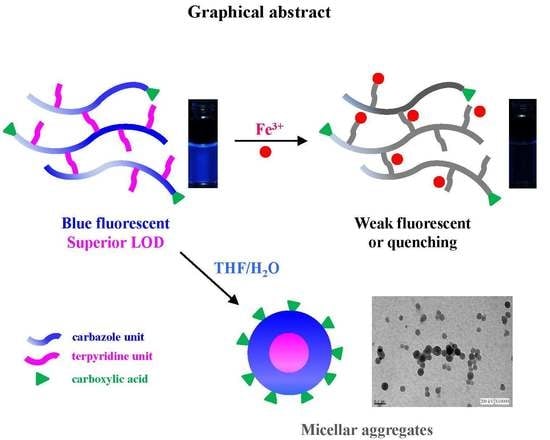
Synthesis, Chemosensory Properties, and Self-Assembly of Terpyridine-Containing Conjugated Polycarbazole through RAFT Polymerization and Heck Coupling Reaction
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Measurements
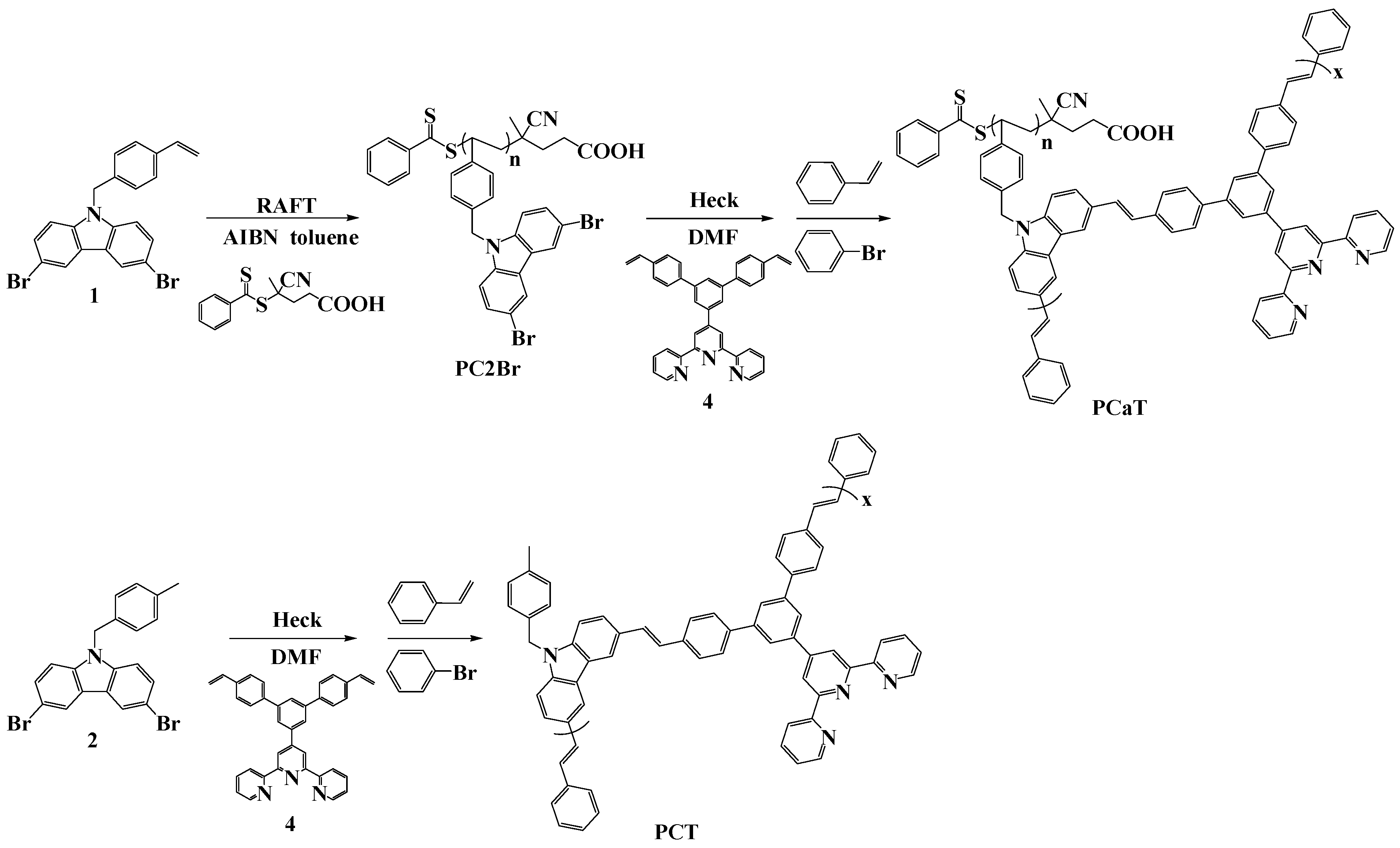
2.3. Synthesis of Intermediates and Monomers (Scheme 1)
2.3.1. Synthesis of 3,6-Dibromo-9-(4-vinylbenzyl)-9H-carbazole (1)
2.3.2. Synthesis of 4’-(3,5-Dibromophenyl)-2,2’:6’,2’’-terpyridine (3)
2.3.3. Synthesis of 4′-(4,4″-Divinyl-[1,1′:3′,1″-terphenyl]-5′-yl)-2,2′:6′,2″-terpyridine (4)
2.4. Synthesis of Polymers (Scheme 2)
2.4.1. Synthesis of Carbazole-Functionalized Polymer (PC2Br) Using the RAFT Method
2.4.2. Synthesis of Carbazole-Functionalized Copolymer (PCaT) Using the Heck Method
2.5. Fluorescent Titration with Metal Ions
2.6. Preparation of Micellar Aggregates
3. Results and Discussion
3.1. Synthesis of Intermediates and Monomers
3.2. Synthesis and Thermal Properties of Polymers
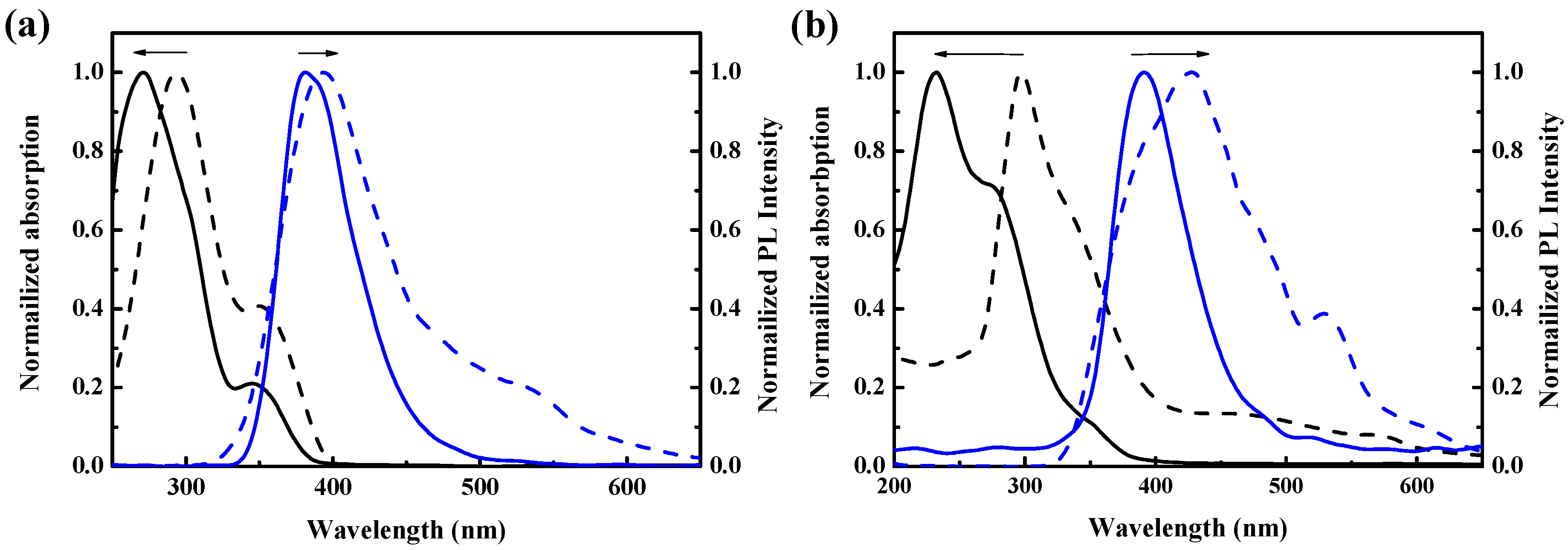
3.3. Optical Properties of Polymers
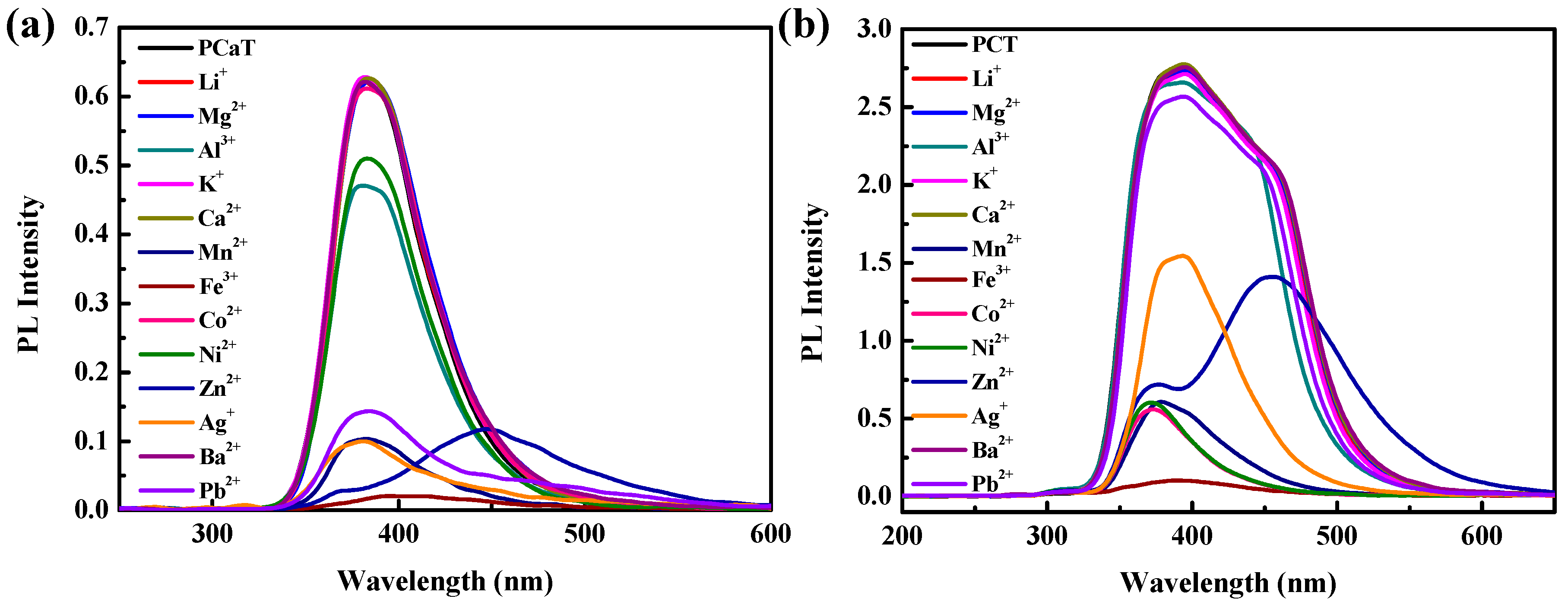
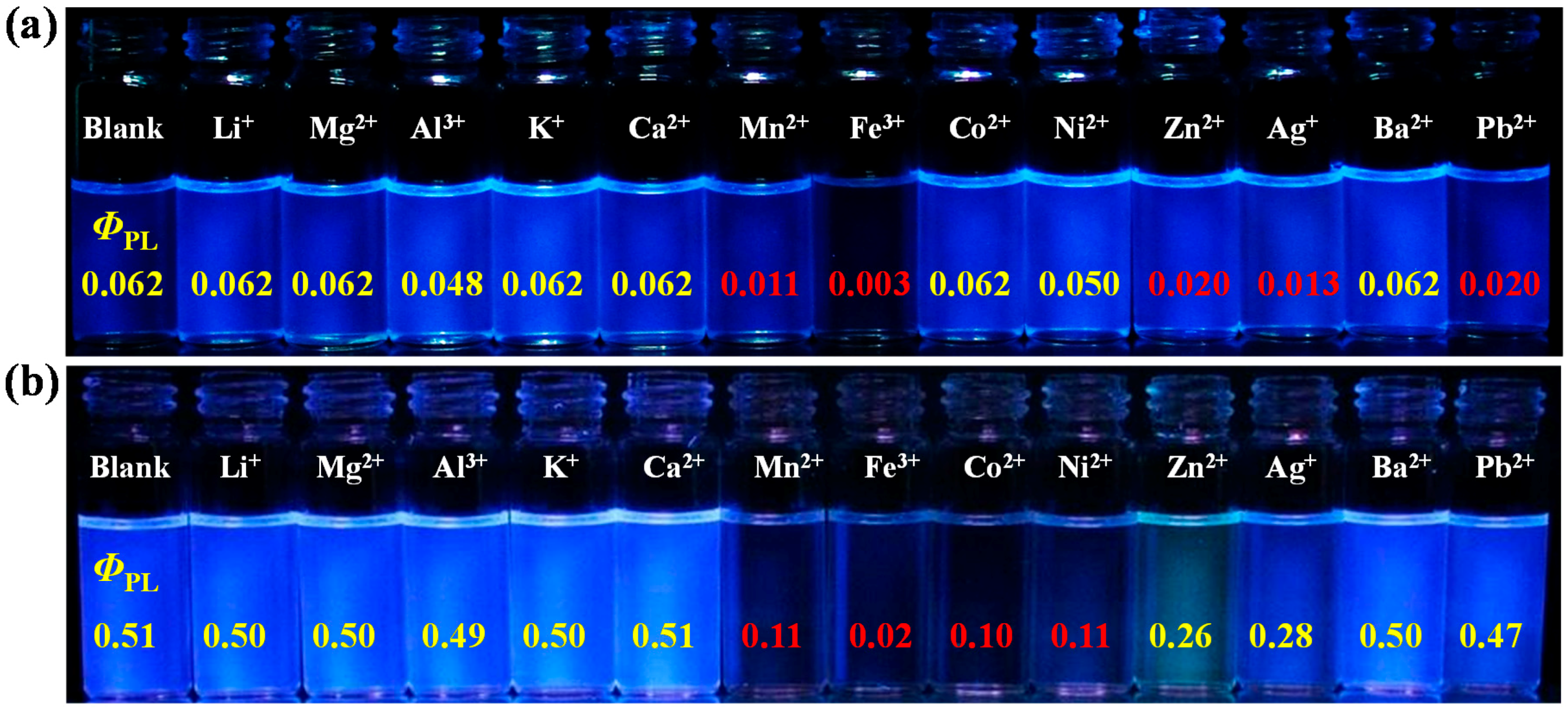
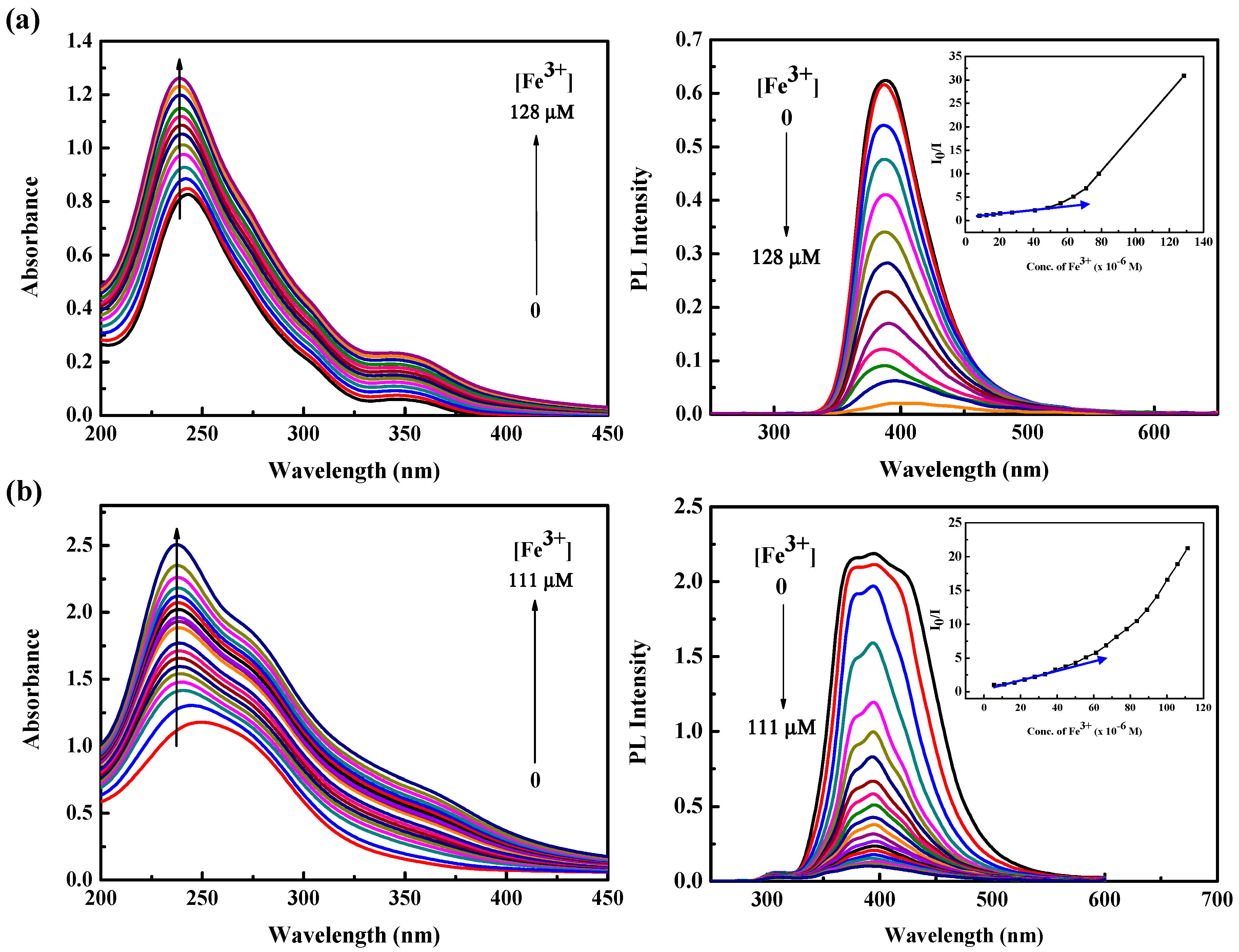
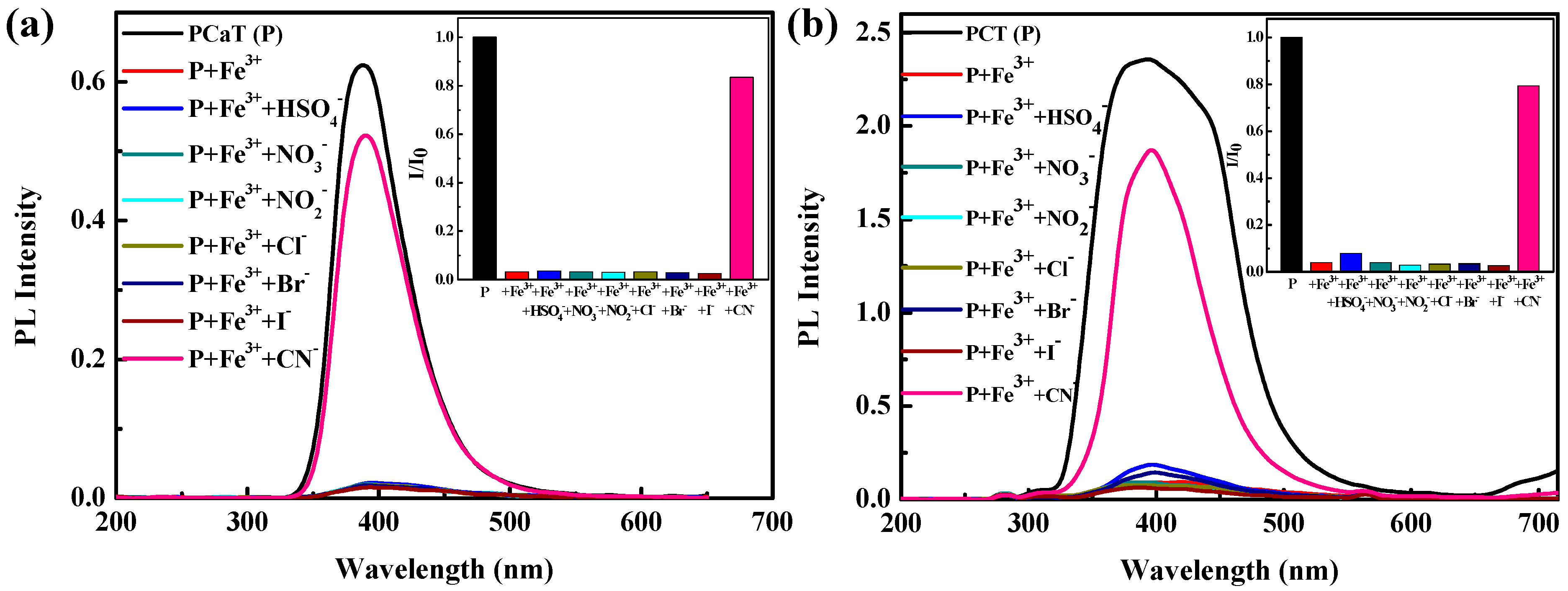
3.4. Ion Sensing Properties
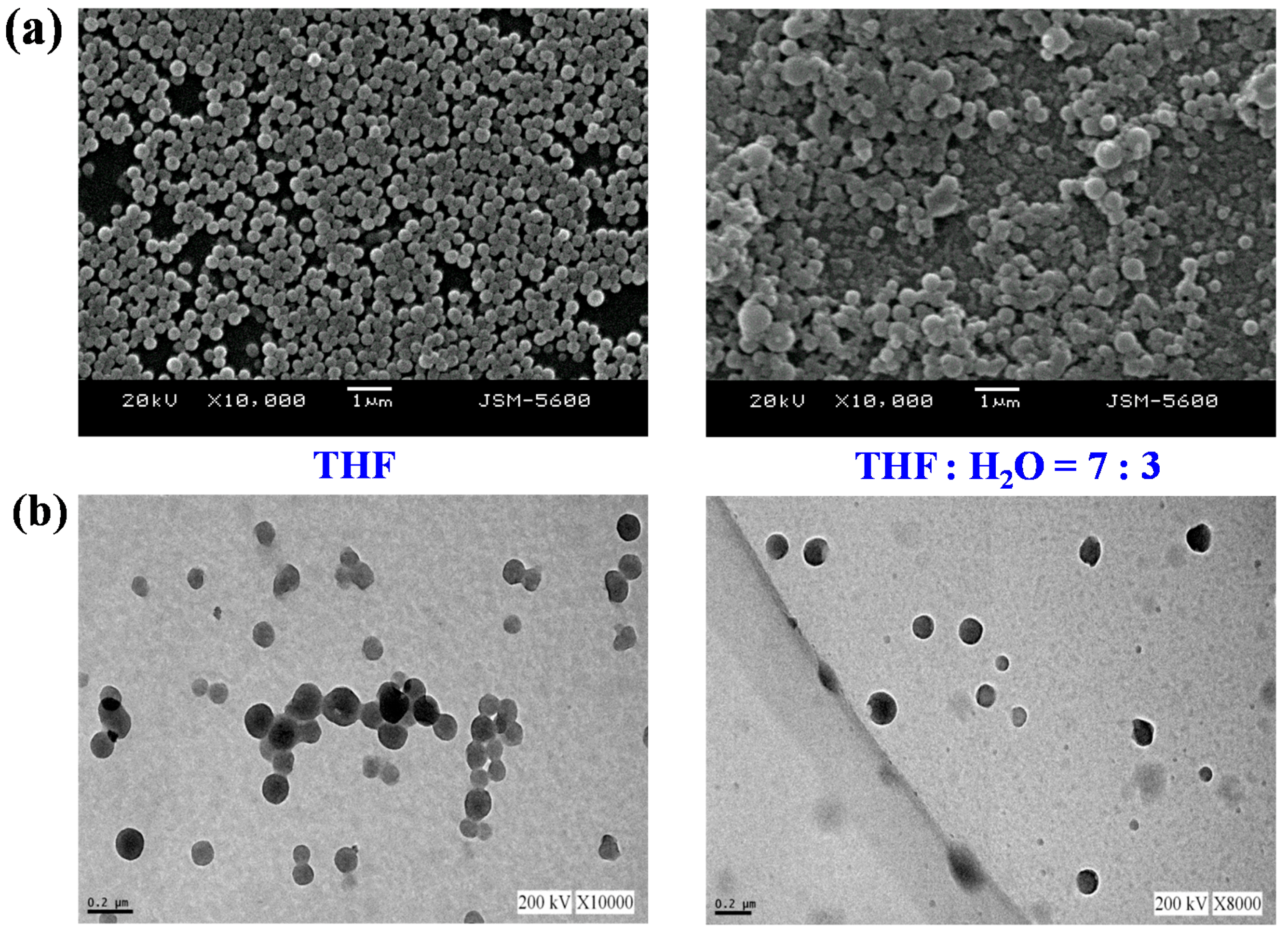
3.5. Self-Assembly of Polymer in THF Solution
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, L.; Lou, X.; Yu, Y.; Qin, J.; Li, Z. A new disubstituted polyacetylene bearing pyridine moieties: convenient synthesis and sensitive chemosensor toward sulfide anion with high selectivity. Macromolecules 2011, 44, 5186–5193. [Google Scholar] [CrossRef]
