Computational Thermomechanical Properties of Silica–Epoxy Nanocomposites by Molecular Dynamic Simulation
Abstract
:1. Introduction
2. Computational Methods
2.1. Establishement of Models
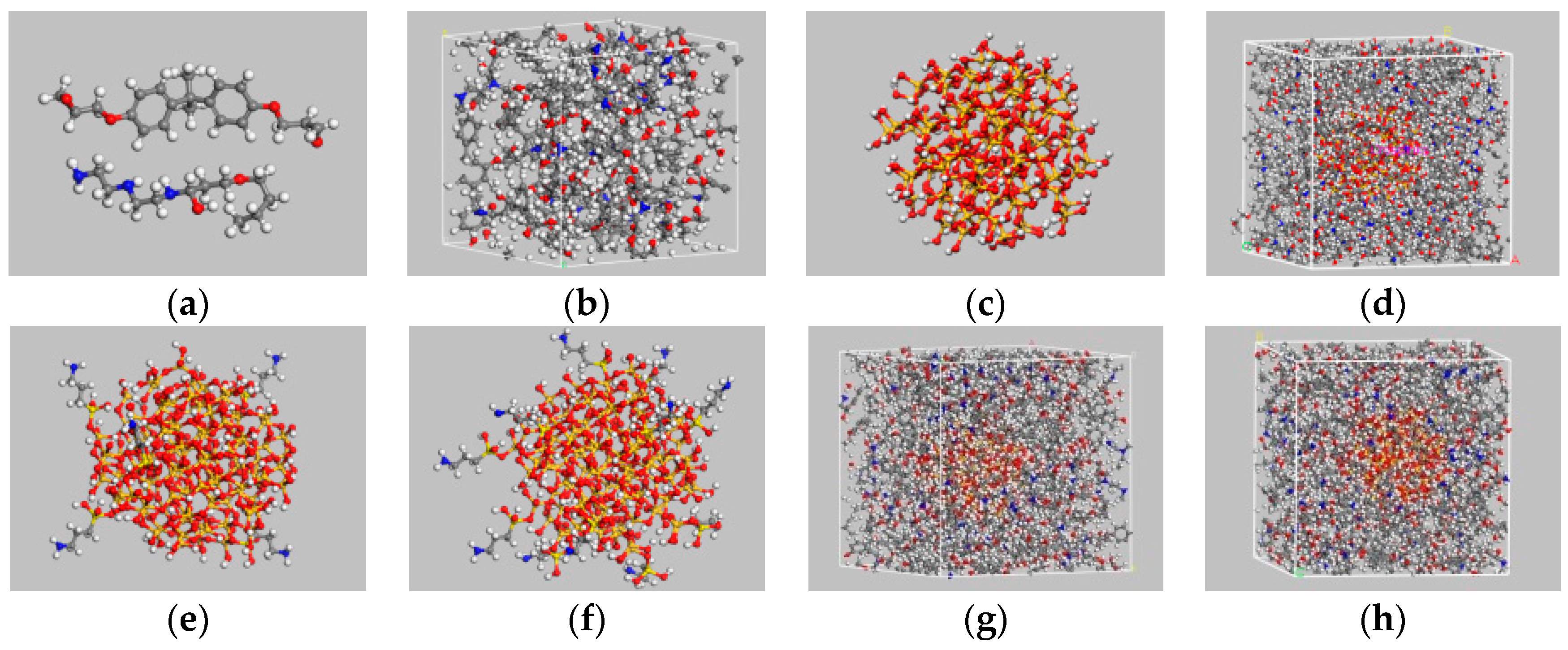
- Cell construction: single molecular models of DGEBA and the curing agent were manually designed (Figure 1a), and the polymerization degree of DGEBA molecules was maintained at a fixed value of 0 to simplify the crosslinking process. A periodic 3D structure consisting of 16:8 molecular mixture of DGEBA and cross linker monomers was then established. The initial density of the unit cell was presumed 0.6 g/cm3.
- For pre-equilibration, a slow stress relaxation procedure was performed to obtain an equilibrated structure. The geometric optimization of 5000 iterations was conducted to acquire a slightly deformed unit cell with a lower total energy than the initial value. Molecular dynamics simulations were subsequently conducted in an NVT (constant volume and temperature) ensemble for 100 ps at 298 K. In the NVT ensemble, forcefield COMPASS was selected and Andersen was used to control temperature. Afterwards, the unit cell was equilibrated twice under the NPT ensemble at 100 ps and 1 atm to obtain the final density of above 1 g/cm3, and a time step of 1 fs should be used. In an NPT (constant pressure and temperature) ensemble, Andersen and Berendsen were chosen to regulate temperature and pressure, respectively. The last equilibrated unit cell was utilized for the subsequent crosslinking step.
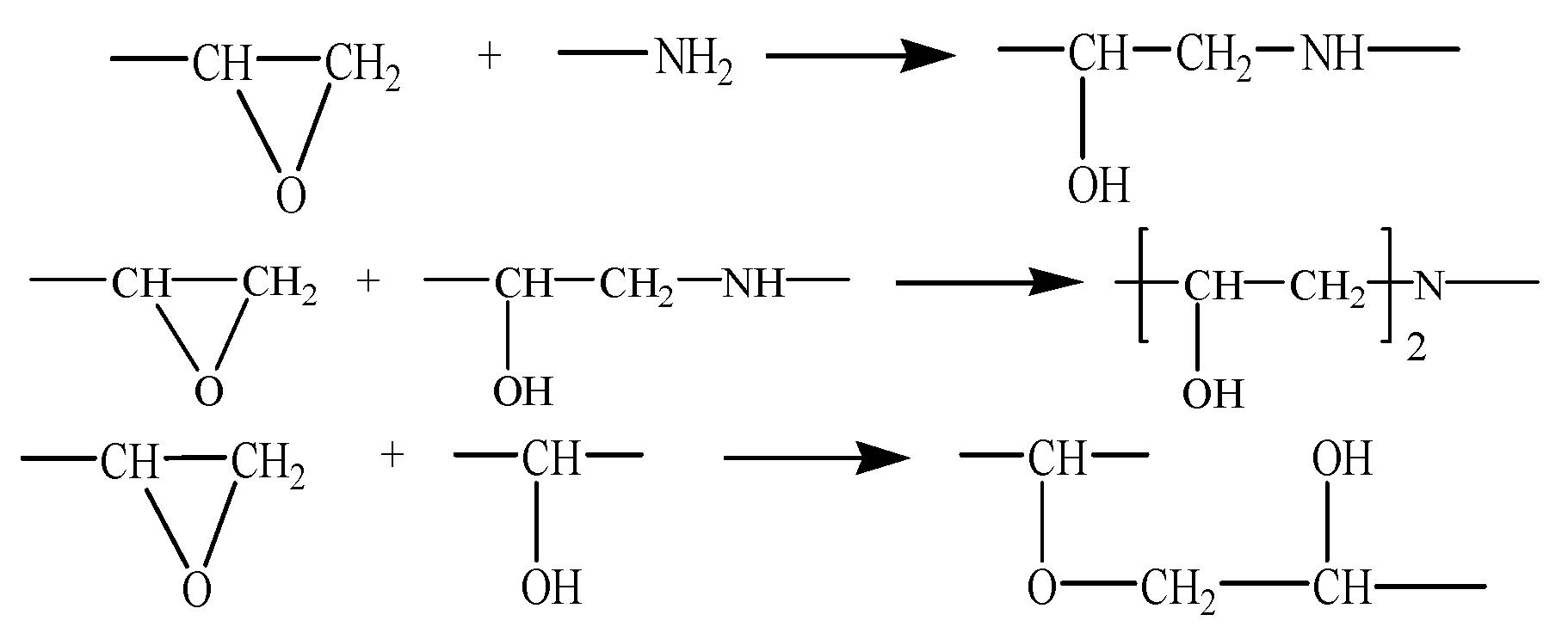
- Three main chemical reactions were involved in the formation of 3D cross-linked thermoset epoxy (Figure 2). Before ring-opening reaction occurs, active –NH·group and –CH(OH)–CH2 group were manually prepared. Then, 16 DGEBA molecules and eight curing agent molecules with active reaction groups formed periodic unit cells and underwent a series of new bond formation processes under the following controlled parameters: initial cutoff distance of 3.5 Å, maximum cutoff distance of 7.0 Å, and conversion degree of 85%. After a specific crosslink PERL language was run, the final model was obtained (Figure 1b). Each final cross-linked structure was equilibrated over 2000 ps NPT dynamics at 298 K and 1 atm to reach the most stable structure before computing thermal and mechanical properties.
