4.2. Freezable Water and Water Sorption Isotherms
4.3. Cristallinity by WAXD Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Biermann, C.J. Handbook of Pulping and Papermaking; Academic Press: New York, NY, USA, 1996. [Google Scholar]
- Froix, M.F.; Nelson, R. The interaction of water with cellulose from nuclear magnetic resonance relaxation times. Macromolecules 1975, 8, 726–730. [Google Scholar] [CrossRef]
- Zografi, G.; Kontny, M.; Yang, A.; Brenner, G. Surface area and water vapor sorption of macrocrystalline cellulose. Int. J. Pharm. 1984, 18, 99–116. [Google Scholar] [CrossRef]
- Villalobos, R.; Hernández-Muñoz, P.; Chiralt, A. Effect of surfactants on water sorption and barrier properties of hydroxypropyl methylcellulose films. Food Hydrocoll. 2006, 20, 502–509. [Google Scholar] [CrossRef]
- Ioelovich, M.; Leykin, A. Study of sorption properties of cellulose and its derivatives. Bioresources 2010, 6, 178–195. [Google Scholar]
- Engelund, E.T.; Thygesen, L.G.; Svensson, S.; Hill, C.A. A critical discussion of the physics of wood–water interactions. Wood Sci. Technol. 2013, 47, 141–161. [Google Scholar] [CrossRef] [Green Version]
- Olsson, A.-M.; Salmén, L. The association of water to cellulose and hemicellulose in paper examined by ftir spectroscopy. Carbohydr. Res. 2004, 339, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Peemoeller, H.; Sharp, A. Nmr study of cellulose-water systems: Water proton spin-lattice relaxation in the rotating reference frame. Polymer 1985, 26, 859–864. [Google Scholar] [CrossRef]
- Hodge, R.; Edward, G.H.; Simon, G.P. Water absorption and states of water in semicrystalline poly (vinyl alcohol) films. Polymer 1996, 37, 1371–1376. [Google Scholar] [CrossRef]
- Ping, Z.; Nguyen, Q.; Chen, S.; Zhou, J.; Ding, Y. States of water in different hydrophilic polymers—dsc and ftir studies. Polymer 2001, 42, 8461–8467. [Google Scholar] [CrossRef]
- Kohler, R.; Alex, R.; Brielmann, R.; Ausperger, B. A new kinetic model for water sorption isotherms of cellulosic materials. Macromol. Symp. 2006, 244, 89–96. [Google Scholar] [CrossRef]
- Gardner, D.J.; Oporto, G.S.; Mills, R.; Samir, M.A.S.A. Adhesion and surface issues in cellulose and nanocellulose. J. Adhes. Sci. Technol. 2008, 22, 545–567. [Google Scholar] [CrossRef]
- Howsmon, J.A. Water sorption and the poly-phase structure of cellulose fibers. Text. Res. J. 1949, 19, 152–162. [Google Scholar] [CrossRef]
- Mihranyan, A.; Llagostera, A.P.; Karmhag, R.; Strømme, M.; Ek, R. Moisture sorption by cellulose powders of varying crystallinity. Int. J. Pharm. 2004, 269, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Hatakeyama, T.; Hatakeyama, H. Studies on bound water of cellulose by differential scanning calorimetry. Text. Res. J. 1981, 51, 607–613. [Google Scholar] [CrossRef]