- Lou, X.; Zhang, Y.; Li, S.; Ou, D.; Wan, Z.; Qin, J.; Li, Z. A new polyfluorene bearing pyridine moieties: A sensitive fluorescent chemosensor for metal ions and cyanide. Polym. Chem. 2012, 3, 1446–1452. [Google Scholar] [CrossRef]
- Cao, S.; Pei, Z.; Xu, Y.; Zhang, R.; Pei, Y. Polytriazole bridged with 2,5-diphenyl-1,3,4-oxadiazole moieties: A highly sensitive and selective fluorescence chemosensor for Ag+. RSC Adv. 2015, 5, 45888–45896. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, G.; Luo, H.; Yao, J.; Liu, Z.; Zhang, D. Highly sensitive thin-film field-effect transistor sensor for ammonia with the DPP-bithiophene conjugated polymer entailing thermally cleavable tert-butoxy groups in the side chains. ACS Appl. Mater. Interfaces 2016, 8, 3635–3643. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, L.; Zeng, G.; Zhang, C.; Zhang, Y.; Xie, X. Current progress in biosensors for heavy metal ions based on DNAzymes/DNA molecules functionalized nanostructures: A review. Sens. Actuators B 2016, 223, 280–294. [Google Scholar] [CrossRef]
- Long, Y.; Chen, H.; Wang, H.; Peng, Z.; Yang, Y.; Zhang, G.; Li, N.; Liu, F.; Pei, J. Highly sensitive detection of nitroaromatic explosives using an electrospun nanofibrous sensor based on a novel fluorescent conjugated polymer. Anal. Chim. Acta 2012, 744, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhao, Z.; Bai, X.; Yuan, X.; Zhang, X.; Masuda, T. Organoborane-containing polyacetylene derivatives: synthesis, characterization, and fluoride-sensing properties. RSC Adv. 2014, 4, 55179–55186. [Google Scholar] [CrossRef]
- Xiang, G.; Wang, L.; Cui, W.; An, X.; Zhou, L.; Li, L.; Cao, D. 2-Pyridine-1H-benzo[d]imidazole based conjugated polymers: A selective fluorescent chemosensor for Ni2+ or Ag+ depending on the molecular linkage sites. Sens. Actuators B 2014, 196, 495–503. [Google Scholar] [CrossRef]