- A spherical SiO2 particle with a diameter of 10 Å was established in accordance with a previously described method [33,34]. Hydroxylation was conducted to produce Si–OH on the surface of the SiO2 particle and consequently achieve a good match with experimental results. Then, one unit cell of a silica–epoxy composite could be established at a controlled density of 0.6 g/cm3. A cross-linked silica–epoxy composite unit cell could be obtained after steps 2 and 3 were repeated (Figure 1d).
- Manual grafting was conducted to remove a fixed number of hydrogen atoms from the total hydroxyl groups and graft some KH550 molecules as the silane coupling agent to connect to SiO2 particles through Si–O bonds [35]. Grafting involved a series of hydrolysis and coupling reactions. Figure 1c shows the particles with a diameter of 10 nm and 104 hydroxyls on the surface. Five and ten KH550 molecules were respectively added onto the surface to form two kinds of SiO2 models with 5% and 10% grafting ratios (Figure 1e,f). The reactions between –NH2 group at the end of KH550 molecules and epoxy groups could be disregarded because of the low active energy of –NH2 groups. Figure 1g,h show the final crosslinking 3D structure of nanocomposites models with 5% and 10% grafting ratios, respectively.
2.2. Tg and CTE
2.3. Thermal Conductivity
2.4. Mechanical Response
3. Results and Discussion
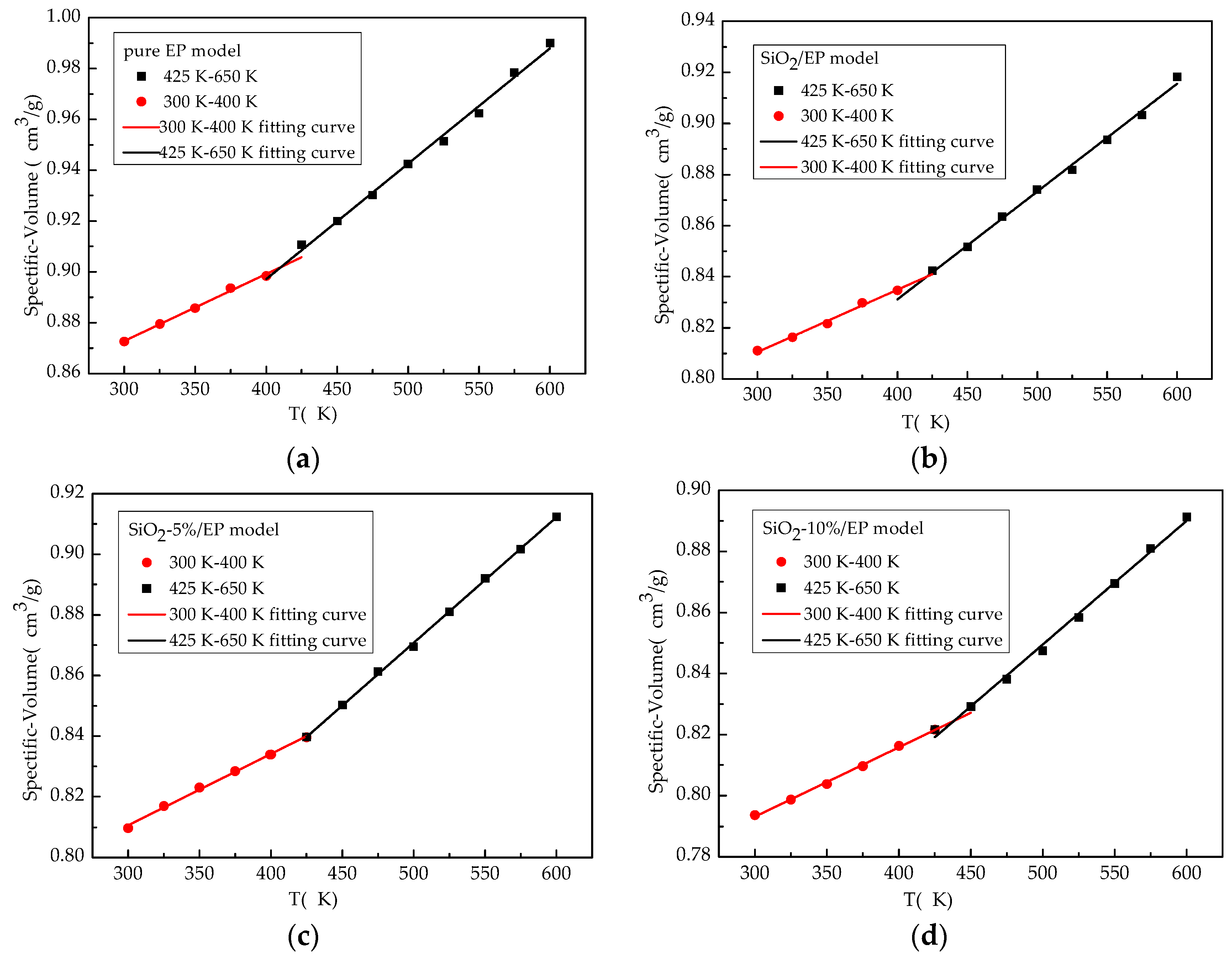
3.1. Tg and CTE
3.2. Thermal Properties
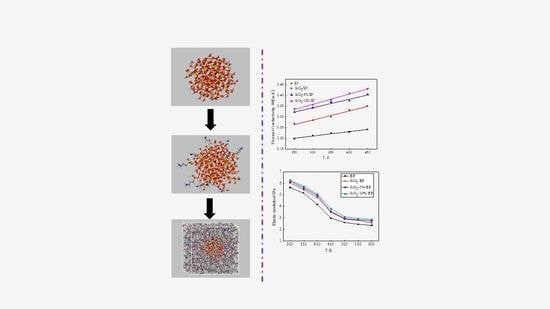
- The four models show a linear increase in thermal conductivity from 300 to 450 K. Incorporation of SiO2 nanoparticles can improve the thermal conductivity of epoxy resin. As temperature increases, without grafting procedure, thermal conductivity increases from 33.04% at 300 K to 44.75% at 450 K compared with that of the pure EP model.