- Agrawal, A.M.; Manek, R.V.; Kolling, W.M.; Neau, S.H. Water distribution studies within microcrystalline cellulose and chitosan using differential scanning calorimetry and dynamic vapor sorption analysis. J. Pharm. Sci. 2004, 93, 1766–1779. [Google Scholar] [CrossRef] [PubMed]
- Bikerman, J.J. Causes of poor adhesion: Weak boundary layers. Ind. Eng. Chem. 1967, 59, 40–44. [Google Scholar] [CrossRef]
- Lee, D.S.; Yam, K.L.; Piergiovanni, L. Food Packaging Science and Technology; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Rampazzo, R.; Alkan, D.; Gazzotti, S.; Ortenzi, M.A.; Piva, G.; Piergiovanni, L. Cellulose nanocrystals from lignocellulosic raw materials, for oxygen barrier coatings on food packaging films. Packag. Technol. Sci. 2017. [Google Scholar] [CrossRef]
- Gicquel, E.; Martin, C.; Yanez, J.G.; Bras, J. Cellulose nanocrystals as new bio-based coating layer for improving fiber-based mechanical and barrier properties. J. Mater. Sci. 2017, 52, 3048–3061. [Google Scholar] [CrossRef]
- Frounchi, M.; Dourbash, A. Oxygen barrier properties of poly(ethylene terephthalate) nanocomposite films. Macromol. Mater. Eng. 2009, 294, 68–74. [Google Scholar] [CrossRef]
- Fukuya, M.N.; Senoo, K.; Kotera, M.; Yoshimoto, M.; Sakata, O. Enhanced oxygen barrier property of poly(ethylene oxide) films crystallite-oriented by adding cellulose single nanofibers. Polymer 2014, 55, 5843–5846. [Google Scholar] [CrossRef]
- Minelli, M.; Baschetti, M.G.; Doghieri, F.; Ankerfors, M.; Lindström, T.; Siró, I.; Plackett, D. Investigation of mass transport properties of microfibrillated cellulose (mfc) films. J. Membr. Sci. 2010, 358, 67–75. [Google Scholar] [CrossRef]
- Aulin, C.; Gallstedt, M.; Lindstrom, T. Oxygen and oil barrier properties of microfibrillated cellulose films and coatings. Cellulose 2010, 17, 559–574. [Google Scholar] [CrossRef]
- Li, F.; Biagioni, P.; Bollani, M.; Maccagnan, A.; Piergiovanni, L. Multi-functional coating of cellulose nanocrystals for flexible packaging applications. Cellulose 2013, 20, 2491–2504. [Google Scholar] [CrossRef]
- Belbekhouche, S.; Bras, J.; Siqueira, G.; Chappey, C.; Lebrun, L.; Khelifi, B.; Marais, S.; Dufresne, A. Water sorption behavior and gas barrier properties of cellulose whiskers and microfibrils films. Carbohydr. Polym. 2011, 83, 1740–1748. [Google Scholar] [CrossRef]
- Aulin, C.; Ahola, S.; Josefsson, P.; Nishino, T.; Hirose, Y.; Osterberg, M.; Wagberg, L. Nanoscale cellulose films with different crystallinities and mesostructures-their surface properties and interaction with water. Langmuir 2009, 25, 7675–7685. [Google Scholar] [CrossRef] [PubMed]
