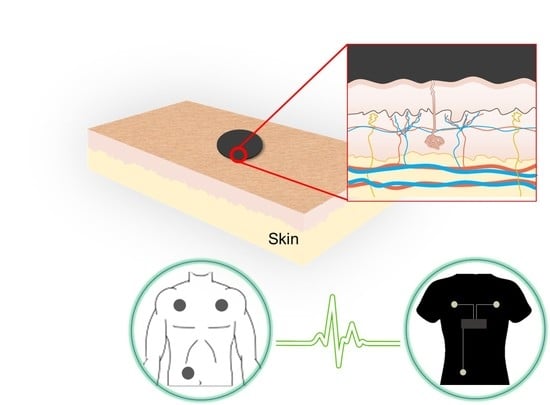
ECG Monitoring Garment Using Conductive Carbon Paste for Reduced Motion Artifacts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of C-Paste
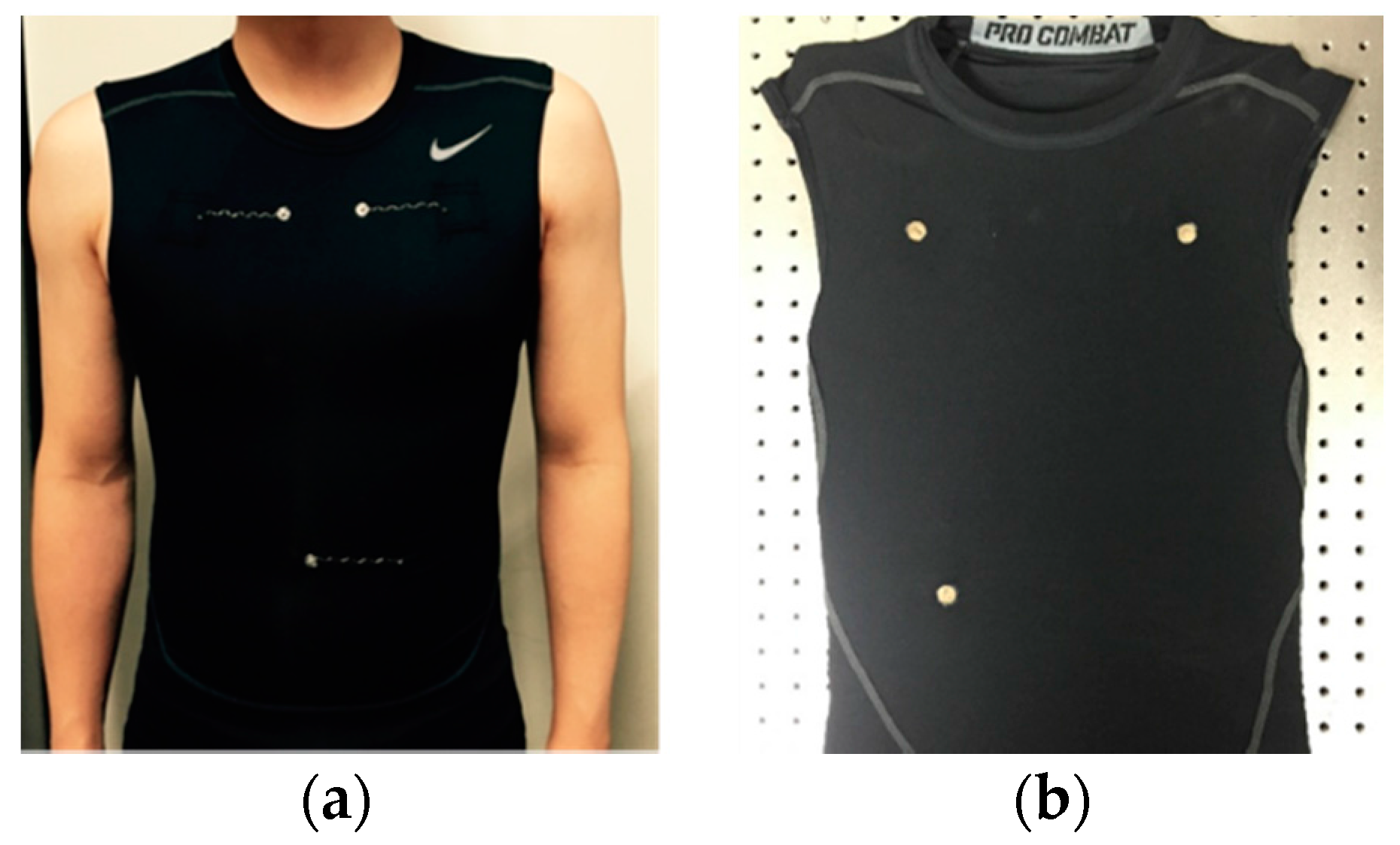
2.2. Preparation of Electrocardiogram (ECG) Measuring Garment
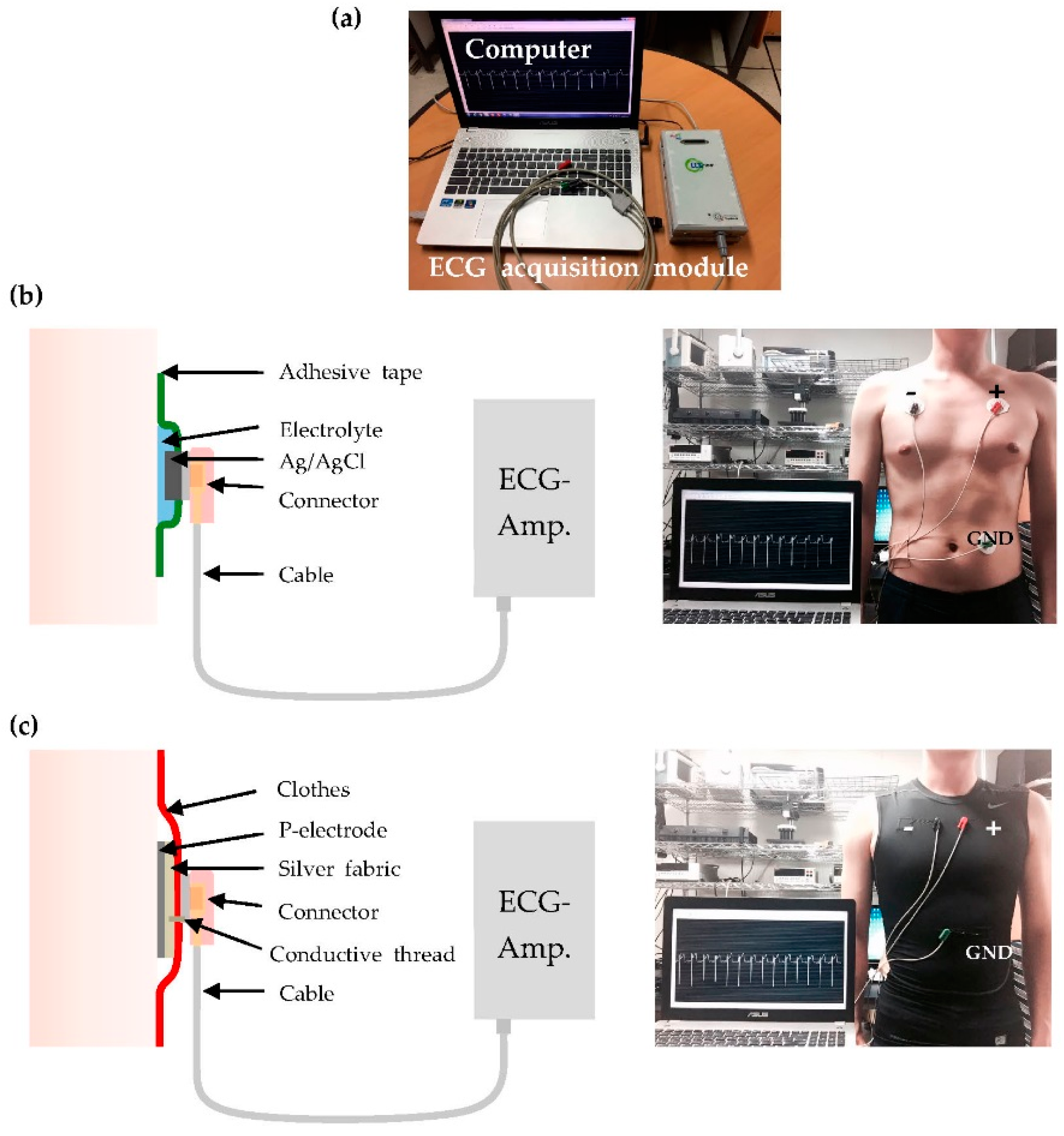
2.3. Measurement Setup
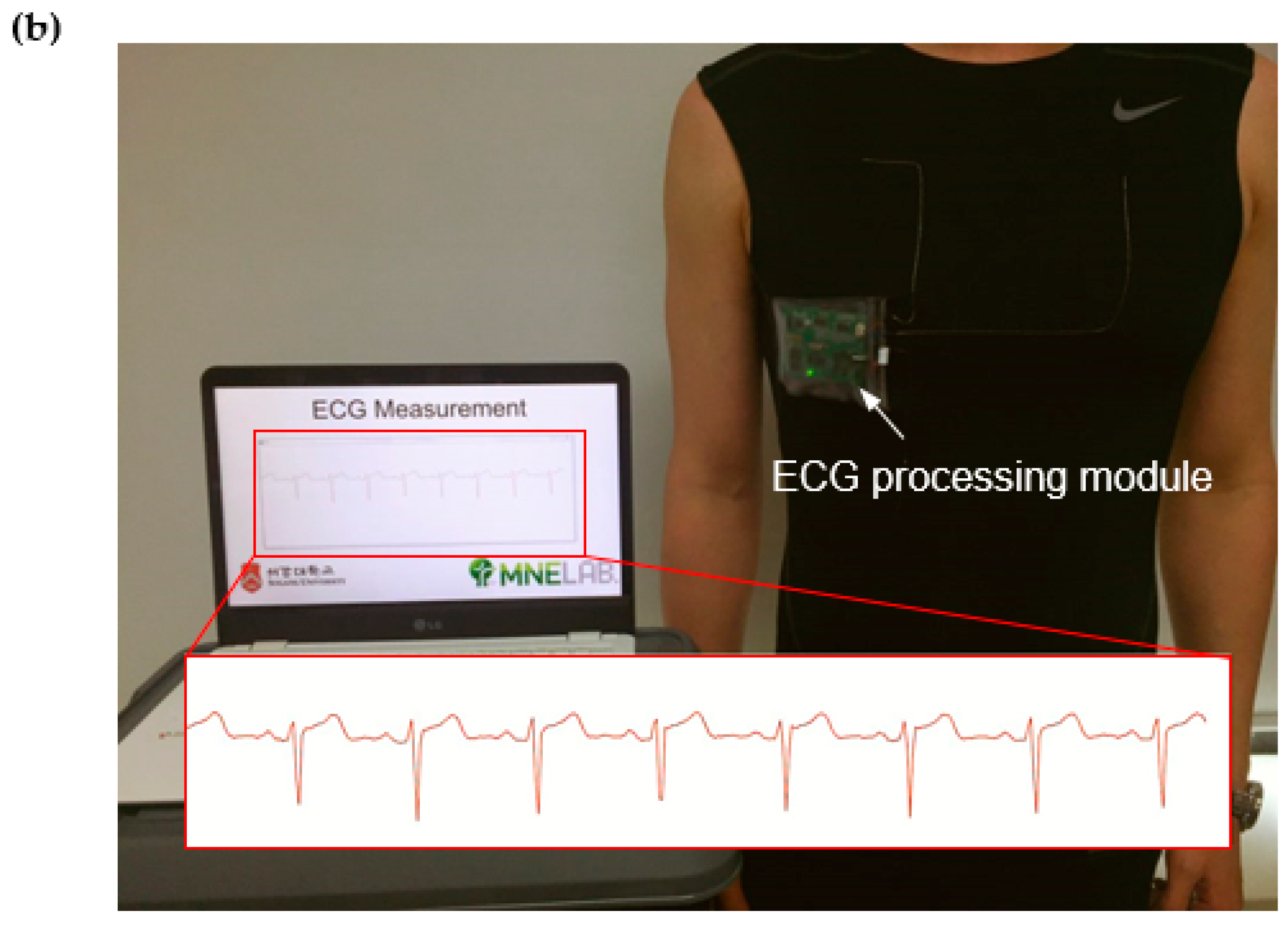
3. Results and Discussion
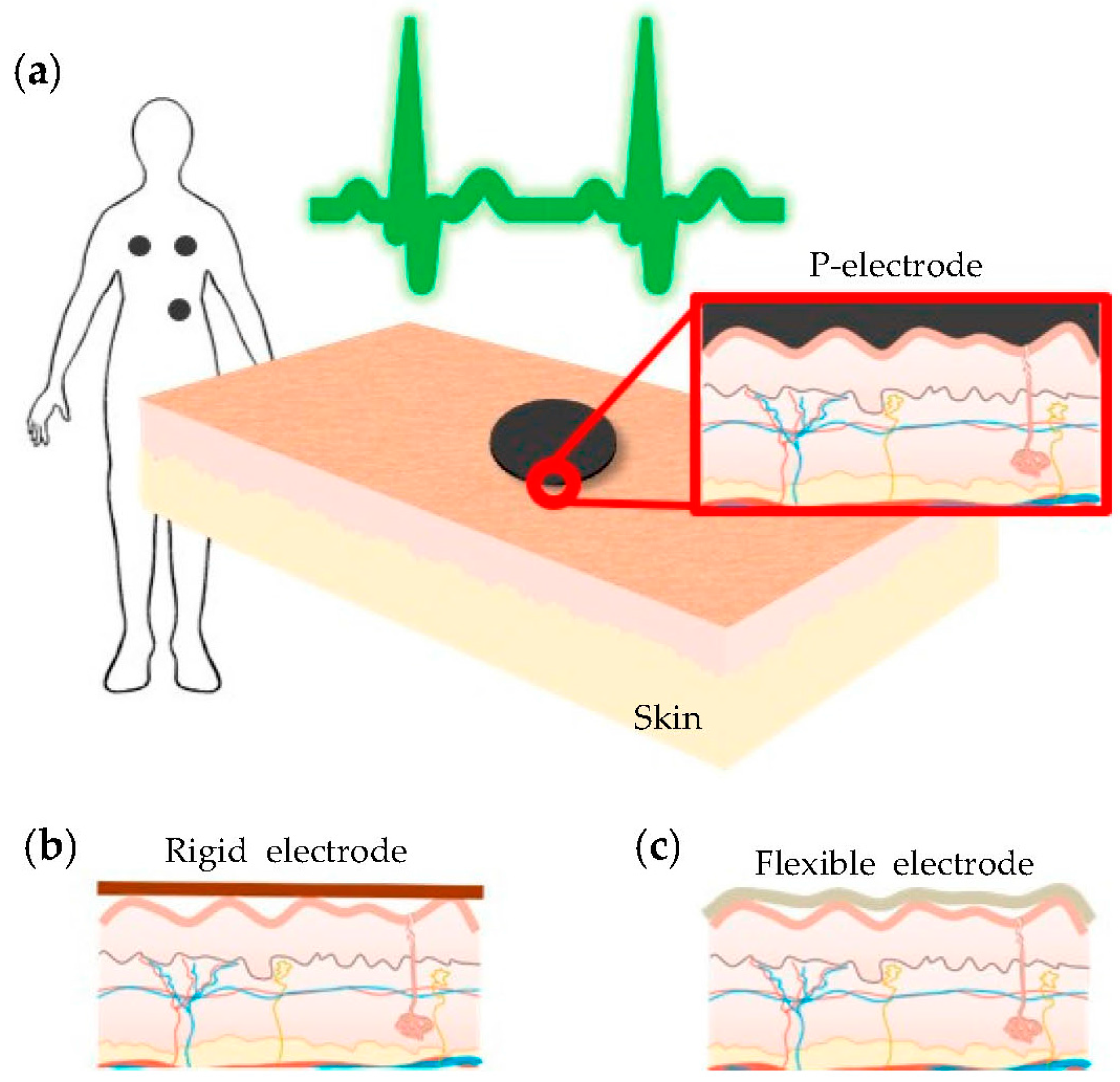
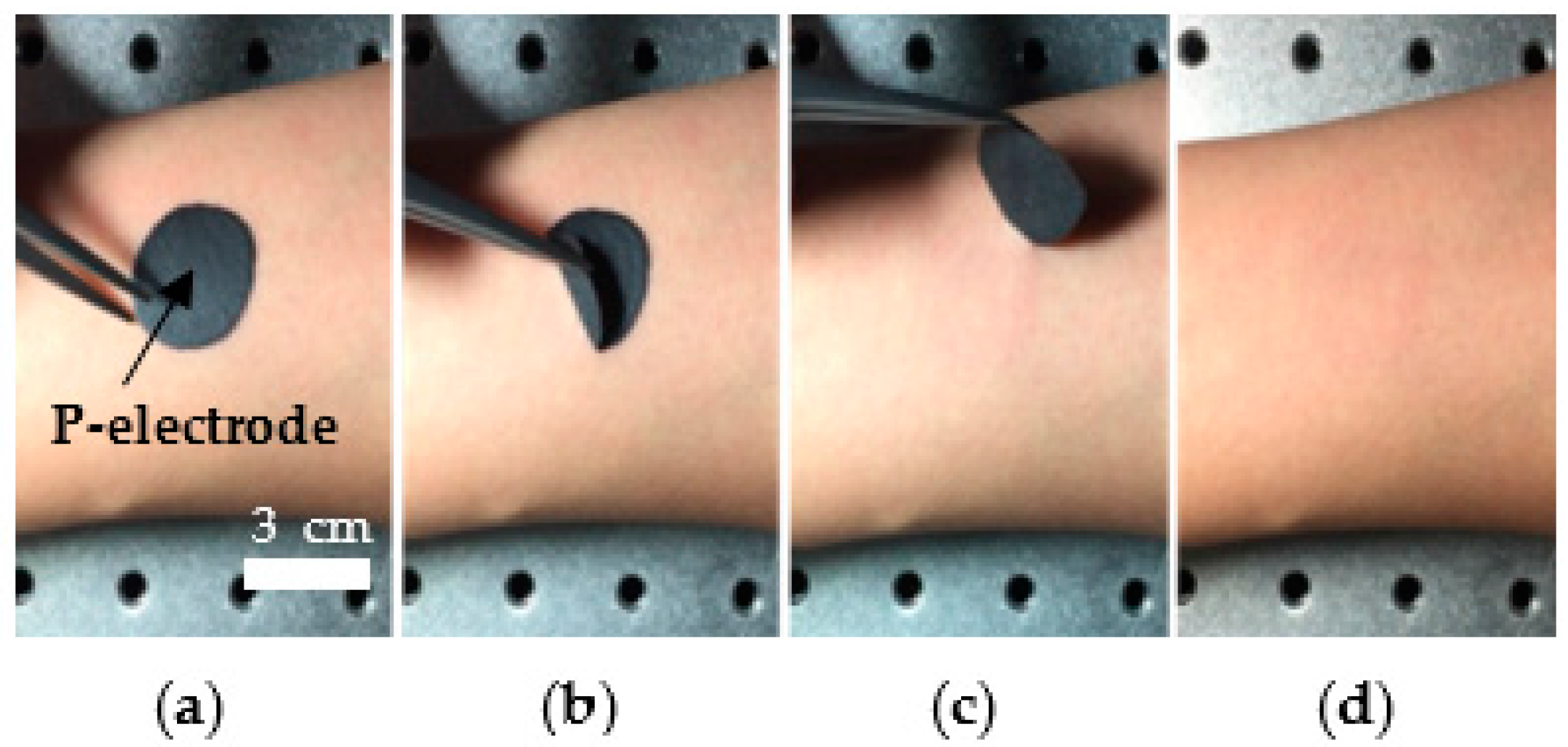
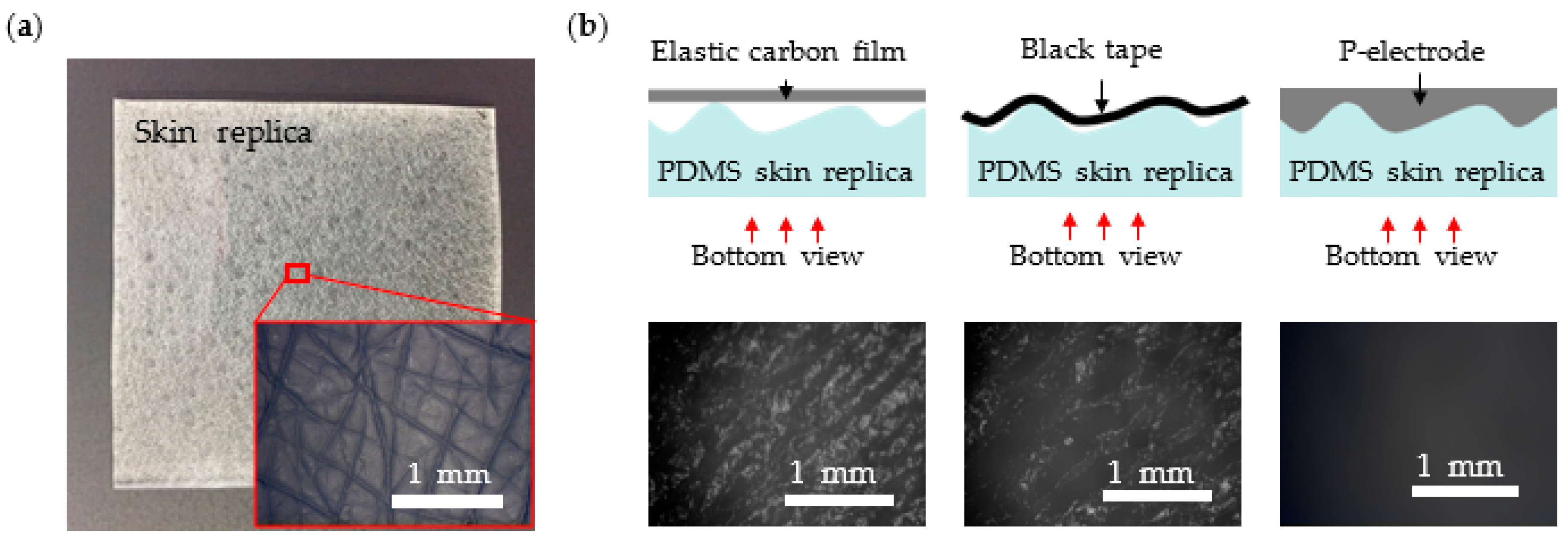
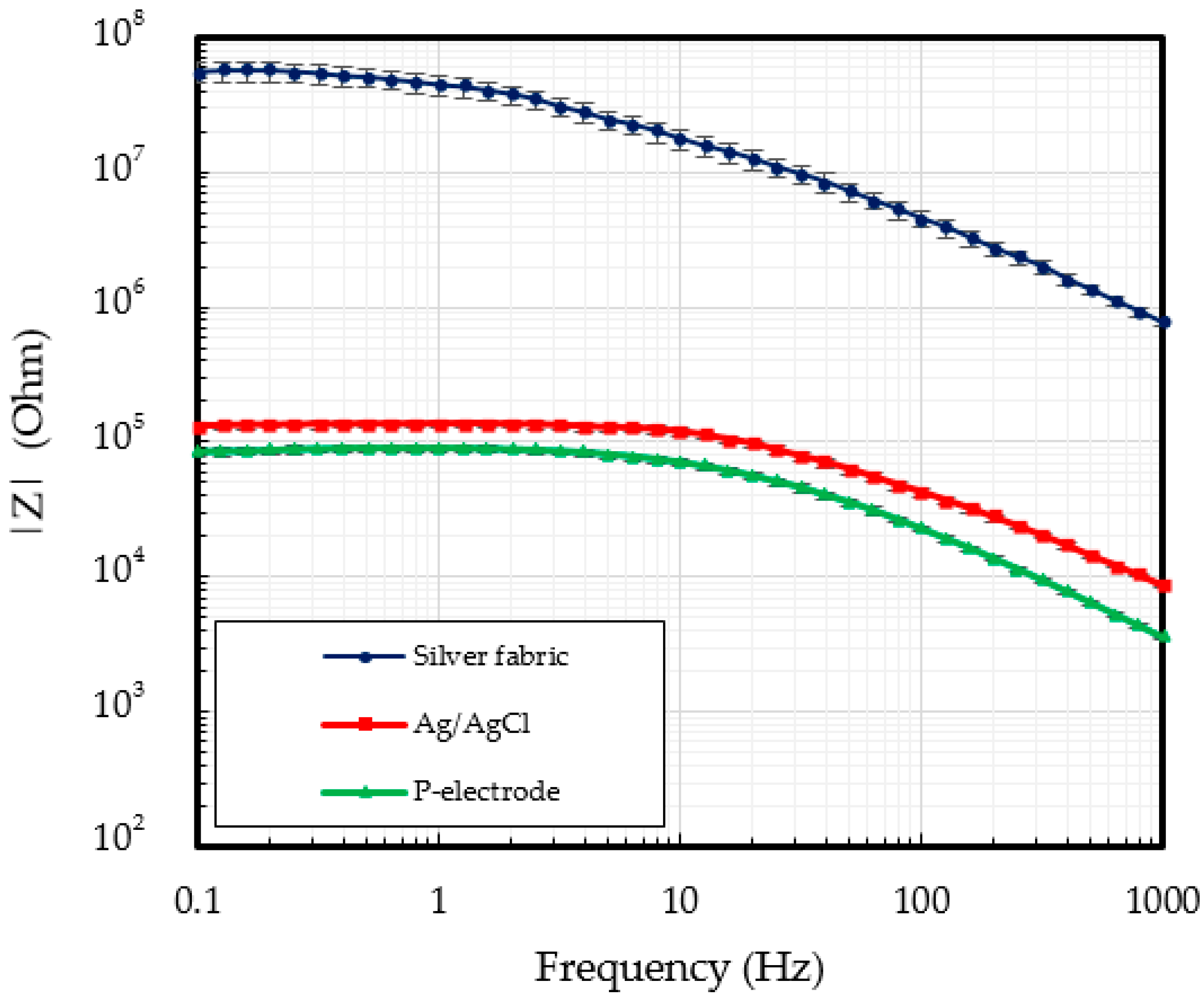
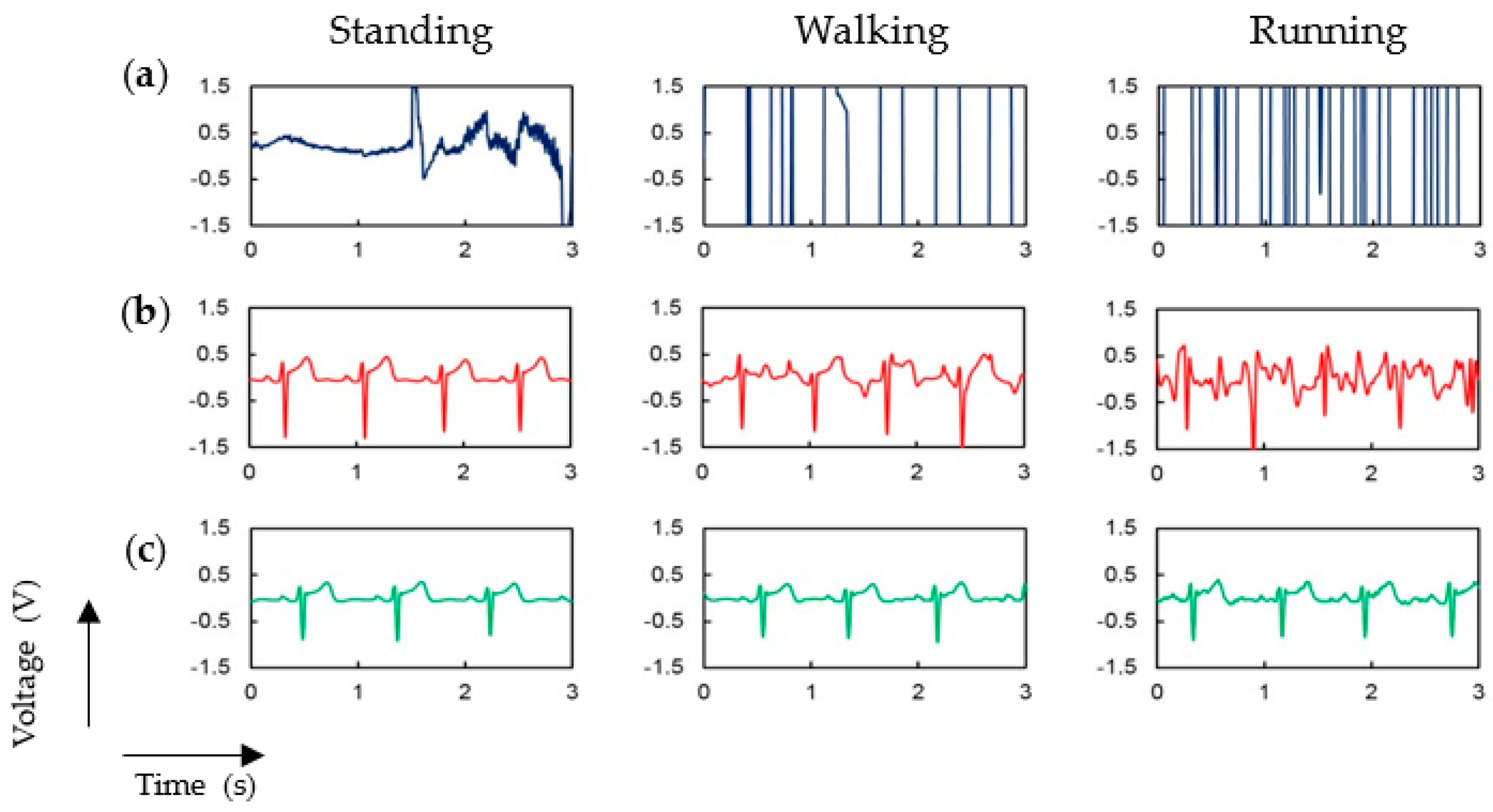
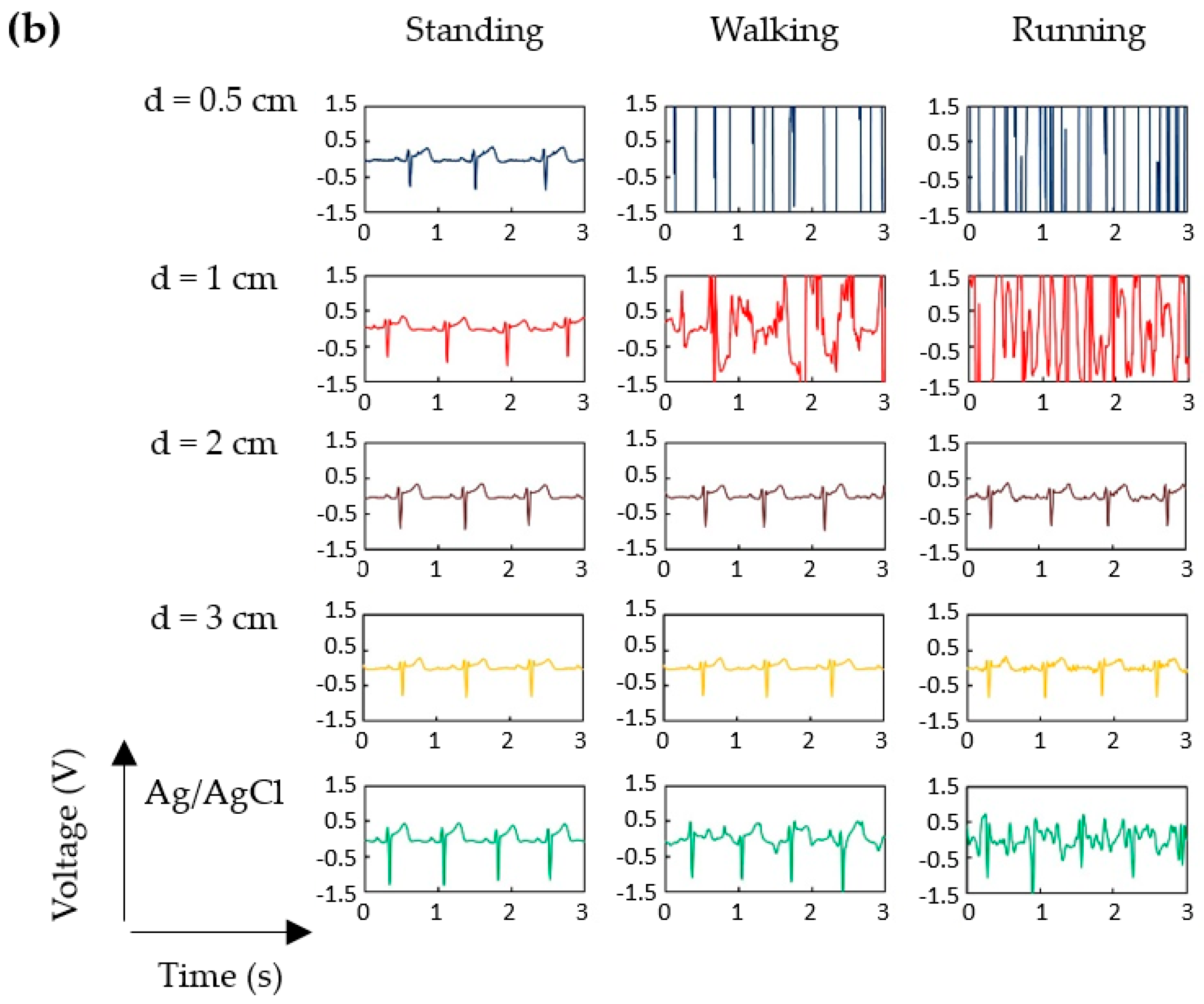
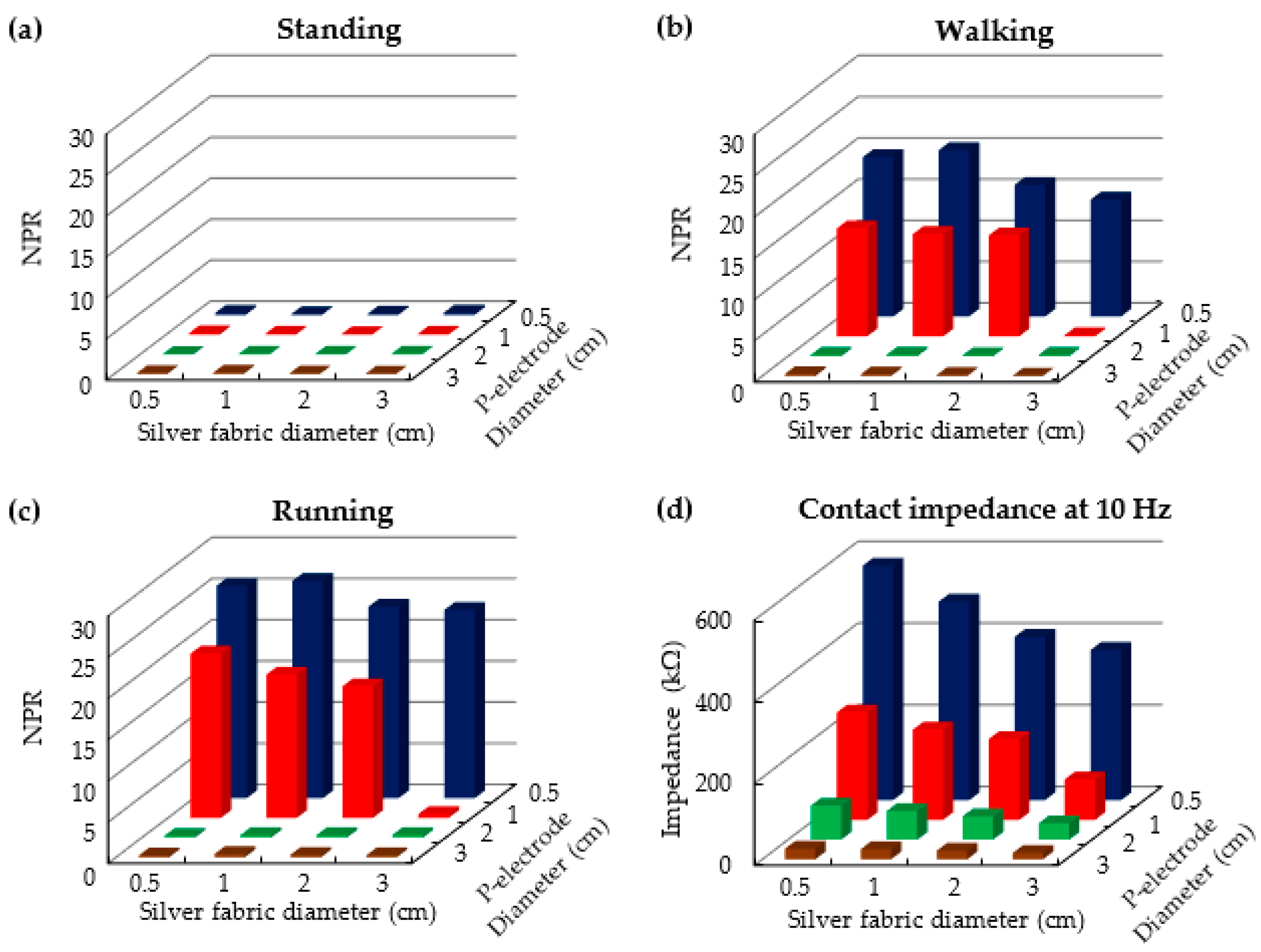
3.1. P-Electrode and Its Contact Properties with Skin
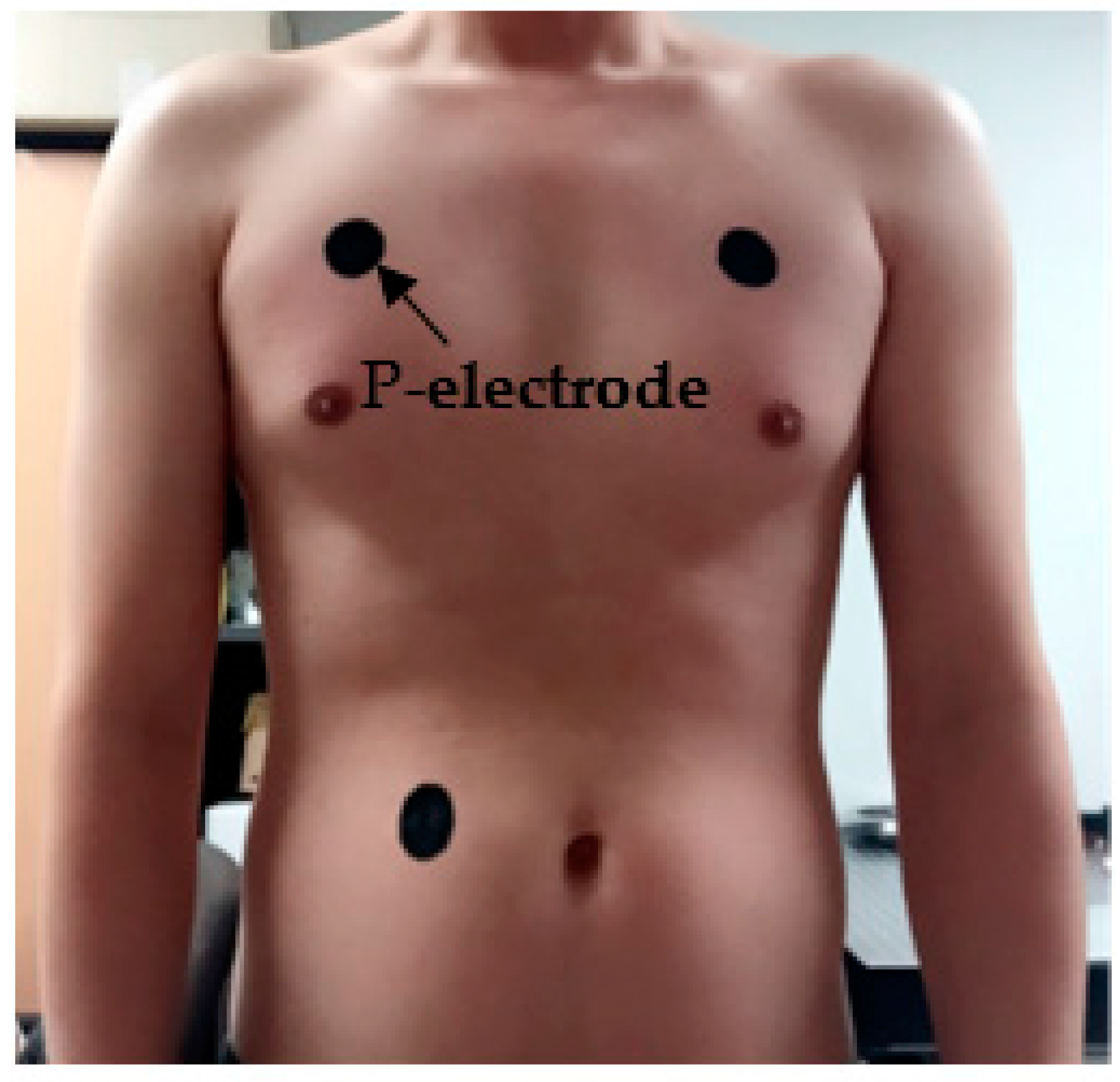
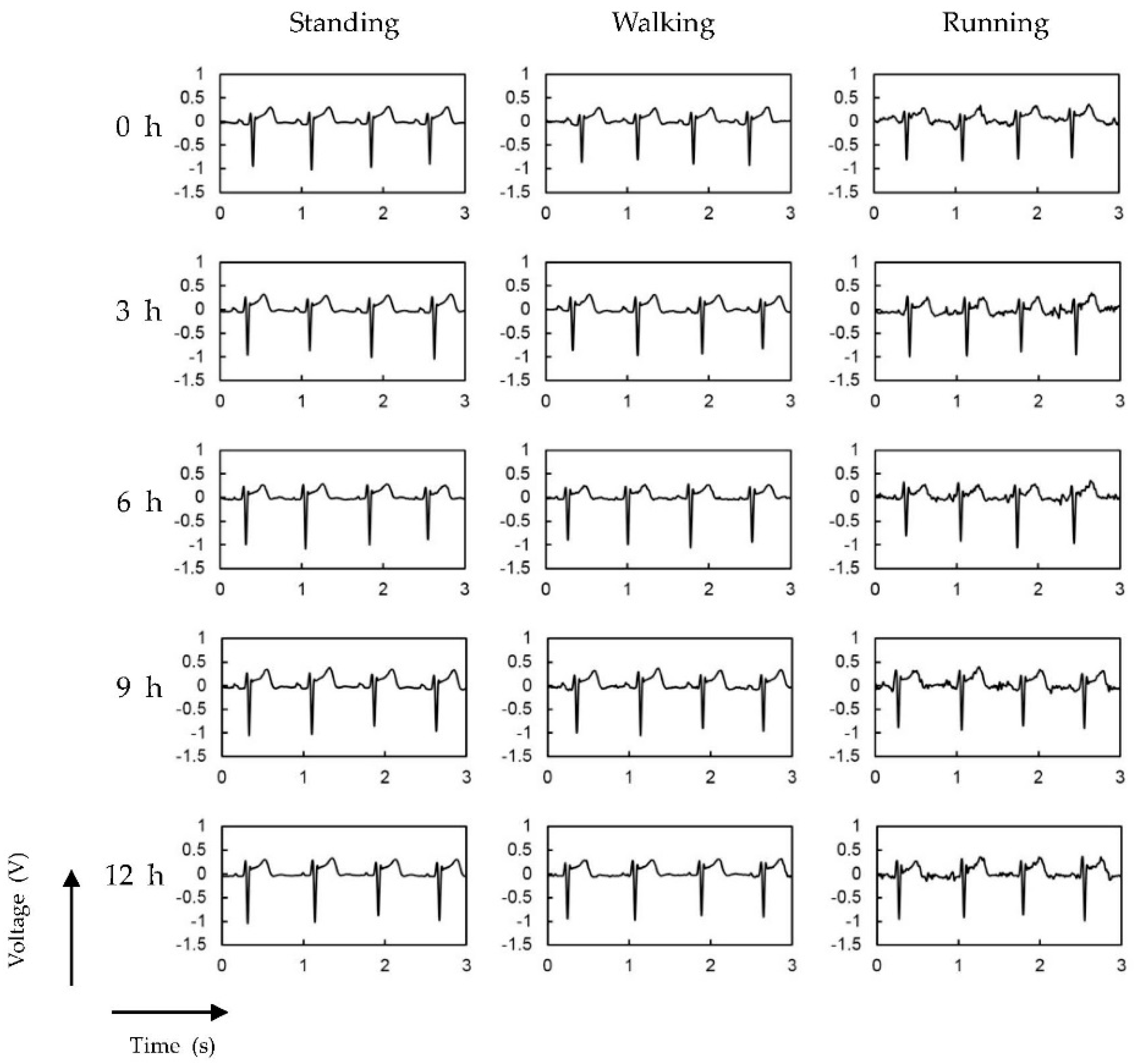
3.2. ECG Measurement Results
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zheng, Y.; Ding, X.; Poon, C.C.Y.; Lo, B.P.L.; Zhang, H.; Zhou, X.; Yang, G.; Zhao, N.; Zhang, Y. Unobtrusive Sensing and Wearable Devices for Health Informatics. IEEE Trans. Biomed. Eng. 2014, 61, 1538–1554. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Son, D.; Lee, M.; Song, C.; Song, J.; Koo, J.H.; Lee, D.J.; Shim, H.J.; Kim, J.H.; Lee, M. A Wearable Multiplexed Silicon Nonvolatile Memory Array using Nanocrystal Charge Confinement. Sci. Adv. 2016, 2, e1501101. [Google Scholar] [CrossRef] [PubMed]
- Lorwongtragool, P.; Sowade, E.; Watthanawisuth, N.; Baumann, R.R.; Kerdcharoen, T. A Novel Wearable Electronic Nose for Healthcare Based on Flexible Printed Chemical Sensor Array. Sensors 2014, 14, 19700–19712. [Google Scholar] [CrossRef] [PubMed]
- Klingeberg, T.; Schilling, M. Mobile Wearable Device for Long Term Monitoring of Vital Signs. Comput. Methods Programs Biomed. 2012, 106, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Oresko, J.J.; Jin, Z.; Cheng, J.; Huang, S.; Sun, Y.; Duschl, H.; Cheng, A.C. A Wearable Smartphone-Based Platform for Real-Time Cardiovascular Disease Detection via Electrocardiogram Processing. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lu, N.; Ma, R.; Kim, Y.S.; Kim, R.H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A.; et al. Epidermal Electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Zucca, A.; Cipriani, C.; Tarantino, S.; Ricci, D.; Mattoli, V.; Greco, F. Tattoo Conductive Polymer Nanosheets for Skin-Contact Applications. Adv. Healthc. Mater. 