- Grafting silane coupling agents on SiO2 nanoparticle surface can considerably enhance the thermal conductivity of composites. A high grafting ratio corresponds to a considerable increase in the thermal conductivity of composite models. At 300 K, the thermal conductivity of SiO2–10%/EP model increases by 67.07%, which is higher than that of the pure EP model.
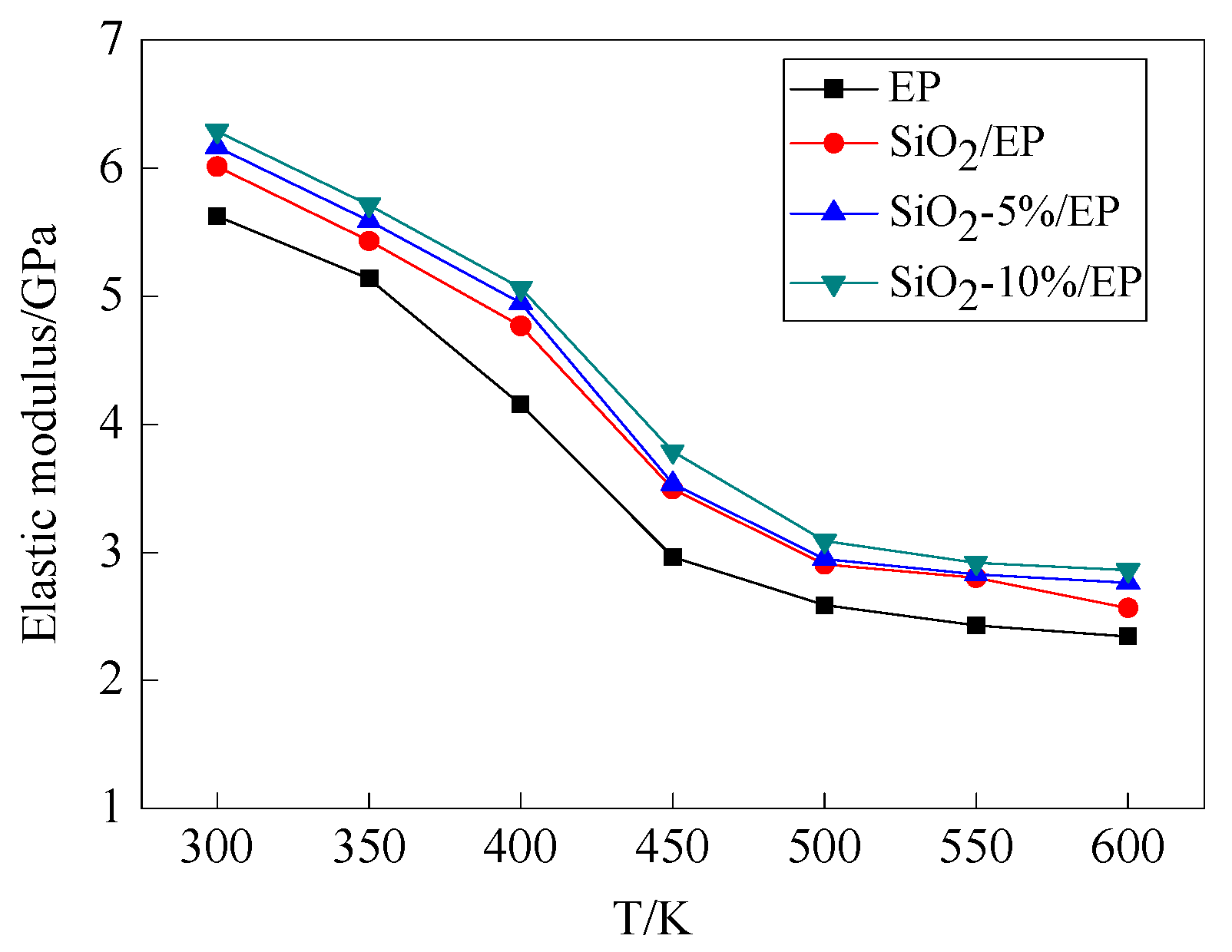
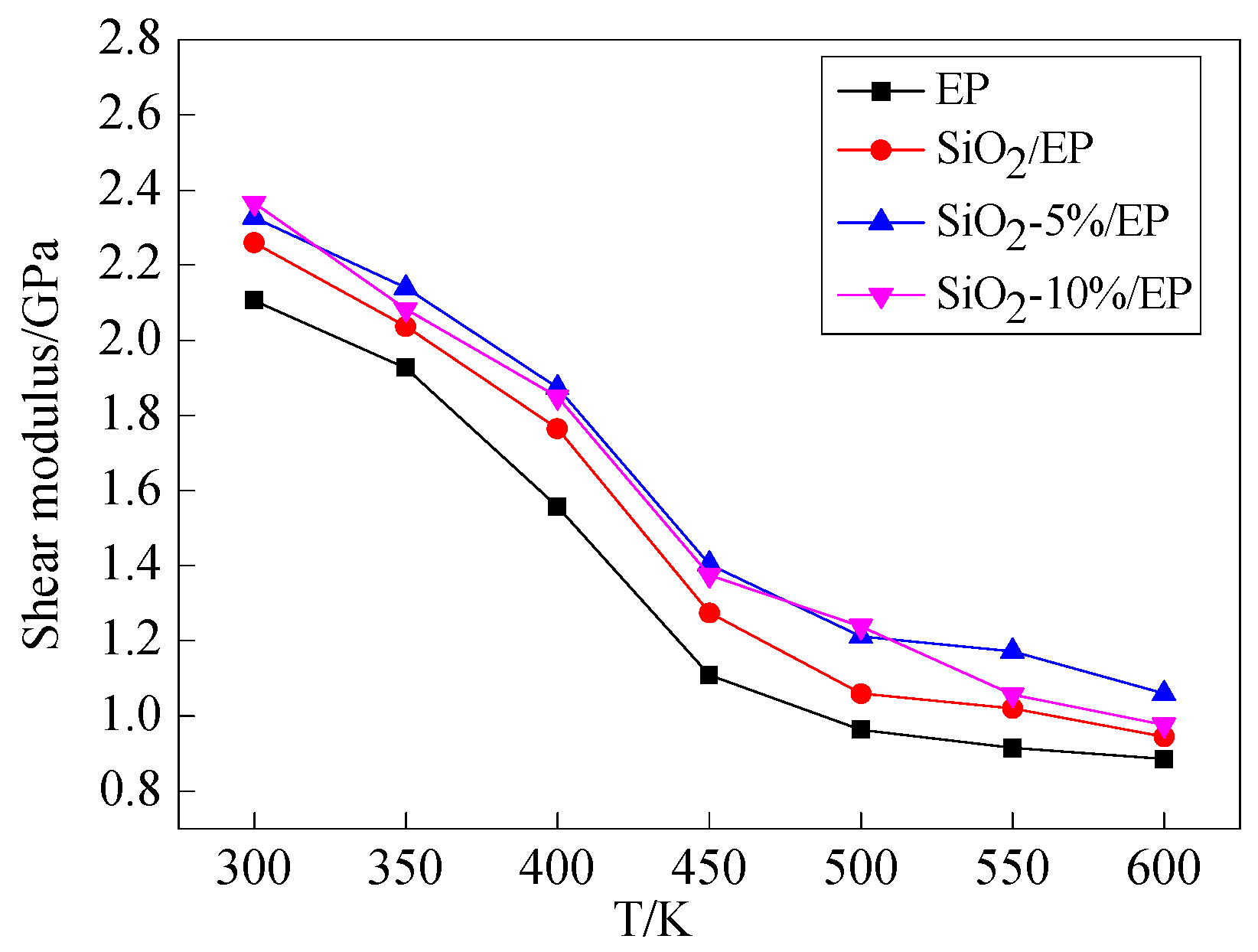
3.3. Mechanical Properties
3.4. Binding Energy
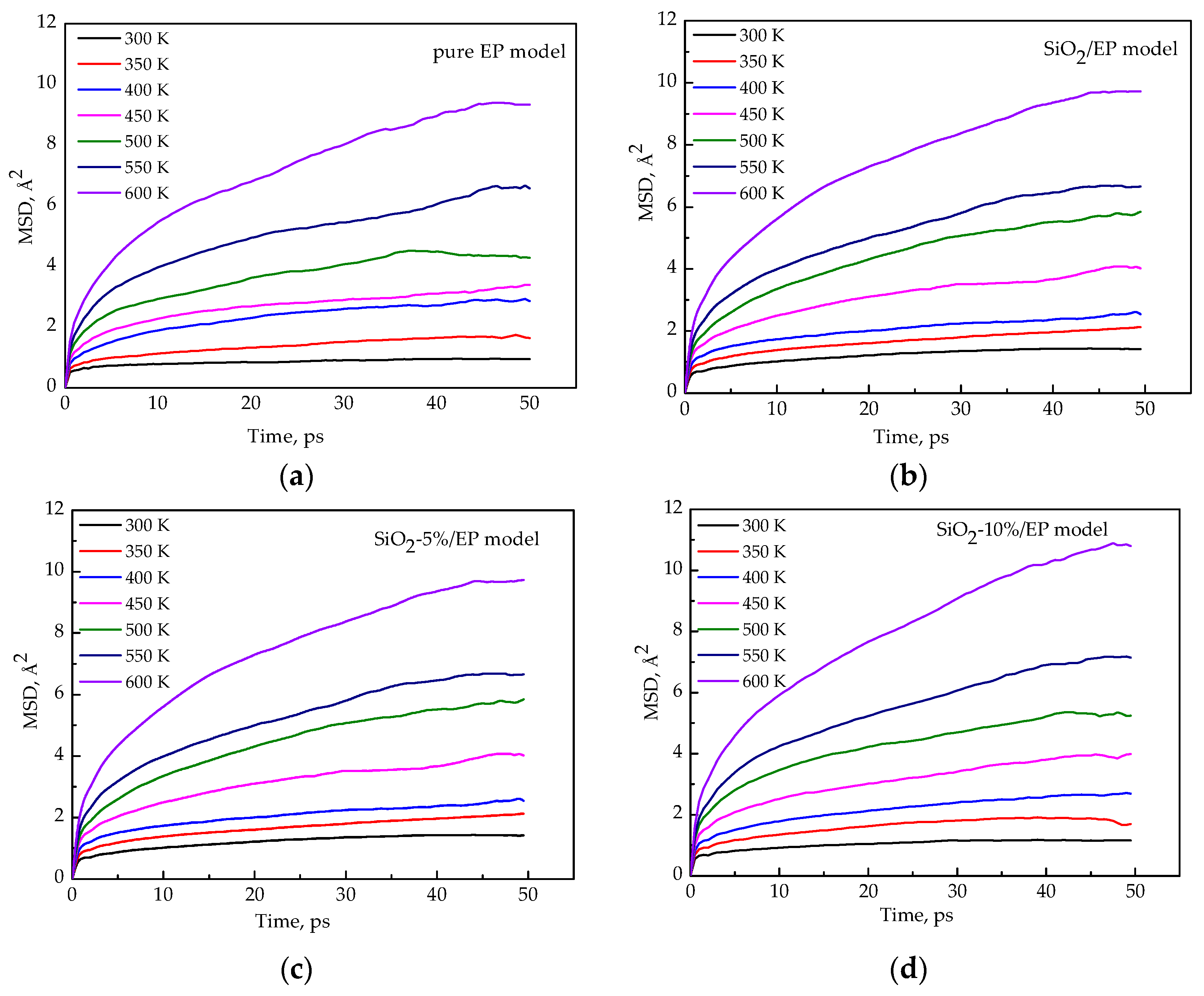