- Auras, R.; Harte, B.; Selke, S. Effect of water on the oxygen barrier properties of poly (ethylene terephthalate) and polylactide films. J. Appl. Polym. Sci. 2004, 92, 1790–1803. [Google Scholar] [CrossRef]
- Lagaron, J.; Gimenez, E.; Gavara, R.; Saura, J. Study of the influence of water sorption in pure components and binary blends of high barrier ethylene–vinyl alcohol copolymer and amorphous polyamide and nylon-containing ionomer. Polymer 2001, 42, 9531–9540. [Google Scholar] [CrossRef]
- Leung, A.; Hrapovic, S.; Lam, E.; Liu, Y.L.; Male, K.B.; Mahmoud, K.A.; Luong, J.H.T. Characteristics and properties of carboxylated cellulose nanocrystals prepared from a novel one-step procedure. Small 2011, 7, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Mascheroni, E.; Rampazzo, R.; Ortenzi, M.A.; Piva, G.; Bonetti, S.; Piergiovanni, L. Comparison of cellulose nanocrystals obtained by sulfuric acid hydrolysis and ammonium persulfate, to be used as coating on flexible food-packaging materials. Cellulose 2016, 23, 779–793. [Google Scholar] [CrossRef]
- Mazeau, K.; Heux, L. Molecular dynamics simulations of bulk native crystalline and amorphous structures of cellulose. J. Phys. Chem. B 2003, 107, 2394–2403. [Google Scholar] [CrossRef]
- Ashiotis, G.; Deschildre, A.; Nawaz, Z.; Wright, J.P.; Karkoulis, D.; Picca, F.E.; Kieffer, J. The fast azimuthal integration python library: Pyfai. J. Appl. Crystallogr. 2015, 48, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, E.B.; Sørensen, H.O.; Wright, J.P.; Goret, G.; Kieffer, J. Fabio: Easy access to two-dimensional X-ray detector images in python. J. Appl. Crystallogr. 2013, 46, 537–539. [Google Scholar] [CrossRef]
- Keen, D.A. A comparison of various commonly used correlation functions for describing total scattering. J. Appl. Crystallogr. 2001, 34, 172–177. [Google Scholar] [CrossRef]
- Juhás, P.; Davis, T.; Farrow, C.L.; Billinge, S.J. Pdfgetx3: A rapid and highly automatable program for processing powder diffraction data into total scattering pair distribution functions. J. Appl. Crystallogr. 2013, 46, 560–566. [Google Scholar] [CrossRef]
- Fischer, H.E.; Barnes, A.C.; Salmon, P.S. Neutron and X-ray diffraction studies of liquids and glasses. Rep. Prog. Phys. 2005, 69, 233. [Google Scholar] [CrossRef]
- Rietveld, H. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Alamprese, C.; Cappa, C.; Ratti, S.; Limbo, S.; Signorelli, M.; Fessas, D.; Lucisano, M. Shelf life extension of whole-wheat breadsticks: Formulation and packaging strategies. Food Chem. 2017, 230, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Tsami, E.; Marinos-Kouris, D.; Maroulis, Z. Water sorption isotherms of raisins, currants, figs, prunes and apricots. J. Food Sci. 1990, 55, 1594–1597. [Google Scholar] [CrossRef]
