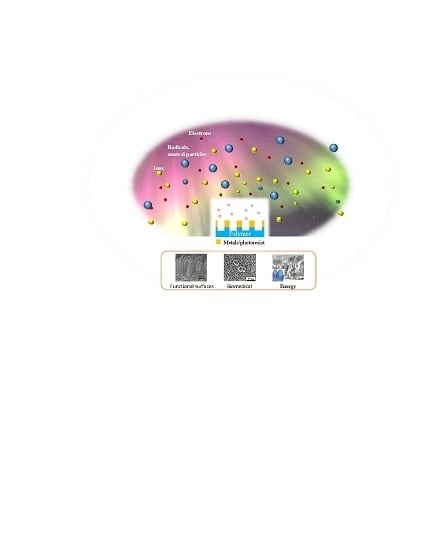
Plasma-Based Nanostructuring of Polymers: A Review
Abstract
:1. Introduction
2. Plasma Technology for Nanostructuring
2.1. Plasma Generation
2.2. Ion Etching
2.3. Gas Precursor Behavior
2.4. Etching Parameters
2.5. Numerical Analysis
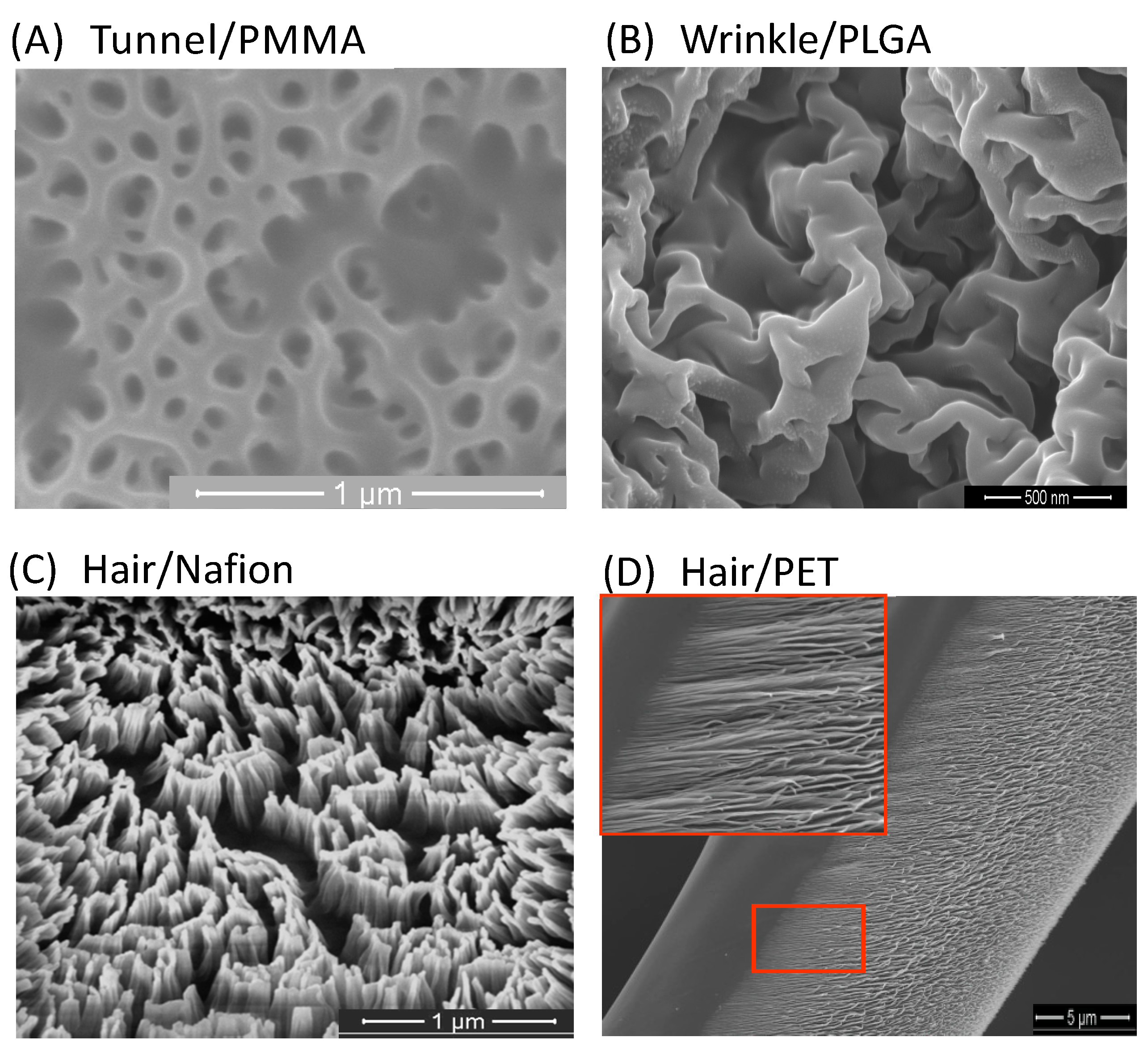
3. Surface Nanostructuring by Plasma
3.1. Plasma Nanostructuring Techniques
3.2. Nanopatterning Techniques by Plasma Etching
3.3. Surface Energy Changes by Plasma Processing
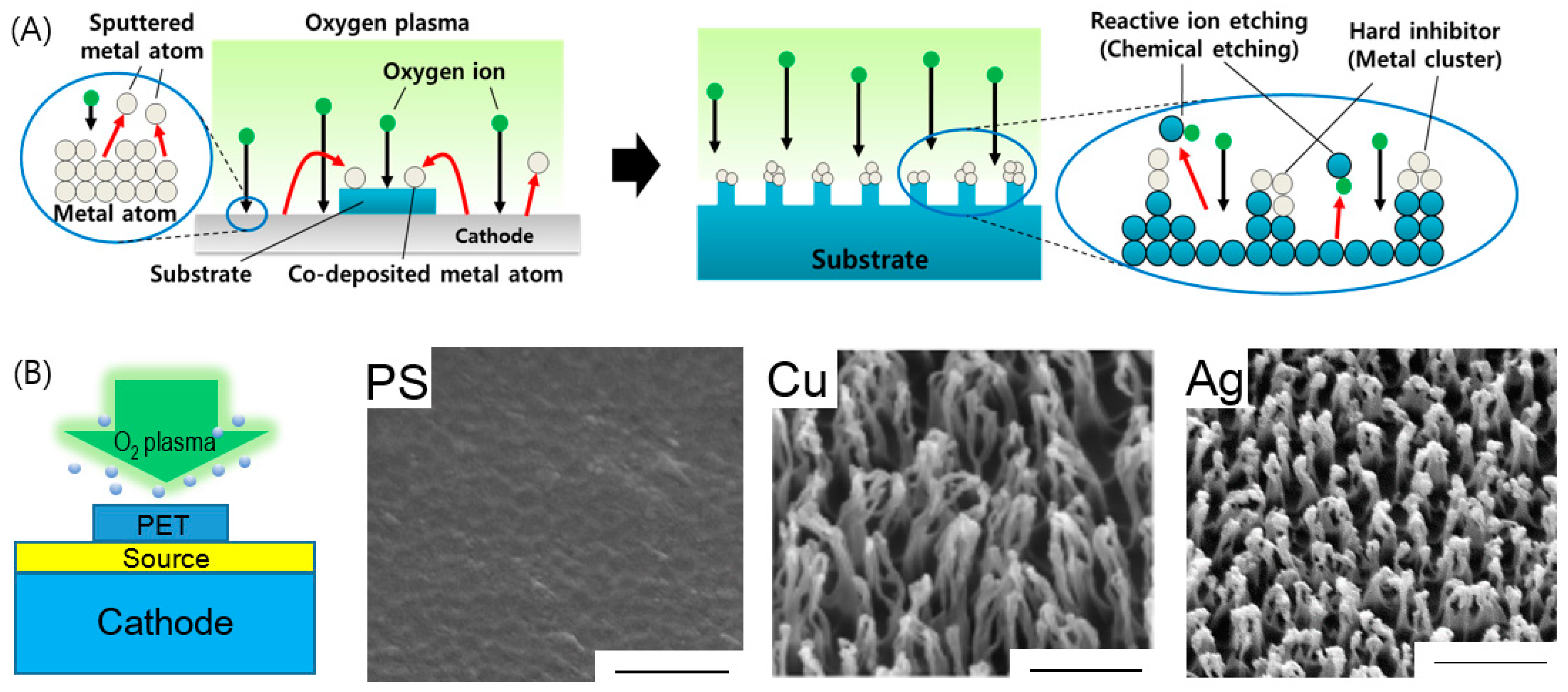
3.4. Selective Etching by Inhibitor, Impurities, and Crystallinity
4. Applications
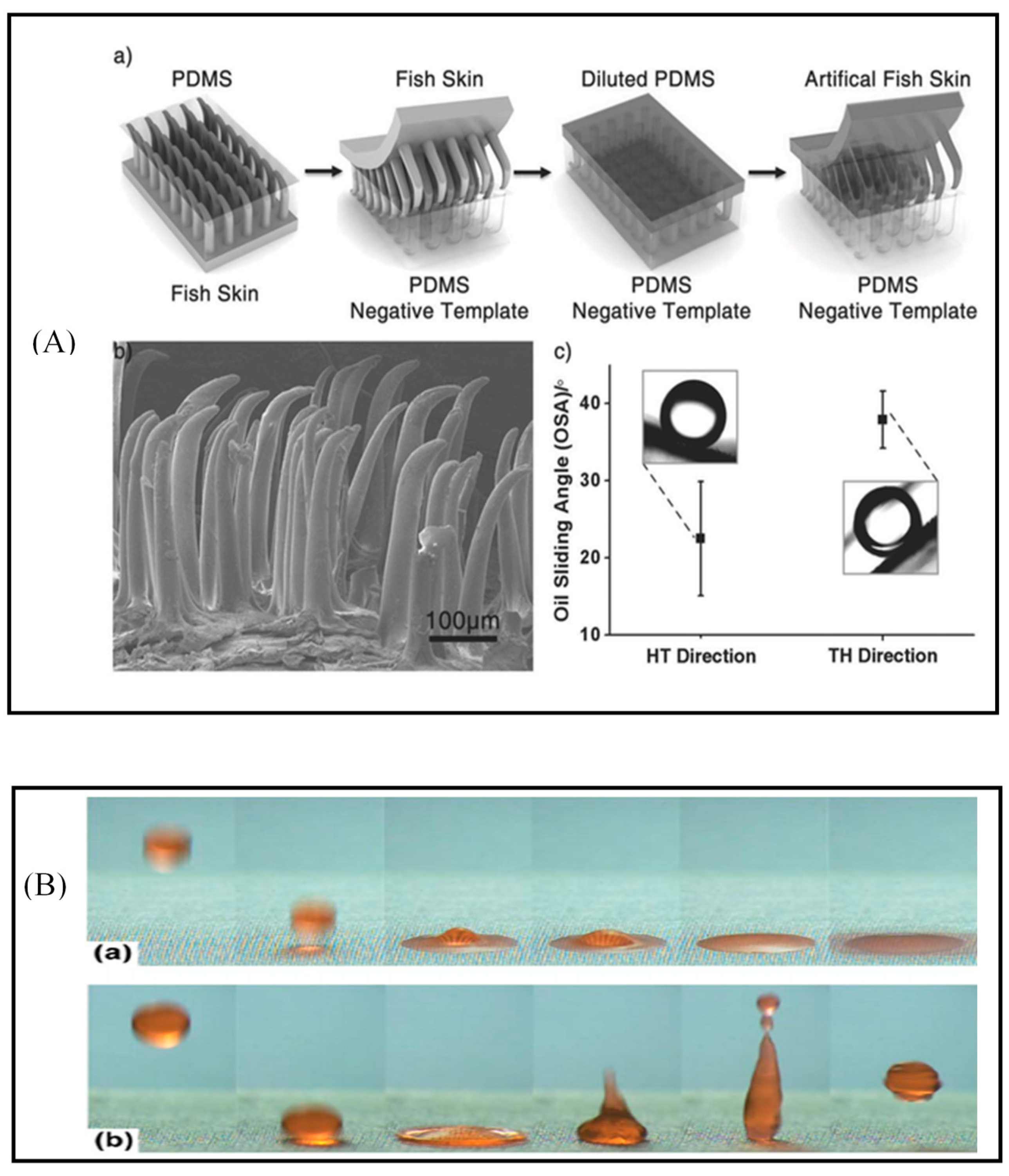
4.1. Wettability
4.1.1. Nanostructured Surface with Special Wettability
- -
- (Super)hydrophobic–(super)oleophilic materials
- -
- (Super)hydrophilic and underwater (super)oleophobic materials
- -
- (Super)hydrophilic- and in air (super)oleophobic materials
- -
- Smart materials with switchable wettability
- -
- Separation oil/water emulsion
4.1.2. Self-Cleaning, Anti-Fouling, Anti-Fogging and Anti-Icing Surface
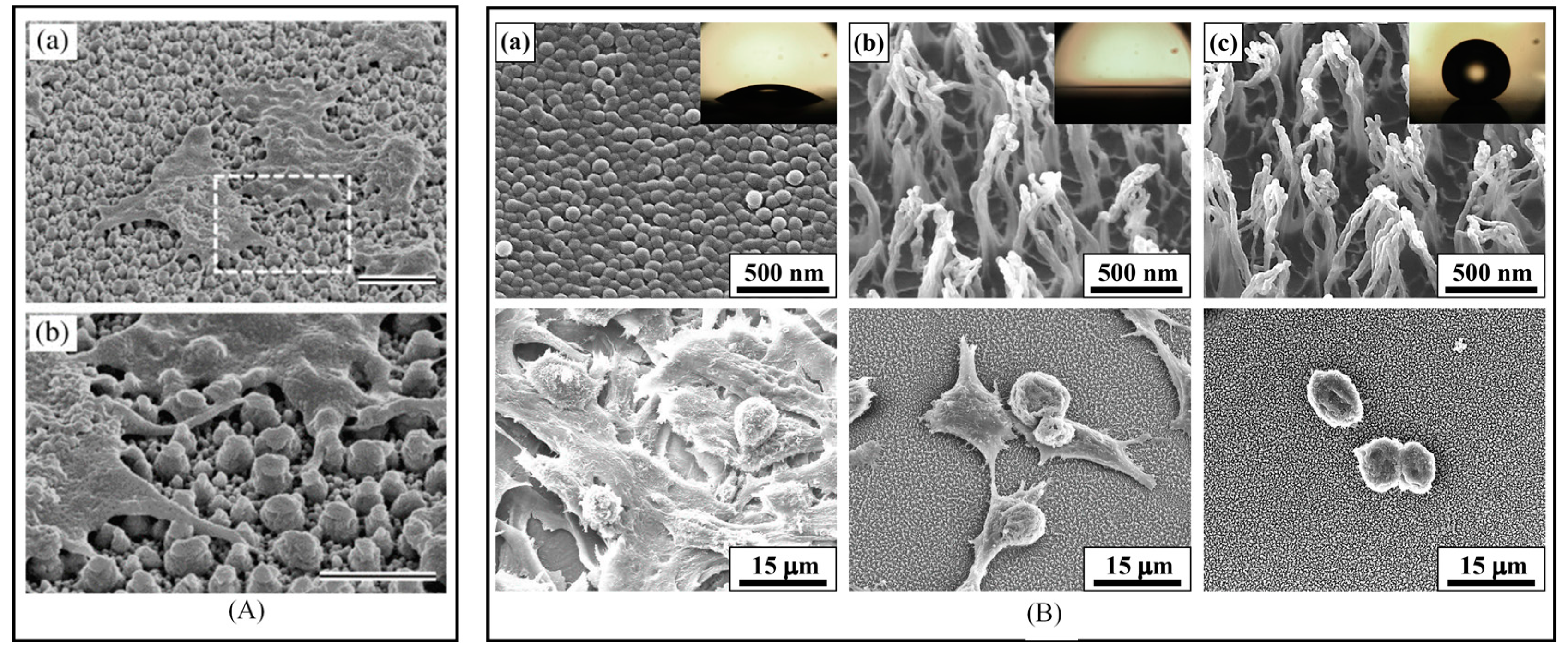
4.2. Bioapplications
4.3. Energy Applications
5. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| a-Si:H | Amorphous Silicone and Hydrogen composition |
| CIGS | Copper indium gallium selenide |
| DNA | DeoxyriboNucleic Acid |
| DLC | Diamond-like carbon |
| D | Dimension |
| DC | Direct current |
| DSSC | Dye-sensitized solar cell |
| F-DLC | Fluorinated DLC |
| FIB | Focused ion beam |
| GDL | Gas diffusion layer |
| HT | Head tail |
| HMDSO | Hexamethyldisiloxane |
| HOPG | Highly oriented pyrolytic graphite |
| HSQ resist | Hydrogen silsesquioxane resist |
| ICP | Inductively coupled plasma |
| ICP-MS | ICP-mass spectrometry |
| IB | Ion beam |
| MD | Molecular dynamic |
| NG | Natural graphite |
| NR-7, SU-8 | Negative Resist |
| OSA | Oil sliding angle |
| PC | Polycarbonate |
| PECVD | Plasma enhance chemical vapor depostion |
| PIII&D | Plasma immersion ion implantation and deposition |
| PES | Polyethersulfone |
| PET | Polyethylene terephthalate |
| PLLA/PLGA | Poly- l-lactic acid/Poly(Lactide-co-Glycolide) |
| PMMA | Poly(methyl methacrylate) |
| PS/PI | Polystyrene/Polyimide |
| PS- b-PDMS | Polystyrene- b-polydimethylsiloxane |
| PS- r-PDSS | Random copolymers of polystyrene and 4-pentamethyldisilylstyrene |
| PVA | Polyvinil acrylate |
| PDMS/PTFE | Polytetrafluoroethylene/Polydimethylsiloxane |
| RF | Radio frequency |
| RgGs | Rabbit γ-Globulins |
| RIE | Reactive ion etching |
| TEM | Transmission electron microscopy |
| UHV | Ultra high voltage |
| UV resist | Ultra violet resist |
| UHMWPE | Ultra-high-molecular-weight polyethylene |
References
- Daniel, M.-C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Zhang, Y.; Fan, L.; Chen, H.; Roco, M.C. Trends in worldwide nanotechnology patent applications: 1991 to 2008. J. Nanopart. Res. 2010, 12, 687–706. [Google Scholar] [CrossRef] [PubMed]
- Pokropivny, V.; Skorokhod, V. Classification of nanostructures by dimensionality and concept of surface forms engineering in nanomaterial science. Mater. Sci. Eng. C 2007, 27, 990–993. [Google Scholar] [CrossRef]
- Fitzpatrick, R. Plasma Physics: An Introduction; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Lim, J.-P.; Uhm, H.S.; Li, S.-Z. Influence of oxygen in atmospheric-pressure argon plasma jet on sterilization of Bacillus atrophaeous spores. Phys. Plasmas 2007, 14, 093504. [Google Scholar] [CrossRef]
- Moisan, M.; Barbeau, J.; Moreau, S.; Pelletier, J.; Tabrizian, M.; Yahia, L.H. Low-temperature sterilization using gas plasmas: A review of the experiments and an analysis of the inactivation mechanisms. Int. J. Pharm. 2001, 226, 1–21. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Pfleging, W.; Kohler, R.; Südmeyer, I.; Rohde, M. Laser micro and nano processing of metals, ceramics, and polymers. In Laser-Assisted Fabrication of Materials; Springer: Berlin/Heidelberg, Germany, 2013; pp. 319–374. [Google Scholar]