- Isaad, J.; Salaün, F. Functionalized poly(vinyl alcohol) polymer as chemodosimeter material for the colorimetric sensing of cyanide in pure water. Sens. Actuators B 2011, 157, 26–33. [Google Scholar] [CrossRef]
- Wu, X.; Xu, B.; Tong, H.; Wang, L. Highly selective and sensitive detection of cyanide by a reaction-based conjugated colymer chemosensor. Macromolecules 2011, 44, 4241–4248. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Y.; Cao, A. Strategy for sensor based on fluorescence emission red shift of conjugated polymers: applications in pH response and enzyme activity detection. Anal. Chem. 2013, 85, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Liu, L.; Yang, Q.; Lv, F.; Wang, S. Dopamine-modified cationic conjugated polymer as a new platform for pH sensing and autophagy imaging. Adv. Funct. Mater. 2013, 23, 764–769. [Google Scholar] [CrossRef]
- Balamurugan, A.; Kumar, V.; Jayakannan, M. Triple action polymer probe: carboxylic distilbene fluorescent polymer chemosensor for temperature, metal-ions and biomolecules. Chem. Commun. 2014, 50, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.M.; Chen, Y. Multifunctional hyperbranched oligo(fluorene vinylene) containing pendant crown ether: synthesis, chemosensory, and electroluminescent properties. Macromolecules 2009, 42, 8052–8061. [Google Scholar] [CrossRef]
- Kim, H.; You, G.R.; Park, G.J.; Choi, J.Y.; Noh, I.; Kim, Y.; Kim, S.J.; Kim, C.; Harrison, R.G. Selective zinc sensor based on pyrazoles and quinoline used to image cells. Dyes Pigments 2015, 113, 723–729. [Google Scholar] [CrossRef]