2015, 4, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.H.; Choi, Y.Y.; Kim, D.J.; Bien, F.; Kim, J.J. Tissue-Informative Mechanism for Wearable Non-Invasive Continuous Blood Pressure Monitoring. Sci. Rep. 2014, 4, 6618. [Google Scholar] [CrossRef] [PubMed]
- Honda, W.; Harada, S.; Arie, T.; Akita, S.; Takei, K. Wearable, Human-Interactive, Health-Monitoring, Wireless Devices Fabricated by Macroscale Printing Techniques. Adv. Funct. Mater. 2014, 24, 3299–3304. [Google Scholar] [CrossRef]
- Yeo, W.; Kim, Y.; Lee, J.; Ameen, A.; Shi, L.; Li, M.; Wang, S.; Ma, R.; Jin, S.H.; Kang, Z. Multifunctional Epidermal Electronics Printed Directly Onto the Skin. Adv. Mater. 2013, 25, 2773–2778. [Google Scholar] [CrossRef] [PubMed]
- Clayton, R.; Murray, A.; Campbell, R.W. Recognition of Ventricular Fibrillation using Neural Networks. Med. Biol. Eng. Comput. 1994, 32, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Barro, S.; Ruiz, R.; Cabello, D.; Mira, J. Algorithmic Sequential Decision-Making in the Frequency Domain for Life Threatening Ventricular Arrhythmias and Imitative Artefacts: A Diagnostic System. J. Biomed. Eng. 1989, 11, 320–328. [Google Scholar] [CrossRef]