- Dai, W.; Kim, S.J.; Seong, W.-K.; Kim, S.H.; Lee, K.-R.; Kim, H.-Y.; Moon, M.-W. Porous carbon nanoparticle networks with tunable absorbability. Sci. Rep. UK 2013, 3, 2524. [Google Scholar] [CrossRef] [PubMed]
- Poncin-Epaillard, F.; Legeay, G. Surface engineering of biomaterials with plasma techniques. J. Biomater. Sci. Polym. Ed. 2003, 14, 1005–1028. [Google Scholar] [CrossRef] [PubMed]
- Tatarova, E.; Bundaleska, N.; Sarrette, J.P.; Ferreira, C. Plasmas for environmental issues: From hydrogen production to 2D materials assembly. Plasma Sources Sci. Technol. 2014, 23, 063002. [Google Scholar] [CrossRef]
- Moreau, M.; Orange, N.; Feuilloley, M. Non-thermal plasma technologies: New tools for bio-decontamination. Biotechnol. Adv. 2008, 26, 610–617. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Yang, Y.; Yuen, M.F.; Chen, X.; Lee, C.S.; Zhang, W. Vertical nanostructure arrays by plasma etching for applications in biology, energy, and electronics. Nano Today 2013, 8, 265–289. [Google Scholar] [CrossRef]
- Skorb, E.V.; Andreeva, D.V. Surface Nanoarchitecture for Bio-Applications: Self-Regulating Intelligent Interfaces. Adv. Funct. Mater. 2013, 23, 4483–4506. [Google Scholar] [CrossRef]
- Cheruthazhekatt, S.; Černák, M.; Slavíček, P.; Havel, J. Gas plasmas and plasma modified materials in medicine. J. Appl. Biomed. 2010, 8, 55–66. [Google Scholar] [CrossRef]
- Lu, T.; Qiao, Y.; Liu, X. Surface modification of biomaterials using plasma immersion ion implantation and deposition. Interface Focus 2012, 2, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, G.; Friedman, G.; Jafri, S.; Schultz, G.; Fridman, A.; Harding, K. Gas plasma: Medical uses and developments in wound care. Plasma Process. Polym. 2010, 7, 194–211. [Google Scholar] [CrossRef]
- Ehlbeck, J.; Schnabel, U.; Polak, M.; Winter, J.; Von Woedtke, T.; Brandenburg, R.; Von dem Hagen, T.; Weltmann, K. Low temperature atmospheric pressure plasma sources for microbial decontamination. J. Phys. D Appl. Phys. 2010, 44, 013002. [Google Scholar] [CrossRef]
- Kylián, O.; Choukourov, A.; Biederman, H. Nanostructured plasma polymers. Thin Solid Films 2013, 548, 1–17. [Google Scholar] [CrossRef]
- Lieberman, M.A.; Lichtenberg, A.J. Principles of Plasma Discharges and Materials Processing; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 1, p. 730. [Google Scholar]
- Coburn, J.; Winters, H.F. Plasma etching-a discussion of mechanisms. Crit. Rev. Solid State Mater. Sci. 1981, 10, 119–141. [Google Scholar] [CrossRef]
- Thi Lan, P.; Jeon, B.-H. Determination of Electron Collision of Cross-Sections for the H2 Molecule for Plasma Discharge Simulation. J. Phys. Soc. Jpn. 2012, 81, 104501. [Google Scholar] [CrossRef]
- Bartschat, K.; Kushner, M.J. Electron collisions with atoms, ions, molecules, and surfaces: Fundamental science empowering advances in technology. Proc. Natl. Acad. Sci. USA 2016, 113, 7026–7034. [Google Scholar] [CrossRef] [PubMed]
- Ostrikov, K.; Cvelbar, U.; Murphy, A.B. Plasma nanoscience: Setting directions, tackling grand challenges. J. Phys. D 2011, 44, 174001. [Google Scholar] [CrossRef]
- Tinck, S.; Bogaerts, A. Computational study of the CF4/CHF3/H2/Cl2/O2/HBr gas phase plasma chemistry. J. Phys. D 2016, 49, 195203. [Google Scholar] [CrossRef]
- Radjenovic, B.; Radmilovic-Radjenovic, M. The Effects of Isotropic Etching on Roughening and Smoothing of Nanostructure. Electron. Mater. Lett. 2012, 8, 491–494. [Google Scholar] [CrossRef]
- Vegh, J.J.; Nest, D.; Graves, D.B.; Bruce, R.; Engelmann, S.; Kwon, T.; Phaneuf, R.J.; Oehrlein, G.S.; Long, B.K.; Willson, C.G. Near-surface modification of polystyrene by Ar(+): Molecular dynamics simulations and experimental validation. Appl. Phys. Lett. 2007, 91, 233113. [Google Scholar] [CrossRef]