- Jin, F.; Shu, W.; Yu, X.; Zhang, Y.; Sun, L.; Liu, Y.; Tao, D.; Zhou, H.; Tian, Y. Crystal structures, two-photon excited fluorescence and bioimaging of Zn(II) complexes based on an extended 2,2′-bipyridine ligand. Dyes Pigments 2015, 121, 379–384. [Google Scholar] [CrossRef]
- Bellusci, A.; Ghedini, M.; Giorgini, L.; Gozzo, F.; Szerb, E.I.; Crispini, A.; Pucci, D. Anion dependent mesomorphism in coordination networks based on 2,2′-bipyridine silver(I) complexes. Dalton Trans. 2009, 7381–7389. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Zheng, C.; Pu, S.; Fan, C.; Liu, G. A highly selective ratiometric fluorescent chemosensor for Hg2+ based on a new diarylethene with a stilbene-linked terpyridine unit. Dyes Pigments 2014, 107, 38–44. [Google Scholar] [CrossRef]
- Zheng, Z.B.; Kang, S.Y.; Zhao, Y.; Zhang, N.; Yi, X.; Wang, K.Z. pH and copper ion luminescence on/off sensing by a dipyrazinylpyridine-appended ruthenium complex. Sens. Actuators B 2015, 221, 614–624. [Google Scholar] [CrossRef]
- Zheng, M.; Tao, H.; Xie, Z.; Zhang, L.; Jing, X.; Sun, Z. Fast response and high sensitivity europium metal organic framework fluorescent probe with chelating terpyridine sites for Fe3+. ACS Appl. Mater. Interfaces 2013, 5, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Maity, D.; Mardanya, S.; Baitalik, S. Pyrene and imidazole functionalized luminescent bimetallic Ru(II) terpyridine complexes as efficient optical chemosensors for cyanide in aqueous, organic and solid media. Dalton Trans. 2015, 44, 18607–18623. [Google Scholar] [CrossRef] [PubMed]
- Ayres, L.; Vos, M.R.J.; Adam, P.J.H.M.; Shklyarevskiy, I.O.; van Hest, J.C.M. Elastin-based side-chain polymers synthesized by ATRP. Macromolecules 2003, 6, 5967–5973. [Google Scholar] [CrossRef]