- Sangwatanaroj, S.; Prechawat, S.; Sunsaneewitayakul, B.; Sitthisook, S.; Tosukhowong, P.; Tungsanga, K. New Electrocardiographic Leads and the Procainamide Test for the Detection of the Brugada Sign in Sudden Unexplained Death Syndrome Survivors and their Relatives. Eur. Heart J. 2001, 22, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Wung, S.; Drew, B. Comparison of 18-Lead ECG and Selected Body Surface Potential Mapping Leads in Determining Maximally Deviated ST Lead and Efficacy in Detecting Acute Myocardial Ischemia during Coronary Occlusion. J. Electrocardiol. 1999, 32, 30–37. [Google Scholar] [CrossRef]
- Nikolic, G.; Bishop, R.L.; Singh, J.B. Sudden Death Recorded during Holter Monitoring. Circulation 1982, 66, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Cheng, C.; Lu, C.; Huang, C.; Chen, Y.; Lin, Y. Adjustable 60 Hz Noise Reduction and ECG Signal Amplification of a Remote Electrocardiogram System. System 2003, 18, 141. [Google Scholar] [CrossRef]
- Chi, Y.M.; Jung, T.; Cauwenberghs, G. Dry-Contact and Noncontact Biopotential Electrodes: Methodological Review. IEEE Rev. Biomed. Eng. 2010, 3, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zhu, Y. Nanomaterial-Enabled Dry Electrodes for Electrophysiological Sensing: A Review. JOM 2016, 68, 1145–1155. [Google Scholar] [CrossRef]