- Timko, H.; Djurabekova, F.; Nordlund, K.; Costelle, L.; Matyash, K.; Schneider, R.; Toerklep, A.; Arnau-Izquierdo, G.; Descoeudres, A.; Calatroni, S.; et al. Mechanism of surface modification in the plasma-surface interaction in electrical arcs. Phys. Rev. B 2010, 81, 184109. [Google Scholar] [CrossRef]
- Du, K.; Wathuthanthri, I.; Liu, Y.; Kang, Y.T.; Choi, C.H. Fabrication of polymer nanowires via maskless O2 plasma etching. Nanotechnology 2014, 25, 165301. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Liu, T.; Ma, J.; Wang, W.; Li, H.; Wang, P.; Bai, J.; Jing, G. Irregular shaping of polystyrene nanosphere array by plasma etching. Mater. Sci. Pol. 2013, 31, 331–337. [Google Scholar] [CrossRef]
- Ko, T.J.; Oh, K.H.; Moon, M.W. Plasma-Induced Hetero-Nanostructures on a Polymer with Selective Metal Co-Deposition. Adv. Mater. Interfaces 2015, 2, 1400431. [Google Scholar] [CrossRef]
- Ting, Y.H.; Liu, C.C.; Park, S.M.; Jiang, H.Q.; Nealey, P.F.; Wendt, A.E. Surface Roughening of Polystyrene and poly(methyl methacrylate) in Ar/O2 plasma etching. Polymers 2010, 2, 649–663. [Google Scholar] [CrossRef]
- Liu, C.H.; Niu, P.L.; Sung, C.K. Integrating anti-reflection and superhydrophobicity of moth-eye-like surface morphology on a large-area flexible substrate. J. Phys. D 2014, 47, 015401. [Google Scholar] [CrossRef]
- Rasmussen, K.H.; Keller, S.S.; Jensen, F.; Jorgensen, A.M.; Hansen, O. SU-8 etching in inductively coupled oxygen plasma. Microelectron. Eng. 2013, 112, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Azarnouche, L.; Sirard, S.M.; Durand, W.J.; Blachut, G.; Gurer, E.; Hymes, D.J.; Ellison, C.J.; Willson, C.G.; Graves, D.B. Plasma and photon interactions with organosilicon polymers for directed self-assembly patterning applications. J. Vac. Sci. Technol. B 2016, 34, 061602. [Google Scholar] [CrossRef]
- Tropmann, A.; Tanguy, L.; Koltay, P.; Zengerle, R.; Riegger, L. Completely Superhydrophobic PDMS Surfaces for Microfluidics. Langmuir 2012, 28, 8292–8295. [Google Scholar] [CrossRef] [PubMed]
- Toma, M.; Loget, G.; Corn, R.M. Flexible teflon nanocone array surfaces with tunable superhydrophobicity for self-cleaning and aqueous droplet patterning. ACS Appl. Mater. Interfaces 2014, 6, 11110–11117. [Google Scholar] [CrossRef] [PubMed]
- Gogolides, E.; Tserepi, A.; Vourdas, N.; Vlachopoulou, M.E.; Tsougeni, K.; Kontziampasis, D. Polymer Nano-Texturing and Stochastic Nano-Patterning Using Plasma Processing. In Proceedings of the AIChE Annual Meeting, Philadelphia, PA, USA, 16–21 November 2008. [Google Scholar]
- Wei, X.; Zhao, B.L.; Li, X.M.; Wang, Z.W.; He, B.Q.; He, T.; Jiang, B. CF4 plasma surface modification of asymmetric hydrophilic polyethersulfone membranes for direct contact membrane distillation. J. Membr. Sci. 2012, 407, 164–175. [Google Scholar] [CrossRef]
- Mattioli, S.; Martino, S.; D‘Angelo, F.; Emiliani, C.; Kenny, J.M.; Armentano, I. Nanostructured Polystyrene Films Engineered by Plasma Processes: Surface Characterization and Stem Cell Interaction. J. Appl. Polym. Sci. 2014, 131, 40427. [Google Scholar] [CrossRef]
- Wan, Y.; Tu, C.; Yang, J.; Bei, J.; Wang, S. Influences of ammonia plasma treatment on modifying depth and degradation of poly (L-lactide) scaffolds. Biomaterials 2006, 27, 2699–2704. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Pal, D.; Neogi, S. Prevention of biofilm attachment by plasma treatment of polyethylene. Surf. Innov. 2016, 4, 33–38. [Google Scholar] [CrossRef]
- Lai, C.Q.; Cheng, H. Versatile fabrication and applications of dense, orderly arrays of polymeric nanostructures over large areas. J. Mater. Chem. B 2014, 2, 5982–5991. [Google Scholar] [CrossRef]
- Ros, O.; Pargon, E.; Fouchier, M.; Gouraud, P.; Barnola, S. Gate patterning strategies to reduce the gate shifting phenomenon for 14 nm fully depleted silicon-on-insulator technology. J. Vac. Sci. Technol. A 2017, 35, 021306. [Google Scholar] [CrossRef]