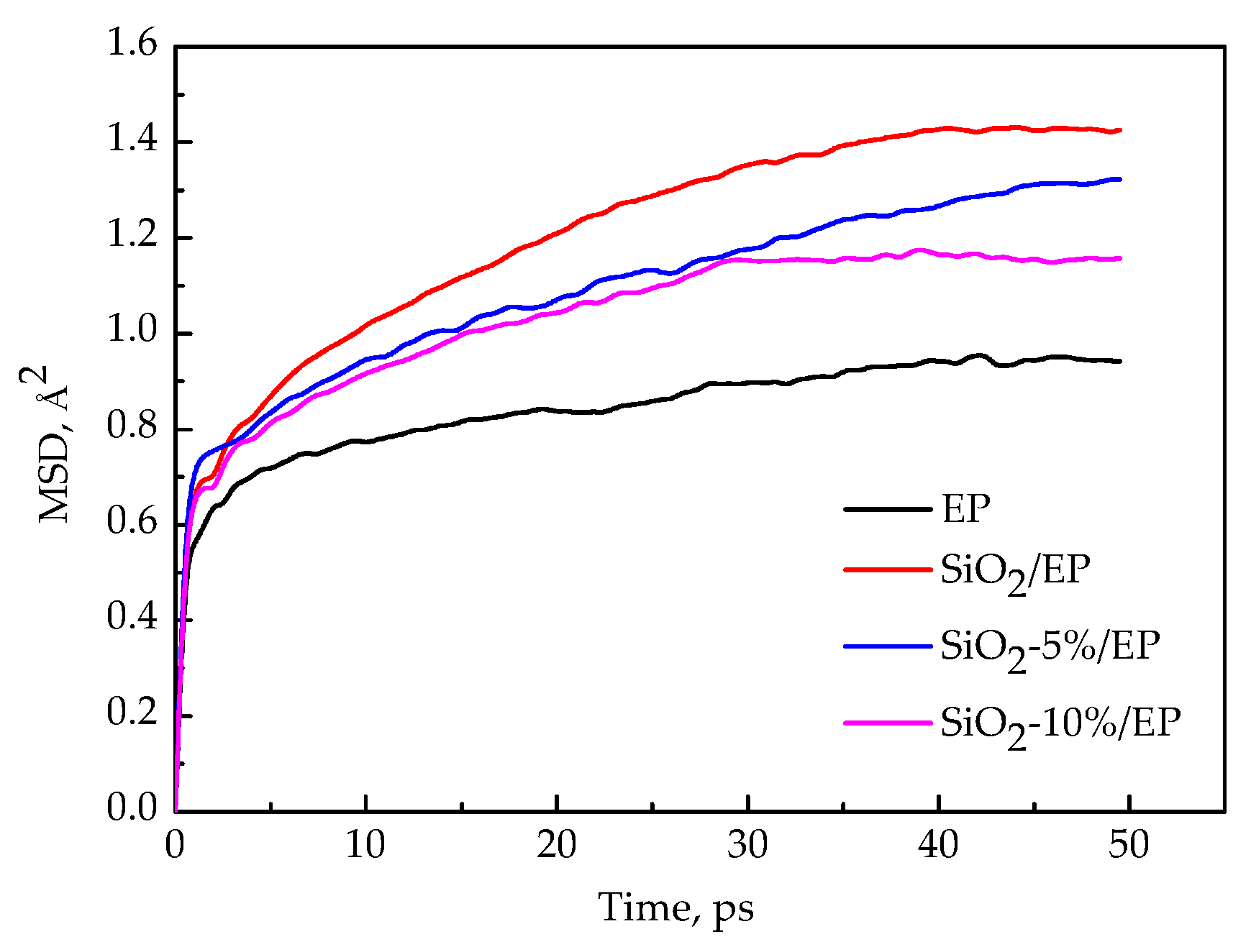
3.5. Segment Movement
- The MSDs of the four models increase with time until a steady state is reached. At a high temperature, especially above 450 K, the value increases faster, which can be attributed to the resultant larger kinetic energy of the molecular chains when the models attain the glassy state. A strong kinetic energy of a molecular chain can lead to a rapid decline of mechanical properties. These results are consistent with the calculated mechanical properties.