- Searle, A.; Kirkup, L. A Direct Comparison of Wet, Dry and Insulating Bioelectric Recording Electrodes. Physiol. Meas. 2000, 21, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Forvi, E.; Bedoni, M.; Carabalona, R.; Soncini, M.; Mazzoleni, P.; Rizzo, F.; O’Mahony, C.; Morasso, C.; Cassarà, D.G.; Gramatica, F. Preliminary Technological Assessment of Microneedles-Based Dry Electrodes for Biopotential Monitoring in Clinical Examinations. Sens. Actuators A Phys. 2012, 180, 177–186. [Google Scholar] [CrossRef]
- Kabiri Ameri, S.; Ho, R.; Jang, H.; Tao, L.; Wang, Y.; Wang, L.; Schnyer, D.M.; Akinwande, D.; Lu, N. Graphene Electronic Tattoo Sensors. ACS Nano 2017, 11, 7634–7641. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.C.; Huang, H.; Zhu, Y. Wearable Silver Nanowire Dry Electrodes for Electrophysiological Sensing. RSC Adv. 2015, 5, 11627–11632. [Google Scholar] [CrossRef]
- Peng, H.; Liu, J.; Dong, Y.; Yang, B.; Chen, X.; Yang, C. Parylene-Based Flexible Dry Electrode for Bioptential Recording. Sens. Actuators B Chem. 2016, 231, 1–11. [Google Scholar] [CrossRef]