- Jiang, S.L.; Shi, T.L.; Gao, Y.; Long, H.; Xi, S.; Tang, Z.R. Fabrication of a 3D micro/nano dual-scale carbon array and its demonstration as the microelectrodes for supercapacitors. J. Micromech. Microeng. 2014, 24, 045001. [Google Scholar] [CrossRef]
- Sun, J.; Iwasaki, T.; Muruganathan, M.; Mizuta, H. Lateral plasma etching enhanced on/off ratio in graphene nanoribbon field-effect transistor. Appl. Phys. Lett. 2015, 106, 033509. [Google Scholar] [CrossRef]
- Yim, S.; Sim, D.M.; Park, W.I.; Choi, M.J.; Choi, J.; Jeon, J.; Kim, K.H.; Jung, Y.S. Surface-Shielding Nanostructures Derived from Self-Assembled Block Copolymers Enable Reliable Plasma Doping for Few-Layer Transition Metal Dichalcogenides. Adv. Funct. Mater. 2016, 26, 5631–5640. [Google Scholar] [CrossRef]
- Andersen, S.; Halvorsen, T.G.; Pedersen-Bjergaard, S.; Rasmussen, K.E.; Tanum, L.; Refsum, H. Stereospecific determination of citalopram and desmethylcitalopram by capillary electrophoresis and liquid-phase microextraction. J. Pharm. Biomed. Anal. 2003, 33, 263–273. [Google Scholar] [CrossRef]
- Her, E.K.; Chung, H.S.; Moon, M.W.; Oh, K.H. An angled nano-tunnel fabricated on poly(methyl methacrylate) by a focused ion beam. Nanotechnology 2009, 20, 285301. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.W.; Lee, S.H.; Sun, J.Y.; Oh, K.H.; Vaziri, A.; Hutchinson, J.W. Wrinkled hard skins on polymers created by focused ion beam. Proc. Natl. Acad. Sci. USA 2007, 104, 1130–1133. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Nagashima, S.; Lee, J.Y.; Lee, K.-R.; Kim, K.-S.; Moon, M.-W. Self-assembled folding of a biaxially compressed film on a compliant substrate. Carbon 2014, 76, 105–112. [Google Scholar] [CrossRef]
- Oh, J.H.; Ko, T.J.; Moon, M.W.; Park, C.H. Nanostructured fabric with robust superhydrophobicity induced by a thermal hydrophobic ageing process. RSC Adv. 2017, 7, 25597–25604. [Google Scholar] [CrossRef]
- Kota, A.K.; Kwon, G.; Tuteja, A. The design and applications of superomniphobic surfaces. NPG Asia Mater. 2014, 6, e109. [Google Scholar] [CrossRef]
- Jorda-Vilaplana, A.; Fombuena, V.; Garcia-Garcia, D.; Samper, M.D.; Sanchez-Nacher, L. Surface modification of polylactic acid (PLA) by air atmospheric plasma treatment. Eur. Polym. J. 2014, 58, 23–33. [Google Scholar] [CrossRef]
- Ko, T.-J.; Jo, W.; Lee, H.J.; Oh, K.H.; Moon, M.-W. Nanostructures formed on carbon-based materials with different levels of crystallinity using oxygen plasma treatment. Thin Solid Films 2015, 590, 324–329. [Google Scholar] [CrossRef]
- Yoon, S.M.; Kim, J.-S.; Yoon, D.; Cheong, H.; Kim, Y.; Lee, H. Effects of polycrystallinity in nano patterning by ion-beam sputtering. J. Appl. Phys. 2014, 116, 024307. [Google Scholar] [CrossRef]
- Macko, S.; Frost, F.; Ziberi, B.; Förster, D.F.; Michely, T. Is keV ion-induced pattern formation on Si (001) caused by metal impurities? Nanotechnology 2010, 21, 085301. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, S.; Jiang, L. Nature-inspired superwettability systems. Nat. Rev. Mater. 2017, 2, 201736. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Jung, Y.C.; Barthlott, W. Fabrication of artificial Lotus leaves and significance of hierarchical structure for superhydrophobicity and low adhesion. Soft Matter 2009, 5, 1386–1393. [Google Scholar] [CrossRef]
- Norgaard, T.; Dacke, M. Fog-basking behaviour and water collection efficiency in Namib Desert Darkling beetles. Front. Zool. 2010, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Zhang, J.; Chen, J.; Meng, G.; Ding, Y.; Dai, Z. Centrifugation-Assisted Fog-Collecting Abilities of Metal-Foam Structures with Different Surface Wettabilities. ACS Appl. Mater. Interfaces 2016, 8, 10005–10013. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Ge, L.; Ci, L.; Ajayan, P.M.; Dhinojwala, A. Gecko-inspired carbon nanotube-based self-cleaning adhesives. Nano Lett. 2008, 8, 822–825. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Yin, W.; Ferguson-McPherson, M.K.; Satija, S.K.; Morris, J.R.; Esker, A.R. Nanoscale Surface Patterns from 103 Single Molecule Helices of Biodegradable Poly (l-lactic acid). Langmuir 2006, 22, 5969–5973. [Google Scholar] [CrossRef] [PubMed]