- Schiraldi, A.; Signorelli, M.; Fessas, D. Knudsen thermogravimetry approach to the thermodynamics of aqueous solutions. J. Chem. Thermodyn. 2013, 62, 79–85. [Google Scholar] [CrossRef]
- Pani, P.; Schiraldi, A.; Signorelli, M.; Fessas, D. Thermodynamic approach to osmo-dehydration. Food Biophys. 2010, 5, 177–185. [Google Scholar] [CrossRef]
- Clausen, P.; Signorelli, M.; Schreiber, A.; Hughes, E.; Plummer, C.J.; Fessas, D.; Schiraldi, A.; Månson, J.-A.E. Equilibrium desorption isotherms of water, ethanol, ethyl acetate, and toluene on a sodium smectite clay. J. Therm. Anal. Calorim. 2009, 98, 833–841. [Google Scholar] [CrossRef]
- Fessas, D.; Schiraldi, A. Water properties in wheat flour dough ii: Classical and knudsen thermogravimetry approach. Food Chem. 2005, 90, 61–68. [Google Scholar] [CrossRef]
- Van den Berg, C. Vapour sorption equilibria and other water-starch interactions. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 1981. [Google Scholar]
- Van den Berg, C. Description of water activity of foods for engineering purposes by means of the gab model of sorption. Eng. Food 1984, 1, 321. [Google Scholar]
- Oswin, C. The kinetics of package life. III. The isotherm. J. Chem. Technol. Biotechnol. 1946, 65, 419–421. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: Oxford, UK, 1979. [Google Scholar]
- Hernandez, R.J.; Gavara, R. Plastics Packaging: Methods for Studying Mass Transfer Interactions: A Literature Review; Pira International: Frankfurt, Germany, 1999. [Google Scholar]
- Fessas, D.; Schiraldi, A. State diagrams of arabinoxylan-water binaries. Thermochim. Acta 2001, 370, 83–89. [Google Scholar] [CrossRef]
- Weinkauf, D.H.; Paul, D.R. Effects of structural order on barrier properties. In Barrier Polymers and Structures; American Chemical Society: Washington, DC, USA, 1990; Volume 423, pp. 60–91. [Google Scholar]
- Polyakova, A.; Stepanov, E.V.; Sekelik, D.; Schiraldi, D.A.; Hiltner, A.; Baer, E. Effect of crystallization on oxygen-barrier properties of copolyesters based on ethylene terephthalate. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 1911–1919. [Google Scholar] [CrossRef]