- Incorporation of SiO2 nanoparticles without surface modification into the epoxy matrix may increase the MSD compared with that of the pure epoxy resin model. However, the silica–epoxy model with 10% grafting ratio of the silane coupling agent can reduce the MSD shown in Figure 9. Therefore, surface modification using a silane coupling agent can create a tighter 3D cross-linked structure to enhance the mechanical and thermal properties of composites. A high grafting ratio can also help complete equilibration processes within a short time. Overall, grafting plays a major role in fabricating flexible new composites.
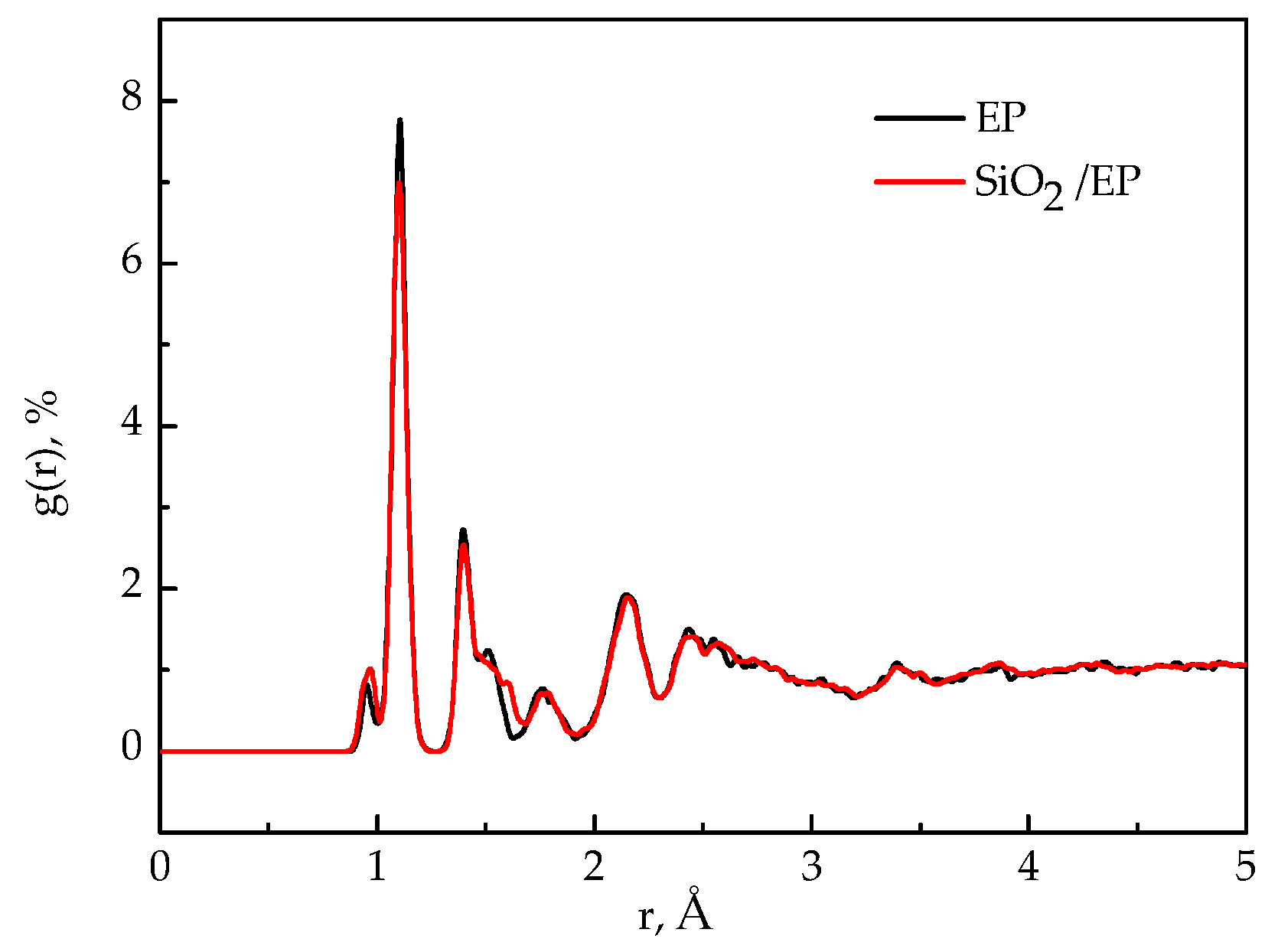
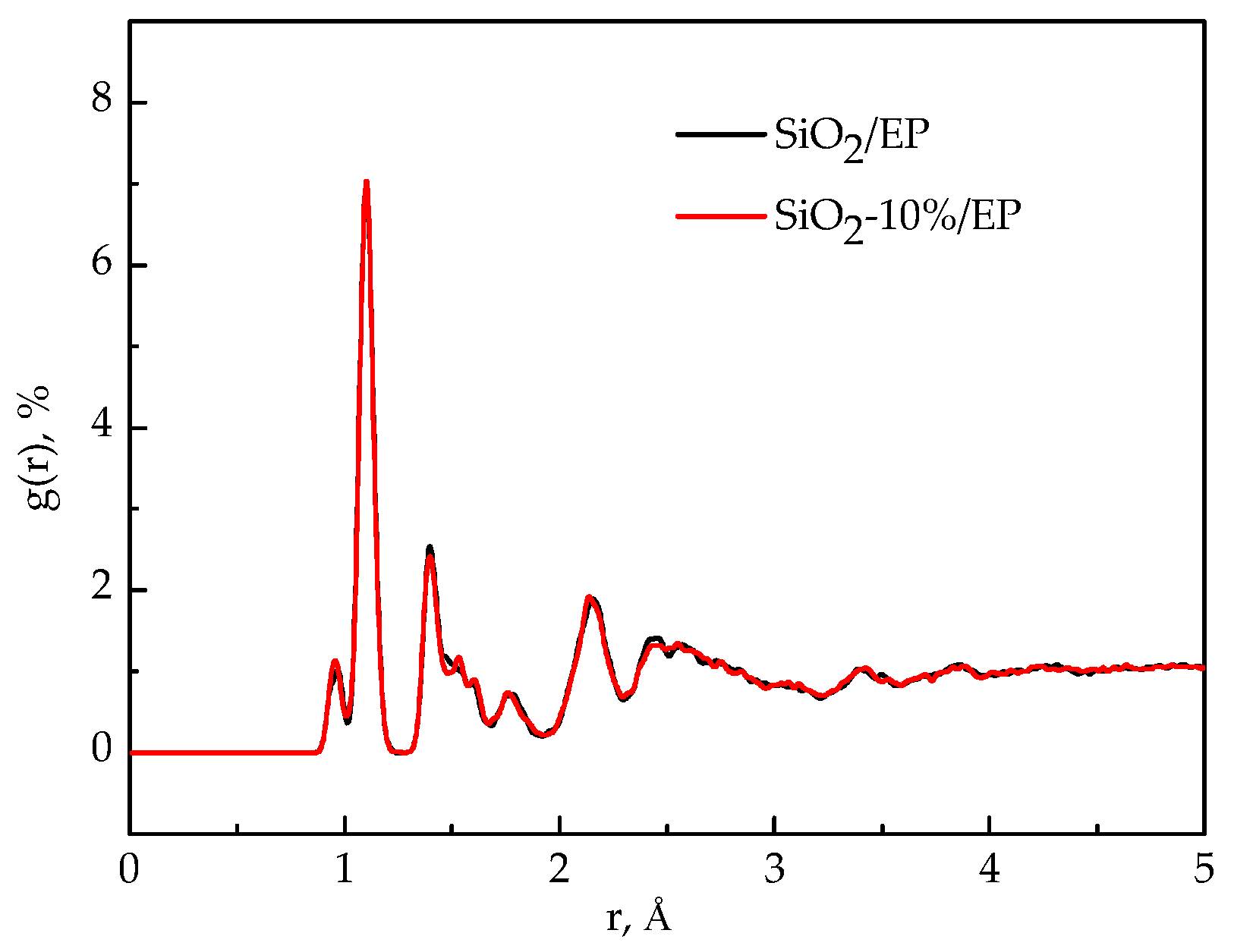
3.6. Radial Distribution Function (RDFs)
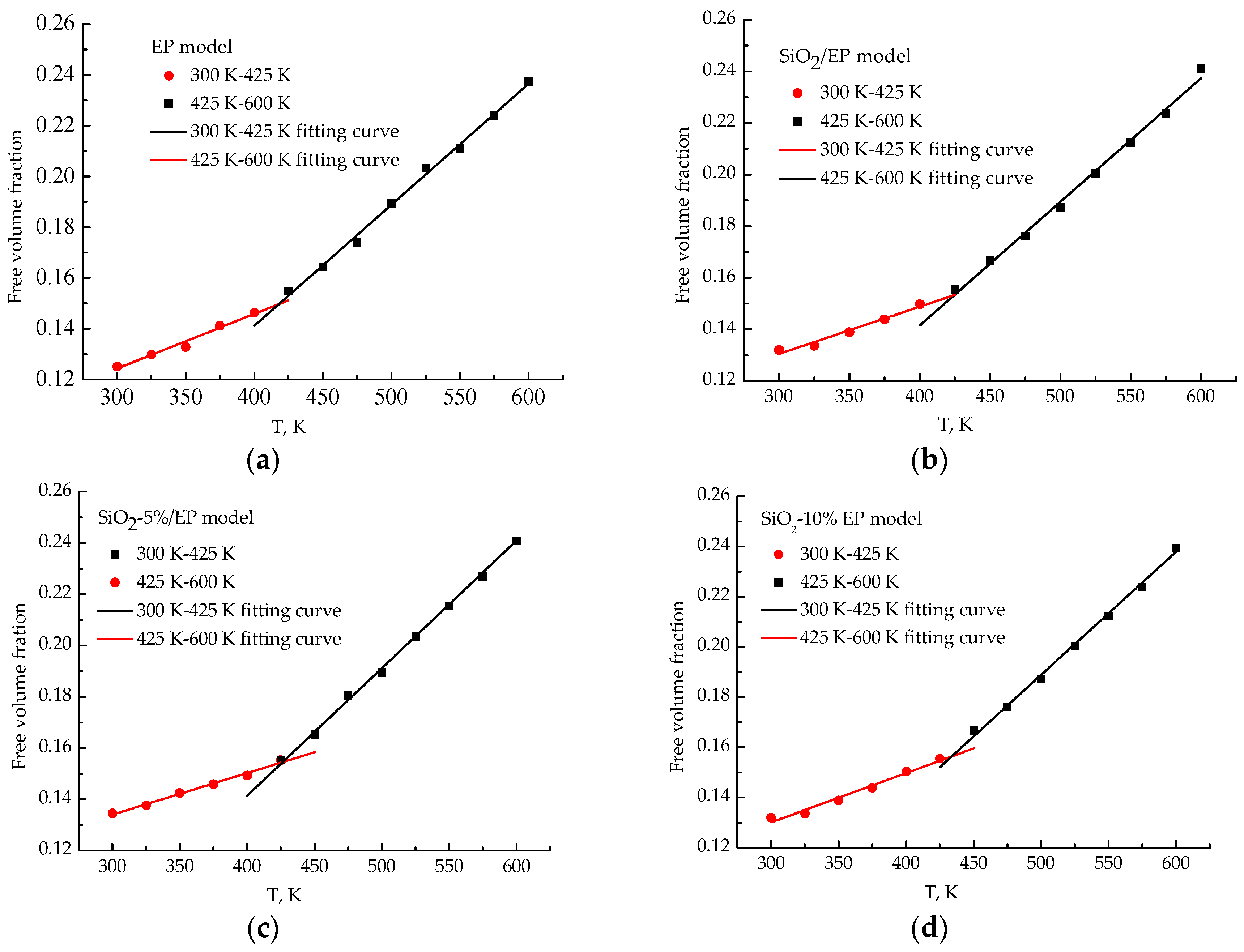
3.7. Free Volume Fractions
4. Conclusions
- Incorporating SiO2 nanoparticles into a polymer host can effectively and proportionally improve Tg, mechanical properties, and thermal conductivity. The surface modification of SiO2 nanoparticles with silane coupling agent KH550 can further enhance the mechanical and thermal properties of composites. The composite model is further improved when the grafting rate reaches 10%. Compared with the pure epoxy resin model, Tg of the model of SiO2–10%/EP increased by 7%. Although the mechanical properties of silica–epoxy composite models likely decline with temperature, the mechanical properties of the silica–epoxy model were also enhanced after incorporating. In the range of 250 to 450 K, thermal conductivity linearly increased. At 300 K, the thermal conductivity of the SiO2–10%/EP model increased by up to 67% at 0.357 W/(m·K), and this value was higher than that of the pure epoxy resin model at 0.214 W/(m·K). The binding energy between SiO2 and epoxy resin also increased by up to 38%, demonstrating a strong interaction between polymer hosts and particles after grafting.