- Howarter, J.A.; Youngblood, J.P. Self-Cleaning and Next Generation Anti-Fog Surfaces and Coatings. Macromol. Rapid Commun. 2008, 29, 455–466. [Google Scholar] [CrossRef]
- Roy, S.; Hussain, C.M.; Mitra, S. Poly (acrylamide-co-acrylic acid) hydrophilization of porous polypropylene membrane for dehumidification. Sep. Purif. Technol. 2013, 107, 54–60. [Google Scholar] [CrossRef]
- Wen, L.; Tian, Y.; Jiang, L. Bioinspired Super-Wettability from Fundamental Research to Practical Applications. Angew. Chem. Int. Ed. 2015, 54, 3387–3399. [Google Scholar] [CrossRef] [PubMed]
- Myung, S.-W.; Jung, S.-C.; Kim, B.-H. Immobilization and controlled release of drug using plasma polymerized thin film. Thin Solid Films 2015, 584, 13–17. [Google Scholar] [CrossRef]
- López-García, J.; Primc, G.; Junkar, I.; Lehocký, M.; Mozetič, M. On the Hydrophilicity and Water Resistance Effect of Styrene-Acrylonitrile Copolymer Treated by CF4 and O2 Plasmas. Plasma Process. Polym. 2015, 12, 1075–1084. [Google Scholar] [CrossRef]
- Skarmoutsou, A.; Charitidis, C.; Gnanappa, A.; Tserepi, A.; Gogolides, E. Nanomechanical and nanotribological properties of plasma nanotextured superhydrophilic and superhydrophobic polymeric surfaces. Nanotechnology 2012, 23, 505711. [Google Scholar] [CrossRef] [PubMed]
- Her, E.K.; Ko, T.-J.; Lee, K.-R.; Oh, K.H.; Moon, M.-W. Bioinspired steel surfaces with extreme wettability contrast. Nanoscale 2012, 4, 2900–2905. [Google Scholar] [CrossRef] [PubMed]
- Ramiasa, M.; Ralston, J.; Fetzer, R.; Sedev, R. The influence of topography on dynamic wetting. Adv. Colloid Interface Sci. 2014, 206, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liang, W.; Guo, Z.; Liu, W. Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: A new strategy beyond nature. Chem. Soc. Rev. 2015, 44, 336–361. [Google Scholar] [CrossRef] [PubMed]
- Simaite, A.; Tondu, B.; Souères, P.; Bergaud, C. Hybrid PVDF/PVDF-graft-PEGMA membranes for improved interface strength and lifetime of PEDOT: PSS/PVDF/ionic liquid actuators. ACS Appl. Mater. Interfaces 2015, 7, 19966–19977. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.S.; Bhushan, B. Bioinspired, roughness-induced, water and oil super-philic and super-phobic coatings prepared by adaptable layer-by-layer technique. Sci. Rep. UK 2015, 5, 14030. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Lin, L.; Xue, Z.; Liu, M.; Wang, S.; Jiang, L. Filefish-Inspired Surface Design for Anisotropic Underwater Oleophobicity. Adv. Funct. Mater. 2014, 24, 809–816. [Google Scholar] [CrossRef]
- Kumar, V.; Pulpytel, J.; Rauscher, H.; Mannelli, I.; Rossi, F.; Arefi-Khonsari, F. Fluorocarbon Coatings Via Plasma Enhanced Chemical Vapor Deposition of 1H, 1H, 2H, 2H-perfluorodecyl Acrylate-2, Morphology, Wettability and Antifouling Characterization. Plasma Process. Polym. 2010, 7, 926–938. [Google Scholar] [CrossRef]
- Gao, S.-H.; Gao, L.-H.; Zhou, K.-S. Super-hydrophobicity and oleophobicity of silicone rubber modified by CF 4 radio frequency plasma. Appl. Surf. Sci. 2011, 257, 4945–4950. [Google Scholar] [CrossRef]
- Choi, Y.S.; Lee, J.S.; Han, J.G. Scratch-resistant hydrophobic and oleophobic coatings prepared by simple PECVD method. J. Mater. Sci. 2014, 49, 4790–4795. [Google Scholar] [CrossRef]
- Oh, J.-H.; Ko, T.-J.; Moon, M.-W.; Park, C.H. Nanostructured superhydrophobic silk fabric fabricated using the ion beam method. RSC Adv. 2014, 4, 38966–38973. [Google Scholar] [CrossRef]
- Di Mundo, R.; Palumbo, F.; d‘Agostino, R. Nanotexturing of polystyrene surface in fluorocarbon plasmas: From sticky to slippery superhydrophobicity. Langmuir 2008, 24, 5044–5051. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-O.; Ko, T.-J.; Yu, E.; Kim, J.; Moon, M.-W.; Park, C.H. Nanostructured self-cleaning lyocell fabrics with asymmetric wettability and moisture absorbency (part I). RSC Adv. 2014, 4, 45442–45448. [Google Scholar] [CrossRef]