- Mei, Y.; Beers, K.L.; Byrd, M.H.C.; VanderHart, D.L.; Washburn, N.R. Solid-phase ATRP synthesis of peptide-polymer hybrids. J. Am. Chem. Soc. 2004, 126, 3472–3476. [Google Scholar] [CrossRef] [PubMed]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef] [PubMed]
- Perrier, S.; Takolpuckdee, P. Macromolecular design via reversible addition–fragmentation chain transfer (RAFT)/xanthates (MADIX) polymerization. J. Polym. Sci. Part A: Polym. Chem. 2005, 43, 5347–5393. [Google Scholar] [CrossRef]
- Favier, A.; Charreyre, M.T. Experimental requirements for an efficient control of free-radical polymerizations via the reversible addition-fragmentation chain transfer (RAFT) process. Macromol. Rapid Commun. 2006, 27, 653–692. [Google Scholar] [CrossRef]
- Lokitz, B.S.; Convertine, A.J.; Ezell, R.G.; Heidenreich, A.; Li, Y.; McCormick, C.L. Responsive nanoassemblies via interpolyelectrolyte complexation of amphiphilic block copolymer micelles. Macromolecules 2006, 39, 8594–8602. [Google Scholar] [CrossRef]
- Lowe, A.B.; McCormick, C.L. Reversible addition–fragmentation chain transfer (RAFT) radical polymerization and the synthesis of water-soluble (co)polymers under homogeneous conditions in organic and aqueous media. Prog. Polym. Sci. 2007, 32, 283–351. [Google Scholar] [CrossRef]
- Goto, A.; Hirai, N.; Nagasawa, K.; Tsujii, Y.; Fukuda, T.; Kaji, H. Phenols and carbon compounds as efficient organic catalysts for reversible chain transfer catalyzed living radical polymerization (RTCP). Macromolecules 2010, 43, 7971–7978. [Google Scholar] [CrossRef]
- Rosen, B.M.; Percec, V. Single-electron transfer and single-electron transfer degenerative chain transfer living radical polymerization. Chem. Rev. 2009, 109, 5069–5119. [Google Scholar] [CrossRef] [PubMed]
