Thermoresponsive and Reducible Hyperbranched Polymers Synthesized by RAFT Polymerisation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Bis(thiobenzoyl) Disulfide, (2-Cyanoprop-2-yl Dithiobenzoate), and Disulfide Diacrylate (DSDA)
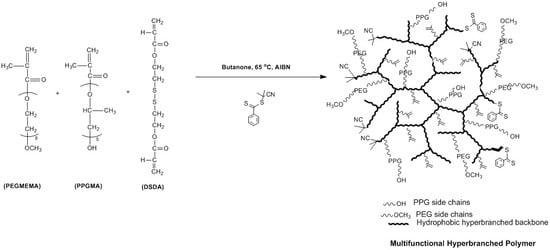
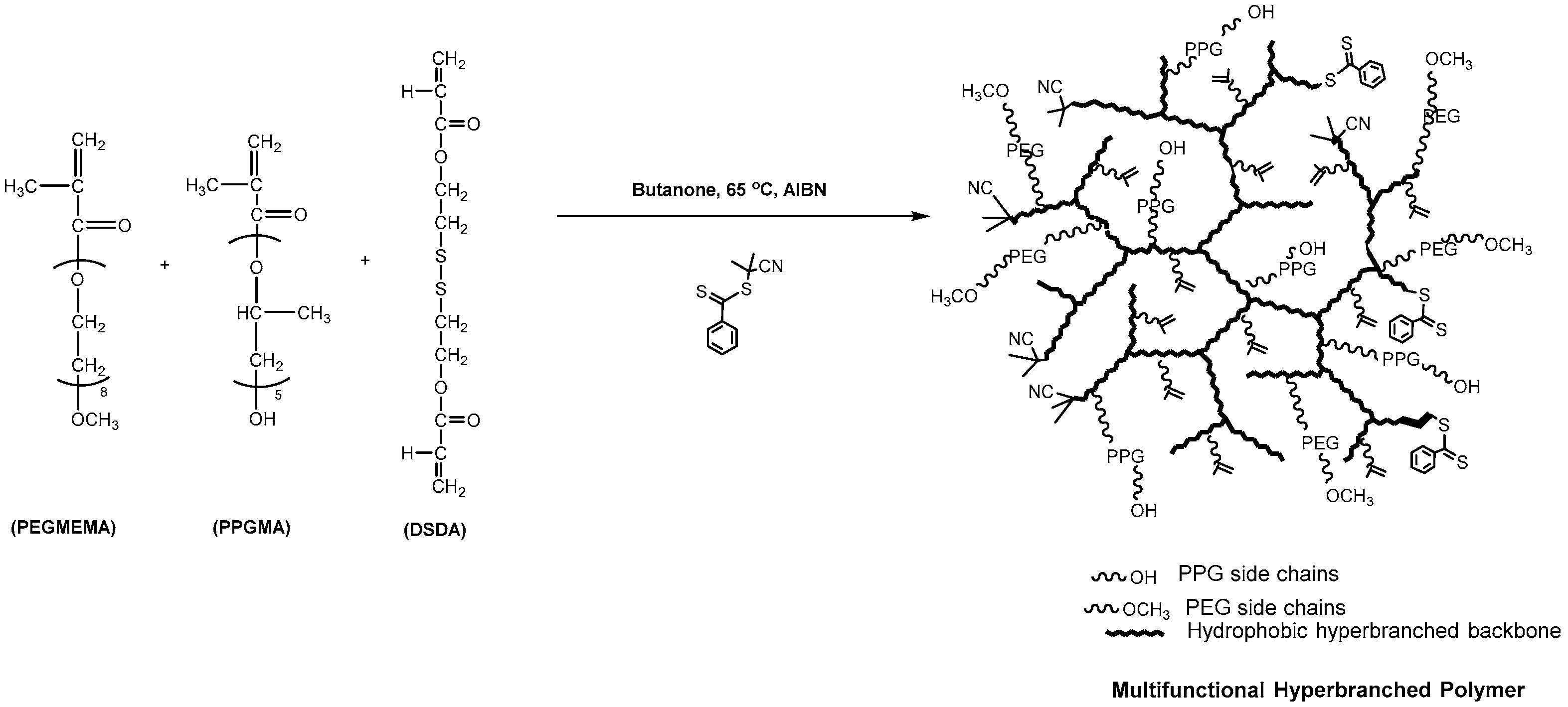
2.2.2. Preparation of PEGMEMA–PPGMA–DSDA Copolymers via One-Pot RAFT Polymerisation
2.2.3. Characterisation of PEGMEMA–PPGMA–DSDA Copolymers
2.2.4. Fabrication of Hydrogels by Thermal Gelation
2.2.5. Fabrication of Hydrogels by Michael Addition Reaction
2.2.6. Swelling Studies
2.2.7. Reducibility Studies
3. Results and Discussion
3.1. Synthesis and Characterisation of Reducible and Thermoresponsive Hyperbranched Polymers via One-Pot RAFT Polymerisation
3.2. Determination of Lower Critical Solution Temperatures (LCST) and the Formation of Physical Gels
3.3. Fabrication of Chemical Cross-Linked Hydrogels from Thiol-Ene Michael Addition Reaction Using PEGMEMA–PPGMA–DSDA Copolymers
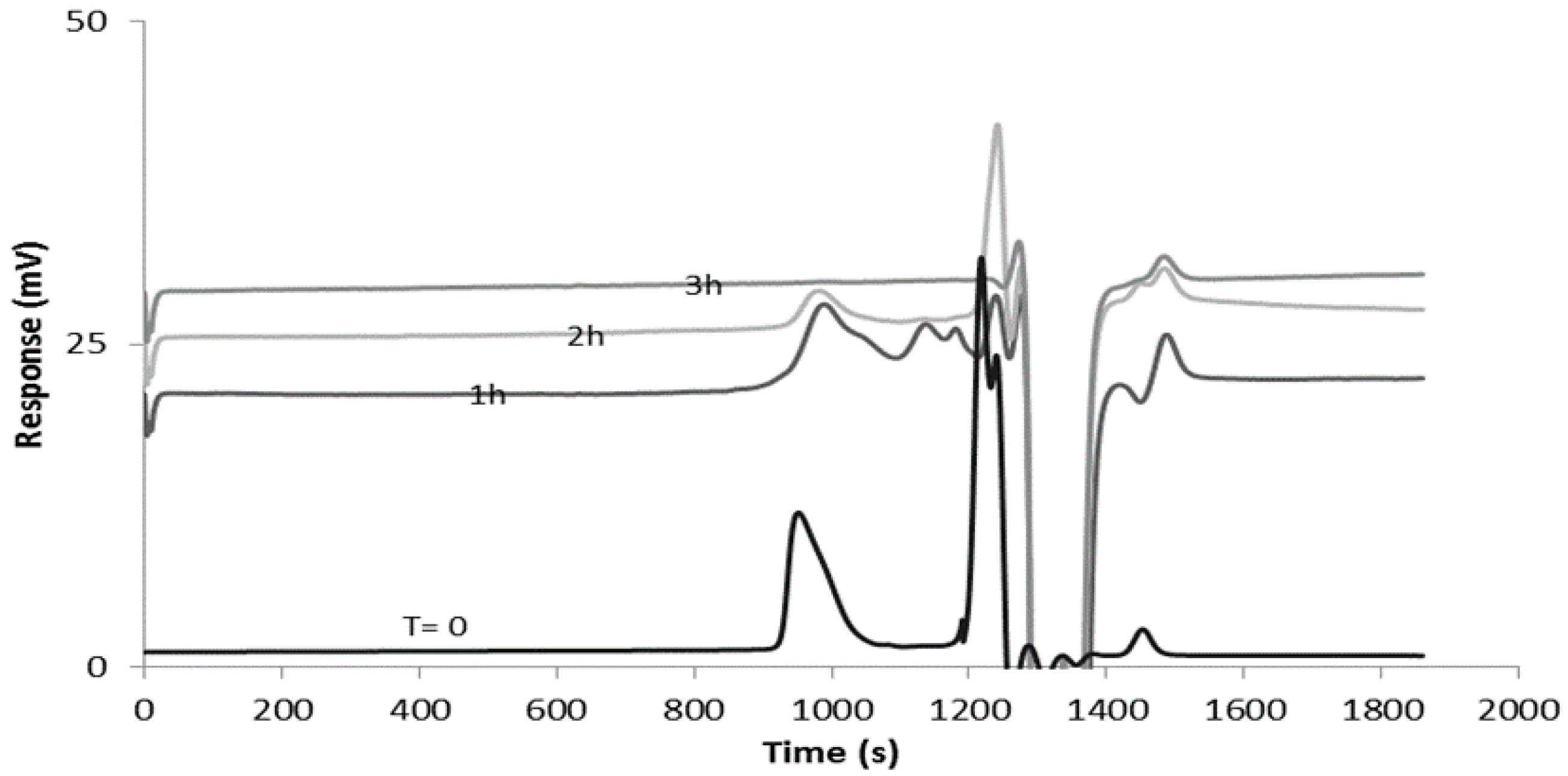
3.4. Swelling
3.5. Reducibility
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tomalia, D.A.; Fréchet, J.M.J. Discovery of dendrimers and dendritic polymers: A brief historical perspective. Polym. Chem. A 2002, 40, 2719–2728. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, T.; Zhu, X.; Yan, D.; Wang, W. Bioapplications of hyperbranched polymers. Chem. Soc. Rev. 2015, 44, 4023–4071. [Google Scholar] [CrossRef] [PubMed]