- Gogolides, E.; Constantoudis, V.; Patsis, G.P.; Tserepi, A. A review of line edge roughness and surface nanotexture resulting from patterning processes. Microelectron. Eng. 2006, 83, 1067–1072. [Google Scholar] [CrossRef]
- Li, J.-H.; Liu, Q.; Wang, Y.-L.; Chen, R.-R.; Takahashi, K.; Li, R.-M.; Liu, L.-H.; Wang, J. Formation of a Corrosion-Resistant and Anti-Icing Superhydrophobic Surface on Magnesium Alloy via a Single-Step Method. J. Electrochem. Soc. 2016, 163, C213–C220. [Google Scholar] [CrossRef]
- Mobarakeh, L.F.; Jafari, R.; Farzaneh, M. The ice repellency of plasma polymerized hexamethyldisiloxane coating. Appl. Surf. Sci. 2013, 284, 459–463. [Google Scholar] [CrossRef]
- Yu, E.; Kim, S.-C.; Lee, H.J.; Oh, K.H.; Moon, M.-W. Extreme wettability of nanostructured glass fabricated by non-lithographic, anisotropic etching. Sci. Rep. UK 2015, 5, 9362. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Moon, M.-W.; Lim, H.; Kim, W.-D.; Kim, H.-Y. Water harvest via dewing. Langmuir 2012, 28, 10183–10191. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Lee, H.J.; Ko, T.-J.; Kim, S.J.; Lee, K.-R.; Oh, K.H.; Moon, M.-W. Hierarchical structures of AlOOH nanoflakes nested on Si nanopillars with anti-reflectance and superhydrophobicity. Nanoscale 2013, 5, 10014–10021. [Google Scholar] [CrossRef] [PubMed]
- Di Mundo, R.; d’Agostino, R.; Palumbo, F. Long-lasting antifog plasma modification of transparent plastics. ACS Appl. Mater. Interfaces 2014, 6, 17059–17066. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yeung, K.W.; Chu, P.K. Functionalization of biomedical materials using plasma and related technologies. Appl. Surf. Sci. 2014, 310, 11–18. [Google Scholar] [CrossRef]
- Nandakumar, D.; Bendavid, A.; Martin, P.J.; Harris, K.D.; Ruys, A.J.; Lord, M.S. Fabrication of Semiordered Nanopatterned Diamond-like Carbon and Titania Films for Blood Contacting Applications. ACS Appl. Mater. Interfaces 2016, 8, 6802–6810. [Google Scholar] [CrossRef] [PubMed]
- Hasebe, T.; Nagashima, S.; Kamijo, A.; Moon, M.-W.; Kashiwagi, Y.; Hotta, A.; Lee, K.-R.; Takahashi, K.; Yamagami, T.; Suzuki, T. Hydrophobicity and non-thrombogenicity of nanoscale dual rough surface coated with fluorine-incorporated diamond-like carbon films: Biomimetic surface for blood-contacting medical devices. Diam. Relat. Mater. 2013, 38, 14–18. [Google Scholar] [CrossRef]
- Kontziampasis, D.; Bourkoula, A.; Petrou, P.; Tserepi, A.; Kakabakos, S.; Gogolides, E. Cell array fabrication by plasma nanotexturing. SPIE Proc. 2013, 8765. [Google Scholar] [CrossRef]
- Malainou, A.; Petrou, P.; Kakabakos, S.; Gogolides, E.; Tserepi, A. Creating highly dense and uniform protein and DNA microarrays through photolithography and plasma modification of glass substrates. Biosens. Bioelectron. 2012, 34, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Tsougeni, K.; Papadakis, G.; Gianneli, M.; Grammoustianou, A.; Constantoudis, V.; Dupuy, B.; Petrou, P.; Kakabakos, S.; Tserepi, A.; Gizeli, E. Plasma nanotextured polymeric lab-on-a-chip for highly efficient bacteria capture and lysis. Lab Chip 2016, 16, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Park, D.; Anghelina, M.; Pécot, T.; Machiraju, R.; Xue, R.; Lannutti, J.J.; Thomas, J.; Cole, S.L.; Moldovan, L. Actin grips: Circular actin-rich cytoskeletal structures that mediate the wrapping of polymeric microfibers by endothelial cells. Biomaterials 2015, 52, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Macgregor-Ramiasa, M.; Vasilev, K. Plasma Polymer Deposition: A Versatile Tool for Stem Cell Research. Adv. Surf. Stem Cell Res. 2016, 199–232. [Google Scholar] [CrossRef]