- Braunecker, W.A.; Matyjaszewski, K. Controlled/living radical polymerization: Features, developments, and perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-catalyzed living radical polymerization. Chem. Rev. 2001, 101, 3689–3746. [Google Scholar] [CrossRef] [PubMed]
- Boyer, C.; Bulmus, V.; Davis, T.P.; Ladmiral, V.; Liu, J.; Perrier, S.B. Bioapplications of RAFT polymerization. Chem. Rev. 2009, 109, 5402–5436. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Ookuma, H.; Nakano, S.; Endo, T. Xanthate-mediated controlled radical polymerization of N-vinylcarbazole. Macromol. Chem. Phys. 2006, 207, 1005–1017. [Google Scholar] [CrossRef]
- Xing, Z.; Zhang, J.; Li, X.; Zhang, W.; Wang, L.; Zhou, N.; Zhu, X. Design and property of thermoresponsive core-shell fluorescent nanoparticles via RAFT polymerization and suzuki coupling reaction. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4021–4030. [Google Scholar] [CrossRef]
- Hiruta, Y.; Funatsu, T.; Matsuura, M.; Wang, J.; Ayano, E.; Kanazawa, H. pH/temperature-responsive fluorescence polymer probe with pH-controlled cellular uptake. Sens. Actuators B 2015, 207, 724–731. [Google Scholar] [CrossRef]
- Juang, R.S.; Yang, P.C.; Wen, H.W.; Lin, C.Y.; Lee, S.C.; Chang, T.W. Synthesis and chemosensory properties of terpyridine-containing diblock polycarbazole through RAFT polymerization. React. Funct. Polym. 2015, 93, 130–137. [Google Scholar] [CrossRef]
- Cho, M.J.; Shin, J.; Jin, J.I.; Kim, Y.M.; Park, Y.W.; Ju, B.K.; Choi, D.H. Photoreactive main chain conjugated polymer containing oxetane moieties in the side chain and its application to green electrophosphorescence devices. Synth. Met. 2009, 159, 2147–2152. [Google Scholar] [CrossRef]