- The analysis of mean square displacement, radial distribution function, and free volume fraction further elucidates the improved mechanism. Temperature drastically influences the three values. The silica–epoxy model with 10% grafting ratio of silane coupling agent could create a tight 3D cross-linked structure and maintain a relatively low MSD value at high temperatures. In the radial distribution function analysis, the peak representing the hydroxyl group at 0.96 Å became stronger than that of the pure epoxy resin model, verifying that the amount of –OH on the modified surface of SiO2 particles increased. The model of SiO2–10%/EP also shows a lower value of free volume fraction than that of the model of SiO2/EP. Overall, grafting procedure proves necessary to control and maintain the improved mechanical and thermal properties.
Author Contributions
Conflicts of Interest
References
- Yang, Q.; Li, X.D.; Shi, L.; Yang, X.P.; Sui, G. The thermal characteristics of epoxy resin: Design and predict by using molecular simulation method. Polymer 2013, 54, 6447–6454. [Google Scholar] [CrossRef]
- Chen, P.; Liu, S.P.; Wang, D.Z. Epoxy Resin and Its Application, 1st ed.; Chemical Industry Publisher: Beijing, China, 2011; pp. 20–100. (In Chinese) [Google Scholar]
- Kang, S.; Hong, S.I.; Choe, C.R.; Park, M. Preparation and characterization of epoxy composites filled with functionalized nanosilica particles obtained via sol–gel process. Polymer 2001, 42, 879–887. [Google Scholar] [CrossRef]
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal conductivity of polymer–based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Choi, J.R.; Park, S.J. Thermal conductivity and thermo–physical properties of nanodiamond–attached exfoliated hexagonal boron nitride/epoxy nanocomposites for microelectronics. Compos. A Appl. Sci. Manuf. 2017, 101, 227–236. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Rhee, K.Y.; Park, S.J. Nanodiamond nanocluster–decorated graphene oxide/epoxy nanocomposites with enhanced mechanical behavior and thermal stability. Compos. B Eng. 2017, 114, 111–120. [Google Scholar] [CrossRef]
- Wattanakul, K.; Manuspiya, H.; Yanumet, N. Effective surface treatments for enhancing the thermal conductivity of BN–filled epoxy composite. J. Appl. Polym. Sci. 2011, 119, 3234–3243. [Google Scholar] [CrossRef]
- Li, Z.; Okamato, K.; Ohki, Y.; Tanaka, T. Effects of Nano–filler Addition on Partial Discharge Resistance and Dielectric Breakdown Strength of Micro–Al2O3/Epoxy Composite. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 653–661. [Google Scholar] [CrossRef]
- Gao, Z.; Zhao, L. Effect of nano–fillers on the thermal conductivity of epoxy composites with micro–Al2O3 particles. Mater. Des. 2015, 66, 176–182. [Google Scholar] [CrossRef]
- Choi, S.; Kim, J. Thermal conductivity of epoxy composites with a binary–particle system of aluminum oxide and aluminum nitride fillers. Compos. B Eng. 2013, 51, 140–147. [Google Scholar] [CrossRef]
- Kharitonov, A.P.; Kharitonova, L.N. Surface modification of polymers by direct fluorination: A convenient approach to improve commercial properties of polymeric articles. Pure Appl. Chem. 2009, 81. [Google Scholar] [CrossRef]
- Du, B.X.; Xiao, M.; Zhang, J.M. Effects of Thermal Conductivity on Tracking Failure of Epoxy/BN Composite under Pulse Strength. In Proceedings of the IEEE International Conference on Solid Dielectrics, Bologna, Italy, 30 June–4 July 2013. [Google Scholar]
- Špitalský, Z.; Krontiras, C.A.; Georga, S.N.; Galiotis, C. Effect of oxidation treatment of multiwalled carbon nanotubes on the mechanical and electrical properties of their epoxy composites. Compos. A Appl. Sci. Manuf. 2009, 40, 778–783. [Google Scholar] [CrossRef]
- Roy, M.; Nelson, J.K.; MacCrone, R.K.; Schadler, L.S.; Reed, C.W. Polymer Nanocomposite Dielectrics—The Role of the Interface. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 629–643. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Park, S.J. In–situ modification of nanodiamonds by mercapto–terminated silane agent for enhancing the mechanical interfacial properties of nitrile butadiene rubber nanocomposites. Polym. Compos. 2017. [Google Scholar] [CrossRef]
- Huang, X.Y.; Jiang, P.K.; Tanaka, T. A Review of Dielectric Polymer Composites With High Thermal Conductivity. IEEE Electr. Insul. Mag. 2011, 27, 8–16. [Google Scholar] [CrossRef]
- Yu, J.H.; Huang, X.Y.; Wu, C.; Wu, X.F.; Wang, G.L.; Jiang, P.K. Interfacial modification of boron nitride nanoplatelets for epoxy composites with improved thermal properties. Polymer 2012, 53, 471–480. [Google Scholar] [CrossRef]
- Xu, J.C.; Zhang, Z.F.; Lin, J. Prediction of the Effective Elastic Moduli of the Nanoparticle–Reinforced Polymer Matrix Composites Considering Interface Effects. Adv. Mater. Res. 2012, 460, 107–118. [Google Scholar] [CrossRef]
- Chang, C.Y.; Ju, S.P.; Chang, J.W.; Huang, S.C.; Yang, H.W. The thermal conductivity and mechanical properties of poly(p-phenylene sulfide)/oxided–graphene and poly(p-phenylene sulfide)/defect–graphene nano–composites by molecular dynamics simulation. RSC Adv. 2014, 4, 26074–26080. [Google Scholar] [CrossRef]
- Mazeau, K.; Heux, L. Molecular Dynamics Simulations of Bulk Native Crystaline and Amorphous Structures of Cellulose. J. Phys. Chem. B 2003, 107, 2394–2403. [Google Scholar] [CrossRef]
- Chen, W.; Lickfield, G.C.; Yang, C.Q. Molecular modeling of cellulose in amorphous state. Part I: Model building and plastic deformation study. Polymer 2004, 45, 1063–1071. [Google Scholar] [CrossRef]
- Stoliarov, S.I.; Lyon, R.E.; Nyden, M.R. A reactive molecular dynamics model of thermal decomposition in polymers. II. Polyisobutylene. Polymer 2004, 45, 8613–8621. [Google Scholar] [CrossRef]
- Taraghi, I.; Fereidoon, A.; Zamani, M.M.; Mohyeddin, A. Mechanical, thermal, and viscoelastic properties of polypropylene/glass hybrid composites reinforced with multiwalled carbon nanotubes. J. Compos. Mater. 2015, 1211, 20–21. [Google Scholar] [CrossRef]