- Yao, S.; Myers, A.; Malhotra, A.; Lin, F.; Bozkurt, A.; Muth, J.F.; Zhu, Y. A Wearable Hydration Sensor with Conformal Nanowire Electrodes. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Yapici, M.K.; Alkhidir, T.; Samad, Y.A.; Liao, K. Graphene-Clad Textile Electrodes for Electrocardiogram Monitoring. Sens. Actuators B Chem. 2015, 221, 1469–1474. [Google Scholar] [CrossRef]
- Takamatsu, S.; Lonjaret, T.; Crisp, D.; Badier, J.M.; Malliaras, G.G.; Ismailova, E. Direct Patterning of Organic Conductors on Knitted Textiles for Long-Term Electrocardiography. Sci. Rep. 2015, 5, 15003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bihar, E.; Roberts, T.; Ismailova, E.; Saadaoui, M.; Isik, M.; Sanchez-Sanchez, A.; Mecerreyes, D.; Hervé, T.; De Graaf, J.B.; Malliaras, G.G. Fully Printed Electrodes on Stretchable Textiles for Long-Term Electrophysiology. Adv. Mater. Techonol. 2017, 2. [Google Scholar] [CrossRef]
- Liu, B.; Luo, Z.; Zhang, W.; Tu, Q.; Jin, X. Carbon Nanotube-Based Self-Adhesive Polymer Electrodes for Wireless Long-Term Recording of Electrocardiogram Signals. J. Biomater. Sci. Polym. Ed. 2016, 27, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Liu, J.; Tian, H.; Xu, B.; Dong, Y.; Yang, B.; Chen, X.; Yang, C. Flexible Dry Electrode Based on Carbon nanotube/polymer Hybrid Micropillars for Biopotential Recording. Sens. Actuators A Phys. 2015, 235, 48–56. [Google Scholar] [CrossRef]
- Jung, H.; Moon, J.; Baek, D.; Lee, J.; Choi, Y.; Hong, J.; Lee, S. CNT/PDMS Composite Flexible Dry Electrodes for Long-Term ECG Monitoring. IEEE Trans. Biomed. Eng. 2012, 59, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Park, J.; Sohn, J.; Cho, D.; Jeon, S. Bioinspired, Highly Stretchable, and Conductive Dry Adhesives Based on 1D–2D Hybrid Carbon Nanocomposites for all-in-One ECG Electrodes. ACS Nano 2016, 10, 4770–4778. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Luo, Z.; Zhang, W.; Tu, Q.; Jin, X. Silver Nanowire-Composite Electrodes for Long-Term Electrocardiogram Measurements. Sens. Actuators A Phys. 2016, 247, 459–464. [Google Scholar] [CrossRef]
- Lee, S.M.; Byeon, H.J.; Lee, J.H.; Baek, D.H.; Lee, K.H.; Hong, J.S.; Lee, S. Self-Adhesive Epidermal Carbon Nanotube Electronics for Tether-Free Long-Term Continuous Recording of Biosignals. Sci. Rep. 2014, 4, 6074. [Google Scholar] [CrossRef] [PubMed]