- Zhang, Y.; Murphy, C.B.; Jones, W.E., Jr. Poly[p-(phenyleneethynylene)-alt-(thienyleneethynylene)] polymers with oligopyridine pendant groups: Highly sensitive chemosensors for transition metal ions. Macromolecules 2002, 35, 630–636. [Google Scholar] [CrossRef]
- Zhou, H.P.; Zhou, F.X.; Wu, P.; Zheng, Z.; Yu, Z.P.; Chen, Y.; Tu, Y.; Kong, L.; Wu, J.; Tian, Y. Three new five-coordinated mercury (II) dyes: structure and enhanced two-photon absorption. Dyes Pigments 2011, 91, 237–247. [Google Scholar] [CrossRef]
- Leriche, P.; Frère, A.; Cravino, A.; Alévêque, O.; Roncali, J. Molecular engineering of the internal charge transfer in thiophene−triphenylamine hybrid π-conjugated systems. J. Org. Chem. 2007, 72, 8332–8336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.J.; Yang, S.H.; Hsu, C.S. Synthesis of conjugated polymers for organic solar cell applications. Chem. Rev. 2009, 109, 5868–5923. [Google Scholar] [CrossRef] [PubMed]
- House, J.E. Inorganic Chemistry; Academic Press/Elsevier: Burlington, MA, USA, 2008. [Google Scholar]
- Cui, Y.; Chen, Q.; Zhang, D.D.; Cao, J.; Han, B.H. Colorimetric naked-eye recognizable anion sensors synthesized via RAFT polymerization. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1551–1556. [Google Scholar]
- Xu, Z.; Yoon, J.; Spring, D.R. Fluorescent chemosensors for Zn2+. Chem. Soc. Rev. 2010, 39, 1996–2006. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Ou, D.; Li, Q.; Li, Z. An indirect approach for anion detection: the displacement strategy and its application. Chem. Commun. 2012, 48, 8462–8477. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Tong, X.; Zhao, Y. Preparation of azobenzene-containing amphiphilic diblock copolymers for light-responsive micellar aggregates. Macromolecules 2004, 37, 8911–8917. [Google Scholar] [CrossRef]