- Yang, S.R.; Qu, J.M. Computing thermomechanical properties of crosslinked epoxy by molecular dynamic simulations. Polymer 2012, 53, 4806–4817. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Valavala, P.K.; Clancy, T.C.; Wise, K.E.; Odegard, G.M. Molecular modeling of crosslinked epoxy polymers: The effect of crosslink density on thermomechanical properties. Polymer 2011, 52, 2445–2452. [Google Scholar] [CrossRef]
- Choi, J.; Yu, S.; Yang, S.; Cho, M. The glass transition and thermoelastic behavior of epoxy–based nanocomposites: A molecular dynamics study. Polymer 2011, 52, 5197–5203. [Google Scholar] [CrossRef]
- Jeyranpour, F.; Alahyarizadeh, G.; Minuchehr, H. The Thermo–Mechanical Properties estimation of Fullerene-Reinforced Resin Epoxy Composites by Molecular Dynamics Simulation—A Comparative Study. Polymer 2016, 88, 9–18. [Google Scholar] [CrossRef]
- Shen, X.; Wang, Z.Y.; Wu, Y.; Liu, X.; He, Y.B.; Kim, J.K. Multilayer Graphene Enables Higher Efficiency in Improving Thermal Conductivities of Graphene/Epoxy Composites–Supporting Information. Nano Lett. 2016, 16, 3585–3593. [Google Scholar] [CrossRef] [PubMed]
- Fasanella, N.A.; Sundararaghavan, V. Atomistic Modeling of Thermal Conductivity of Epoxy Nanotube Composites. JOM 2016, 68, 1396–1410. [Google Scholar] [CrossRef]
- Yao, X.F. The Structural Characterization and Properties of SiO2–Epoxy Nanocomposites. Sens. Actuators B 2006, 204, 791–798. [Google Scholar] [CrossRef]
- Ponyrko, S.; Kobera, L.; Brus, J.; Matějka, L. Epoxy–silica hybrids by nonaqueous sol–gel process. Polymer 2013, 54, 6271–6282. [Google Scholar] [CrossRef]
- Kim, B.; Choi, J.; Yang, S.; Yu, S.; Cho, M. Influence of crosslink density on the interfacial characteristics of epoxy nanocomposites. Polymer 2015, 60, 186–197. [Google Scholar] [CrossRef]
- Wang, Z.; Lv, Q.; Chen, S.; Li, C.; Sun, S.; Hu, S. Effect of Interfacial Bonding on Interphase Properties in SiO2/Epoxy Nanocomposite: A Molecular Dynamics Simulation Study. ACS Appl. Mater. Interfaces 2016, 8, 7499–7508. [Google Scholar] [CrossRef] [PubMed]
- Griebel, M.; Hamaekers, J. Molecular dynamics simulations of the elastic moduli of polymer–carbon nanotube composites. Comput. Method Appl. Mech. 2004, 193, 1773–1788. [Google Scholar] [CrossRef]
- Feyel, F.; Chaboche, J.L. FE 2 multiscale approach for modeling the elastoviscoplastic behaviour of long fiber SiC/Ti composite materials. Comput. Method Appl. Mech. 2000, 183, 309–330. [Google Scholar] [CrossRef]
- Pozuelo, J.; Baselga, J. Glass transition temperature of low molecular weight poly(3-aminopropyl methyl siloxane). A molecular dynamics study. Polymer 2002, 43, 6049–6055. [Google Scholar] [CrossRef]
- Yu, S.; Yang, S.; Cho, M. Multi-scale modeling of cross-linked epoxy nanocomposites. Polymer 2009, 50, 945–952. [Google Scholar] [CrossRef]
- Agari, Y.; Uno, T. Estimation on Thermal Conductivities of Filled Polymers. J. Appl. Polym. Sci. 2010, 32, 5705–5712. [Google Scholar] [CrossRef]
- Zhang, M.M.; Lussetti, E.; de Souza, L.E.; Müllerplathe, F. Thermal Conductivities of Molecular Liquids by Reverse Nonequilibrium Molecular Dynamics. J. Phys. Chem. B 2005, 109, 15060–15067. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.P.; Haung, T.J.; Liao, C.H.; Chang, J.W. Investigation of thermal conductivity of graphite flake/poly (p–phenylene sulfide) composite by experimental measurement and non–equilibrium molecular dynamics simulation. Polymer 2013, 54, 4702–4709. [Google Scholar] [CrossRef]
- Choi, J.; Yang, S.; Yu, S.; Shin, H.; Cho, M. Method of scale bridging for thermoelasticity of cross-linked epoxy/SiC nanocomposites at a wide range of temperatures. Polymer 2012, 53, 5178–5189. [Google Scholar] [CrossRef]
- Sirk, T.W.; Khare, K.S.; Karim, M.; Lenhart, J.L.; Andzelm, J.W.; McKenna, G.B.; Khare, R. High strain rate mechanical properties of a cross-linked epoxy across the glass transition. Polymer 2013, 54, 7048–7057. [Google Scholar] [CrossRef]
- Zhang, X.X.; Wen, H.; Chen, X.Y.; Wu, Y.J.; Xiao, S. Study on the Thermal and Dielectric Properties of SrTiO3/Epoxy Nanocomposites. Energies 2017, 10, 692. [Google Scholar] [CrossRef]

| Structure models | 600 K | 500 K | 400 K | 300 K |
|---|---|---|---|---|
| EP | 31.25 | 30.90 | 30.63 | 30.55 |
| SiO2/EP | 40.71 | 40.09 | 39.61 | 39.41 |
| SiO2–5%/EP | 40.96 | 40.11 | 39.69 | 39.33 |
| SiO2–10%/EP | 40.58 | 40.04 | 39.52 | 39.32 |
| Structure models | 600 K | 500 K | 400 K | 300 K |
|---|---|---|---|---|
| EP | 1.015 | 1.081 | 1.098 | 1.13 |
| SiO2/EP | 1.108 | 1.164 | 1.207 | 1.225 |
| SiO2–5%/EP | 1.094 | 1.167 | 1.204 | 1.237 |
| SiO2–10%/EP | 1.124 | 1.17 | 1.217 | 1.235 |
| Structure models | Tg | Tg in Ref. | CTE below Tg (250–350 K) | CTE above Tg (450–650 K) | CTE below Tg in Ref. | CTE above Tg in Ref. |
|---|---|---|---|---|---|---|
| EP | 410 | 462 ± 9 [33] | 218 | 375 | 90 [41] | 188 [41] |
| SiO2/EP | 421 | 456 ± 11 [33] 414 [3] | 206 | 360 | ||
| SiO2–5%/EP | 427 | 198 | 321 | |||
| SiO2–10%/EP | 438 | 192 | 295 |
| Temperature | EP | SiO2/EP | SiO2–5%/EP | SiO2–10%/EP |
|---|---|---|---|---|
| 250 K | 0.201 | 0.265 | 0.323 | 0.340 |
| 300 K | 0.214 | 0.284 | 0.342 | 0.357 |
| 350 K | 0.225 | 0.301 | 0.368 | 0.379 |
| 400 K | 0.231 | 0.332 | 0.377 | 0.410 |
| 450 K | 0.242 | 0.350 | 0.404 | 0.431 |
| Temperature | SiO2/EP | SiO2–5%/EP | SiO2–10%/EP |
|---|---|---|---|
| 300 K | −423 | −461 | −585 |
| 400 K | −347 | −414 | −531 |
| 500 K | −345 | −384 | −481 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wen, H.; Wu, Y. Computational Thermomechanical Properties of Silica–Epoxy Nanocomposites by Molecular Dynamic Simulation. Polymers 2017, 9, 430. https://doi.org/10.3390/polym9090430
Zhang X, Wen H, Wu Y. Computational Thermomechanical Properties of Silica–Epoxy Nanocomposites by Molecular Dynamic Simulation. Polymers. 2017; 9(9):430. https://doi.org/10.3390/polym9090430
Chicago/Turabian StyleZhang, Xiaoxing, Hao Wen, and Yunjian Wu. 2017. "Computational Thermomechanical Properties of Silica–Epoxy Nanocomposites by Molecular Dynamic Simulation" Polymers 9, no. 9: 430. https://doi.org/10.3390/polym9090430