- Mokwena, K.K.; Tang, J. Ethylene vinyl alcohol: A review of barrier properties for packaging shelf stable foods. Crit. Rev. Food Sci. Nutr. 2012, 52, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Guinault, A.; Sollogoub, C.; Ducruet, V.; Domenek, S. Impact of crystallinity of poly (lactide) on helium and oxygen barrier properties. Eur. Polym. J. 2012, 48, 779–788. [Google Scholar] [CrossRef] [Green Version]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Tanaka, K.; Naruse, H.; Morikawa, H.; Marumo, F. Phase-transition process of Na2SO4 (iii) to Na2SO4 (i) and anharmonic thermal vibration. Acta Crystallogr. Sect. B Struct. Sci. 1991, 47, 581–588. [Google Scholar] [CrossRef]
- Zachariasen, W.; Ziegler, G. The crystal structure of anhydrous sodium sulfate Na2SO4. Z. Kristallogr. Cryst. Mater. 1932, 81, 92–101. [Google Scholar] [CrossRef]
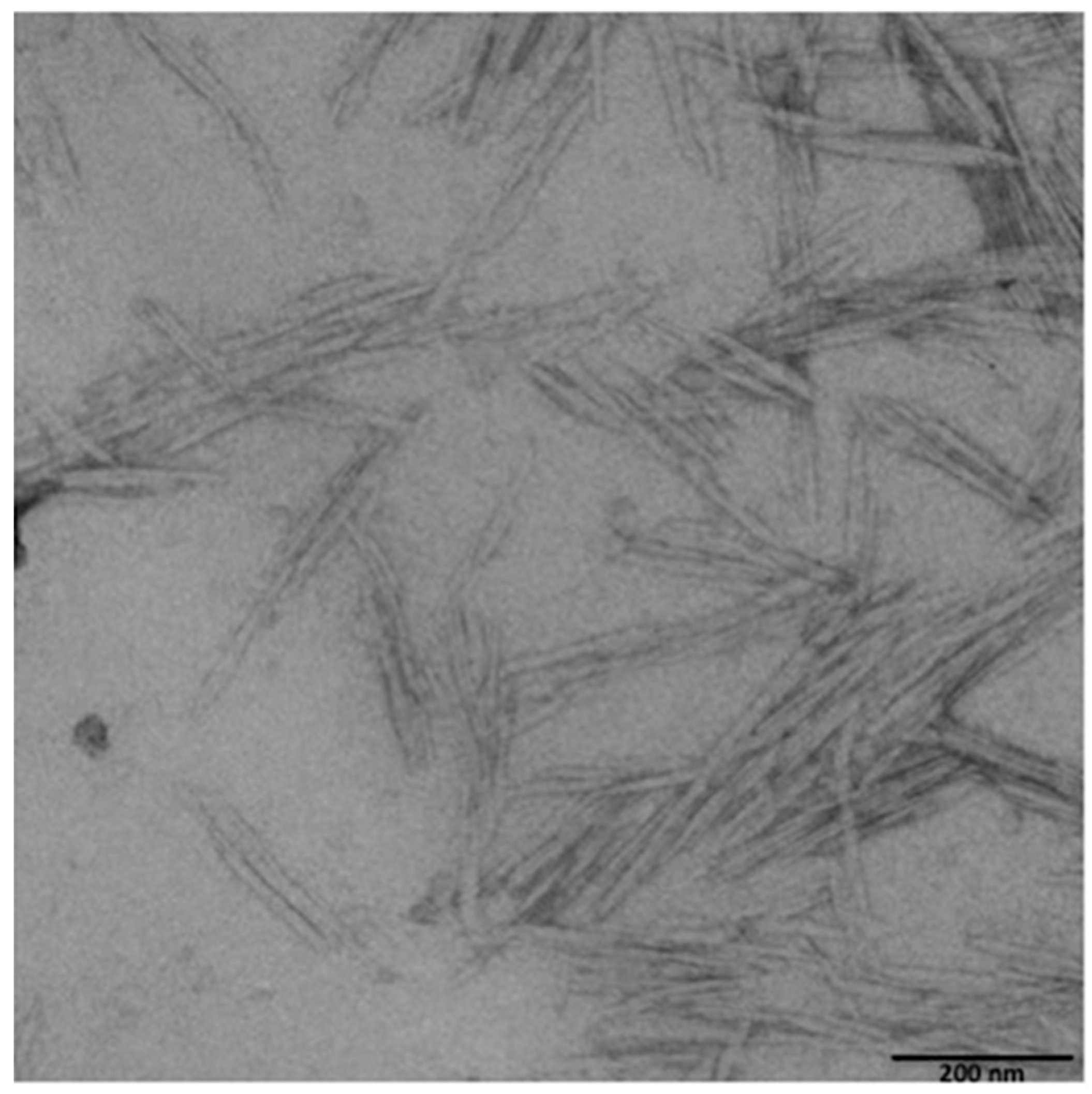

| Property of CNCs | Value 1 |
|---|---|
| Hydrodynamic diameter (nm) | 101.15 ± 3.65 |
| Average dimensions (length, L) from TEM measures | 139 ± 33 |
| Average dimensions (diameter, D) from TEM measures | 16 ± 5 |
| Aspect ratio (L/D) | 9 ± 4 |
| Zeta potential (mV) | −44.40 ± 4.12 |
| Conductivity (mS cm−1) | 0.095 ± 0.024 |
| Polydispersity index | 22.95 ± 0.63 |
| aw after freeze drying | 0.26 ± 0.01 |
| Property of CNCs coated PET film | |
| aw after coating and drying | 0.46 ± 0.05 |
| Thickness of PET film (µm) | 12.0 ± 1 |
| Thickness of CNCs coating (nm) | 756.3 ± 22.3 |
| Transparency (T% at 550 nm) | 85.67 ± 0.3 |
| Haze (%) | 1.89 ± 0.1 |
| Optical contact angle (water) at 57% RH | 11.23 ± 0.41 |
| Optical contact angle (water) at 81% RH | 9.33 ± 0.56 |
| Optical contact angle (water) at 97% RH | 8.05 ± 0.31 |
| CNCs humidity % (g H2O/g sample)·100 | Freezable water % ± 5% |
|---|---|
| 85 | 100 |
| 75 | 100 |
| 52 | 75 |
| 46 | 65 |
| 8 | 0 |
| Coefficient | Adsorption | Desorption |
|---|---|---|
| GAB equation | ||
| R2 | 0.989 | 0.810 |
| k | 1.27 | 1.95 |
| C | 0.14 | 0.35 |
| Mo | 0.45 | 0.08 |
| OSWIN equation | ||
| c1 | 1.3 | 1.23 |
| c2 | 1.57 | 1.90 |
| R2 | 0.97 | 0.90 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotie, G.; Rampazzo, R.; Ortenzi, M.A.; Checchia, S.; Fessas, D.; Piergiovanni, L. The Effect of Moisture on Cellulose Nanocrystals Intended as a High Gas Barrier Coating on Flexible Packaging Materials. Polymers 2017, 9, 415. https://doi.org/10.3390/polym9090415
Fotie G, Rampazzo R, Ortenzi MA, Checchia S, Fessas D, Piergiovanni L. The Effect of Moisture on Cellulose Nanocrystals Intended as a High Gas Barrier Coating on Flexible Packaging Materials. Polymers. 2017; 9(9):415. https://doi.org/10.3390/polym9090415
Chicago/Turabian StyleFotie, Ghislain, Riccardo Rampazzo, Marco Aldo Ortenzi, Stefano Checchia, Dimitrios Fessas, and Luciano Piergiovanni. 2017. "The Effect of Moisture on Cellulose Nanocrystals Intended as a High Gas Barrier Coating on Flexible Packaging Materials" Polymers 9, no. 9: 415. https://doi.org/10.3390/polym9090415