- Shen, H.W.; Eisenberg, A. Block length dependence of morphological phase diagrams of the ternary system of PS-b-PAA/dioxane/H2O. Macromolecules 2000, 33, 2561–2572. [Google Scholar] [CrossRef]

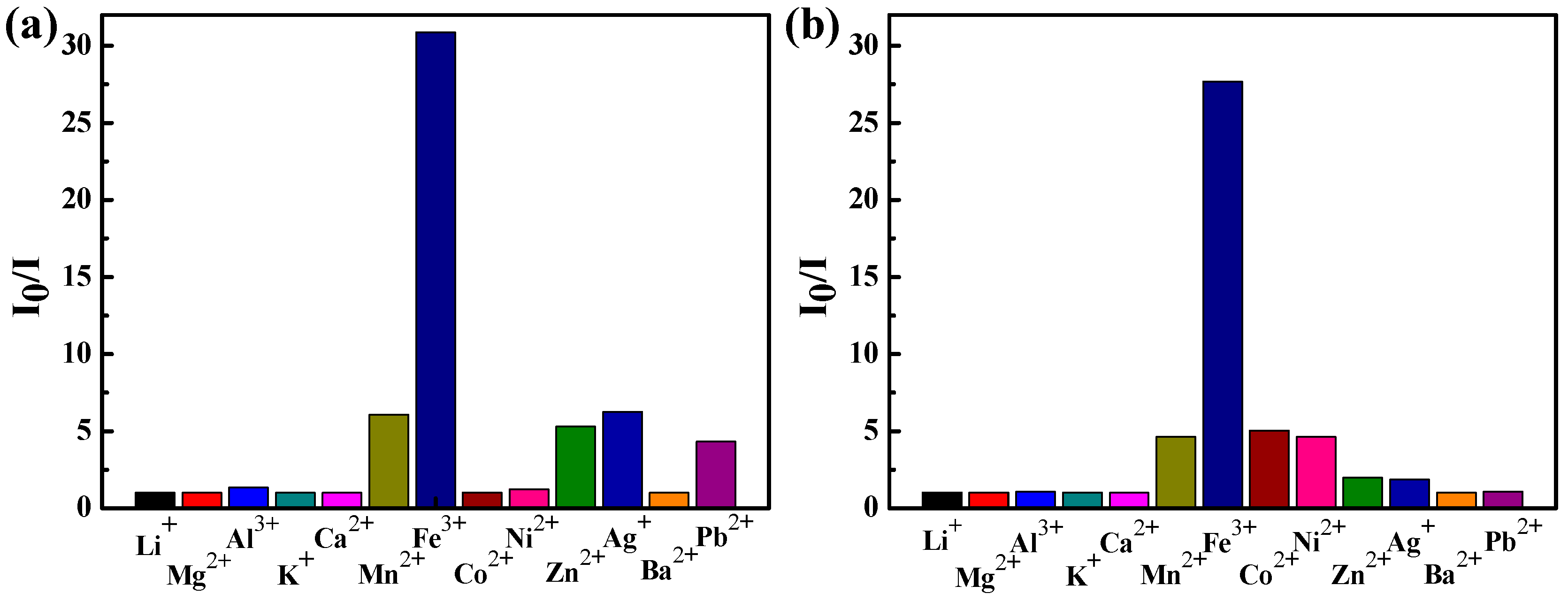
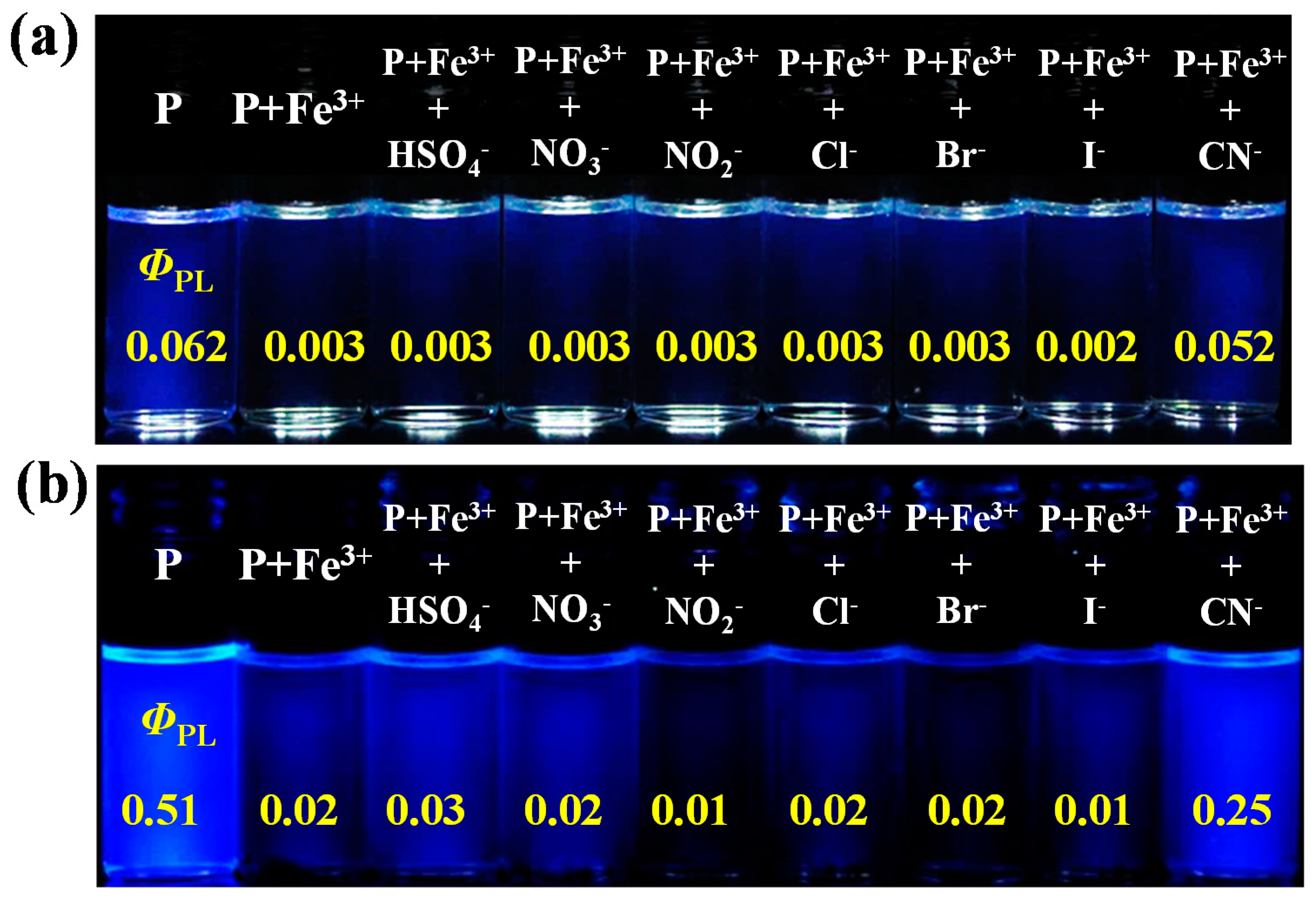
| Polymer | Yield (%) | Mw (×103) a | PDI a | Tg (°C) b | Td (°C) c | Molar ratio (x:y) d |
|---|---|---|---|---|---|---|
| PC2Br | 18.4 | 6.78 | 1.12 | 186.6 | 314.3 | ― |
| PCaT | 10.3 | 7.92 | 1.14 | 198.3 | 343.0 | 94:6 |
| PCT | 35.6 | 12.2 | 1.65 | 159.8 | 268.7 | 55:45 |
| Polymer | UV-vis λmax sol’n (nm) a | UV-vis λmax film (nm) a | PL λmax sol’n (nm) b | PL λmax film (nm) b | Stokes shift c | ΦPL d |
|---|---|---|---|---|---|---|
| PCaT | 271, 346s | 293, 350s | 381 | 394 | 110 | 0.062 |
| PCT | 232, 275s | 297 | 391 | 428, 529s | 159 | 0.51 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, P.-C.; Li, S.-Q.; Chien, Y.-H.; Tao, T.-L.; Huang, R.-Y.; Chen, H.-Y. Synthesis, Chemosensory Properties, and Self-Assembly of Terpyridine-Containing Conjugated Polycarbazole through RAFT Polymerization and Heck Coupling Reaction. Polymers 2017, 9, 427. https://doi.org/10.3390/polym9090427
Yang P-C, Li S-Q, Chien Y-H, Tao T-L, Huang R-Y, Chen H-Y. Synthesis, Chemosensory Properties, and Self-Assembly of Terpyridine-Containing Conjugated Polycarbazole through RAFT Polymerization and Heck Coupling Reaction. Polymers. 2017; 9(9):427. https://doi.org/10.3390/polym9090427
Chicago/Turabian StyleYang, Po-Chih, Si-Qiao Li, Yueh-Han Chien, Ta-Lun Tao, Ruo-Yun Huang, and Hsueh-Yu Chen. 2017. "Synthesis, Chemosensory Properties, and Self-Assembly of Terpyridine-Containing Conjugated Polycarbazole through RAFT Polymerization and Heck Coupling Reaction" Polymers 9, no. 9: 427. https://doi.org/10.3390/polym9090427