- Cardu, R.; Leong, P.H.; Jin, C.T.; McEwan, A. Electrode Contact Impedance Sensitivity to Variations in Geometry. Physiol. Meas. 2012, 33, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Kaitainen, S.; Kutvonen, A.; Suvanto, M.; Pakkanen, T.T.; Lappalainen, R.; Myllymaa, S. Liquid Silicone Rubber (LSR)-Based Dry Bioelectrodes: The Effect of Surface Micropillar Structuring and Silver Coating on Contact Impedance. Sens. Actuators A Phys. 2014, 206, 22–29. [Google Scholar] [CrossRef]
- Jonathan, E. In Vivo Sweat Film Layer Thickness Measured with Fourier-Domain Optical Coherence Tomography (FD-OCT). Opt. Lasers Eng. 2008, 46, 424–427. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-W.; Yun, K.-S. ECG Monitoring Garment Using Conductive Carbon Paste for Reduced Motion Artifacts. Polymers 2017, 9, 439. https://doi.org/10.3390/polym9090439
Lee J-W, Yun K-S. ECG Monitoring Garment Using Conductive Carbon Paste for Reduced Motion Artifacts. Polymers. 2017; 9(9):439. https://doi.org/10.3390/polym9090439
Chicago/Turabian StyleLee, Jin-Woo, and Kwang-Seok Yun. 2017. "ECG Monitoring Garment Using Conductive Carbon Paste for Reduced Motion Artifacts" Polymers 9, no. 9: 439. https://doi.org/10.3390/polym9090439