- Intranuovo, F.; Favia, P.; Sardella, E.; Ingrosso, C.; Nardulli, M.; d’Agostino, R.; Gristina, R. Osteoblast-like cell behavior on plasma deposited micro/nanopatterned coatings. Biomacromolecules 2010, 12, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Ko, T.-J.; Kim, E.; Nagashima, S.; Oh, K.H.; Lee, K.-R.; Kim, S.; Moon, M.-W. Adhesion behavior of mouse liver cancer cells on nanostructured superhydrophobic and superhydrophilic surfaces. Soft Matter 2013, 9, 8705–8711. [Google Scholar] [CrossRef]
- Garcia, L.E.G.; MacGregor-Ramiasa, M.; Visalakshan, R.M.; Vasilev, K. Protein Interactions with Nanoengineered Polyoxazoline Surfaces Generated via Plasma Deposition. Langmuir 2017, 33, 7322–7331. [Google Scholar] [CrossRef] [PubMed]
- Tsougeni, K.; Petrou, P.; Awsiuk, K.; Marzec, M.; Ioannidis, N.; Petrouleas, V.; Tserepi, A.; Kakabakos, S.; Gogolides, E. Direct covalent biomolecule immobilization on plasma-nanotextured chemically stable substrates. ACS Appl. Mater. Interfaces 2015, 7, 14670–14681. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Cho, Y.; Jung, M.S.; Shin, H.A.; Moon, M.W.; Han, H.N.; Nam, K.T.; Joo, Y.C.; Choi, I.S. Fatigue-Free, Electrically Reliable Copper Electrode with Nanohole Array. Small 2012, 8, 3300–3306. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.-K.; Chuang, S.-I.; Bao, Q.; Liao, Y.-T.; Duh, J.-G. One-step argon/nitrogen binary plasma jet irradiation of Li4Ti5O12 for stable high-rate lithium ion battery anodes. J. Power Sources 2015, 275, 660–667. [Google Scholar] [CrossRef]
- Rahman, M.M.; Sultana, I.; Chen, Z.; Srikanth, M.; Li, L.H.; Dai, X.J.; Chen, Y. Ex situ electrochemical sodiation/desodiation observation of Co3O4 anchored carbon nanotubes: A high performance sodium-ion battery anode produced by pulsed plasma in a liquid. Nanoscale 2015, 7, 13088–13095. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Han, Y.-J.; Seo, Y.D.; Nakabayashi, K.; Miyawaki, J.; Santamaría, R.; Menéndez, R.; Yoon, S.-H.; Jang, J. C4F8 plasma treatment as an effective route for improving rate performance of natural/synthetic graphite anodes in lithium ion batteries. Carbon 2016, 103, 28–35. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, Q.; Ma, Z.; Liu, Q.; Wu, Z.; Wang, S. Oxygen plasma modified separator for lithium sulfur battery. RSC Adv. 2015, 5, 79473–79478. [Google Scholar] [CrossRef]
- Li, X.; He, J.; Wu, D.; Zhang, M.; Meng, J.; Ni, P. Development of plasma-treated polypropylene nonwoven-based composites for high-performance lithium-ion battery separators. Electrochim. Acta 2015, 167, 396–403. [Google Scholar] [CrossRef]
- Le Thai, M.; Chandran, G.T.; Dutta, R.K.; Li, X.; Penner, R.M. 100k Cycles and Beyond: Extraordinary Cycle Stability for MnO2 Nanowires Imparted by a Gel Electrolyte. ACS Energy Lett. 2016, 1, 57–63. [Google Scholar] [CrossRef]
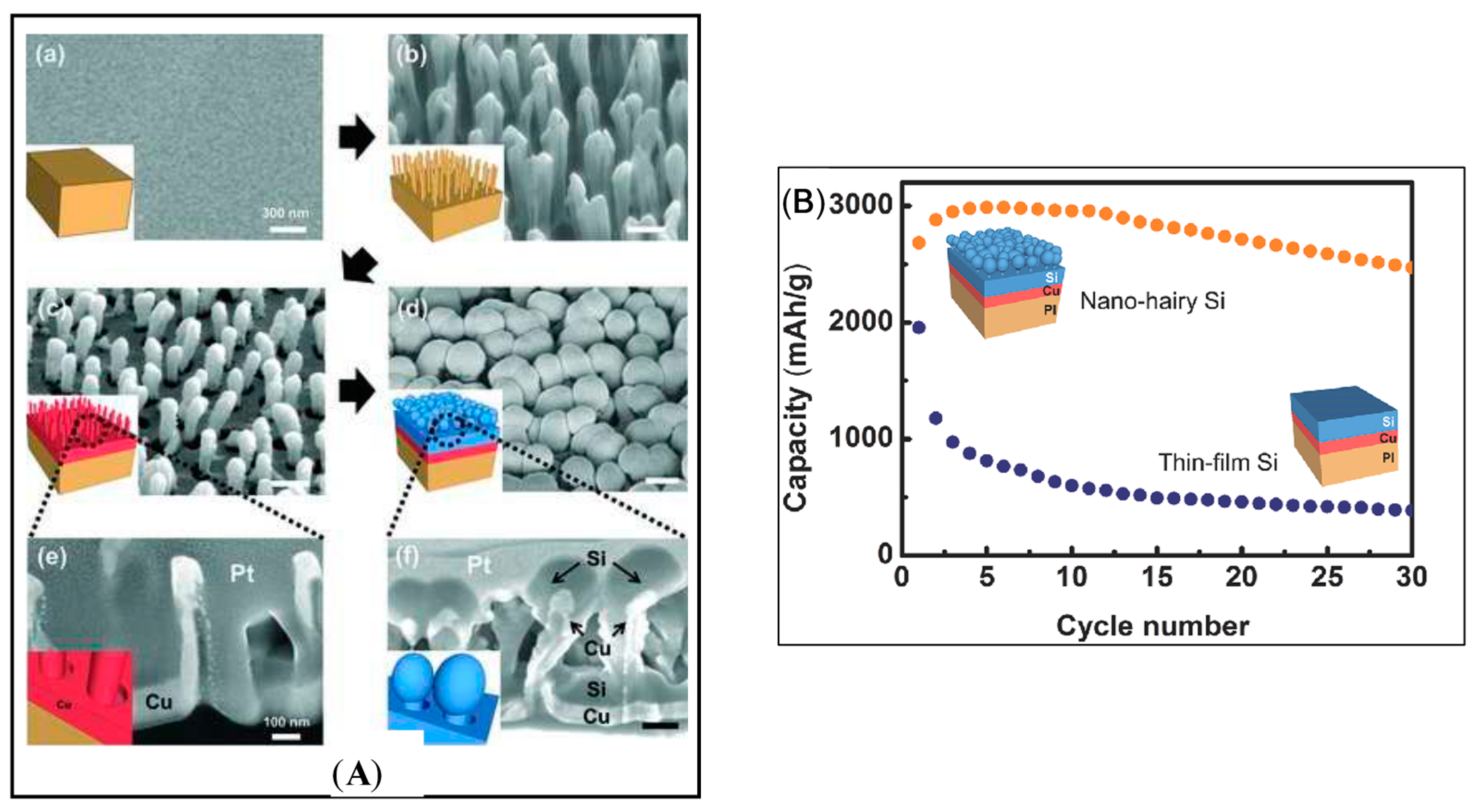
- Jung, M.S.; Seo, J.H.; Moon, M.W.; Choi, J.W.; Joo, Y.C.; Choi, I.S. A Bendable Li-Ion Battery with a Nano-Hairy Electrode: Direct Integration Scheme on the Polymer Substrate. Adv. Energy Mater. 2015, 5, 1400611. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.; Yin, H.; Liu, Z.; Luan, E.; Zhao, F.; Tang, Z.; Liu, S. Three-dimensional graphene/Pt nanoparticle composites as freestanding anode for enhancing performance of microbial fuel cells. Sci. Adv. 2015, 1, e1500372. [Google Scholar] [CrossRef] [PubMed]
- Ko, T.-J.; Kim, S.H.; Hong, B.K.; Lee, K.-R.; Oh, K.H.; Moon, M.-W. High Performance Gas Diffusion Layer with Hydrophobic Nanolayer under a Supersaturated Operation Condition for Fuel Cells. ACS Appl. Mater. Interfaces 2015, 7, 5506–5513. [Google Scholar] [CrossRef] [PubMed]
- Di Mundo, R.; Ambrico, M.; Ambrico, P.F.; d‘Agostino, R.; Italiano, F.; Palumbo, F. Single-Step Plasma Process Producing Anti-Reflective and Photovoltaic Behavior on Crystalline Silicon. Plasma Process. Polym. 2011, 8, 239–245. [Google Scholar] [CrossRef]
- Kovalenko, A.; Ashcheulov, P.; Guerrero, A.; Heinrichová, P.; Fekete, L.; Vala, M.; Weiter, M.; Kratochvílová, I.; Garcia-Belmonte, G. Diamond-based electrodes for organic photovoltaic devices. Sol. Energy Mater. Sol. Cells 2015, 134, 73–79. [Google Scholar] [CrossRef]
- Russell, H.; Andriotis, A.; Menon, M.; Jasinski, J.; Martinez-Garcia, A.; Sunkara, M. Direct Band Gap Gallium Antimony Phosphide (GaSbxP1−x) Alloys. Sci. Rep. UK 2016, 6, 20822. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Park, S.J.; Kim, S.H.; Lee, M.W.; Han, J.; Kim, J.Y.; Kim, H.; Hwang, Y.J.; Lee, D.-K.; Min, B.K. Monolithic DSSC/CIGS tandem solar cell fabricated by a solution process. Sci. Rep. UK 2015, 5, 8970. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-Y.; Wang, W.-Y.; Chou, S.-W. Flexible carbon nanotube/polypropylene composite plate decorated with poly (3,4-ethylenedioxythiophene) as efficient counter electrodes for dye-sensitized solar cells. J. Power Sources 2015, 282, 348–357. [Google Scholar] [CrossRef]
- Togonal, A.S.; Foldyna, M.; Chen, W.; Wang, J.X.; Neplokh, V.; Tchernycheva, M.; Nassar, J.; Cabarrocas, P.R. Core–shell heterojunction solar cells based on disordered silicon nanowire arrays. J. Phys. Chem. C 2016, 120, 2962–2972. [Google Scholar] [CrossRef]
- Cao, L.; Bu, W.; Zheng, J.; Pan, S.; Wang, Z.; Uchida, S. Plutonium determination in seawater by inductively coupled plasma mass spectrometry: A review. Talanta 2016, 151, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Thiry, D.; Konstantinidis, S.; Cornil, J.; Snyders, R. Plasma diagnostics for the low-pressure plasma polymerization process: A critical review. Thin Solid Films 2016, 606, 19–44. [Google Scholar] [CrossRef]
| Applicable Field | Substrate Materials | Plasma Source/Techniques | Gas | Nanostructures | Refs. |
|---|---|---|---|---|---|
| Morphology | NR-7, SU-8 and PMMA | RIE | O2 | Vertical nanowire structures, single level (non-hierarchical array of nanowire structures) | [30] |
| PS sphere | a plasma etching | Air, Ar/O2 | Surface roughness | [31] | |
| PET | RF-PECVD | O2 | pillar- or hair-like nanostructure | [32] | |
| PS, PMMA | RIE | Ar/O2 | Nanoroughening | [33] | |
| UV resist | Molding and plasma ashing | Ar–O2 | moth-eye-like surface morphology | [34] | |
| Su-8 | ICP-RIE | O2, SF6 | lower roughness and higher etch rate | [35] | |
| PS-r-PDSS | RF-ICP | O2, H2/N2, or H2 | Directed self-assembly nanopattern | [36] | |
| Wettability | PDMS/PTFE | Glow discharge | CF4/O2 | Nanoparticles/Superhydrophobic | [37] |
| Teflon film | Glow discharge | O2 | nanocone arrays | [38] | |
| PMMA | Helicon Plasma reactor | O2 | Nanoroughness | [39] | |
| PDMS | Helicon Plasma reactor | SF6 | nanotexturing | [39] | |
| PES | (RF) glow discharge power | CF4 | Nanosized hollow porous structure | [40] | |
| Bio and medical | PS | Glow discharge, RF | O2 | micropatterned grooves and nanostructured roughness | [41] |
| PLLA | IC-RF-glow discharge plasma | NH3 | Super hydrophilic | [42] | |
| UHMWPE | Plasma etching- | N2 | Rough surface | [43] | |
| PS | Glow discharge, RF | CF4/O2 | Dense, orderly arrays nanostructures | [44] | |
| Energy and Electronic | photoresist | ICP reactor | Cl2/O2; CF4/CH2F2; SO2/O2 | 24 nm wide gate patterns | [45] |
| SU-8 | Plasma etching | O2 | Hemispherical pattern and nano-hairy structures | [46] | |
| HSQ resist | Plasma etching | O2 | 28 nm HSQ mask with lower width of Graphene nanowire | [47] | |
| PS-b-PDMS | RIE | CF4, O2, Ar | Surface masking nanostructures, and plasma induced doping | [48] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, L.T.; Yoon, S.M.; Moon, M.-W. Plasma-Based Nanostructuring of Polymers: A Review. Polymers 2017, 9, 417. https://doi.org/10.3390/polym9090417
Phan LT, Yoon SM, Moon M-W. Plasma-Based Nanostructuring of Polymers: A Review. Polymers. 2017; 9(9):417. https://doi.org/10.3390/polym9090417
Chicago/Turabian StylePhan, Lan Thi, Sun Mi Yoon, and Myoung-Woon Moon. 2017. "Plasma-Based Nanostructuring of Polymers: A Review" Polymers 9, no. 9: 417. https://doi.org/10.3390/polym9090417