- Voit, B.I.; Lederer, A. Hyperbranched and highly branched polymer architectures—Synthetic strategies and major characterization aspects. Chem. Rev. 2009, 109, 5924–5973. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, D. Supramolecular self-assembly of amphiphilic hyperbranched polymers at all scales and dimensions: Progress, characteristics and perspectives. Chem. Commun. 2009, 10, 1172–1188. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.Y.; Huang, W.; Liu, J.; Zhu, X.; Yan, D. Self-assembly of hyperbranched polymers and its biomedical applications. Adv. Mater. 2010, 22, 4567–4590. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, H. Recent Progress on Hyperbranched polymers synthesized via radical-based self-condensing vinyl polymerization. Polymers 2017, 9, 188. [Google Scholar] [CrossRef]
- Fairbanks, B.D.; Gunatillake, P.A.; Meagher, L. Biomedical applications of polymers derived by reversible addition—Fragmentation chain-transfer (RAFT). Adv. Drug Deliv. Rev. 2015, 91, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J. Molecular size distribution in three dimensional polymers. I. Gelation. J. Am. Chem. Soc. 1941, 63, 3083–3090. [Google Scholar] [CrossRef]
- Matsumoto, A. Free-radical crosslinking polymerization and copolymerization of multivinyl compounds. Synth. Photosynth. 1995, 123, 41–80. [Google Scholar]
- Li, W.W.; Gao, H.F.; Matyjaszewski, K. Influence of initiation efficiency and polydispersity of primary chains on gelation during atom transfer radical copolymerization of monomer and cross-Linker. Macromolecules 2009, 42, 927–932. [Google Scholar] [CrossRef]
- England, R.M.; Rimmer, S. Hyper/highly-branched polymers by radical polymerisations. Polym. Chem. 2010, 10, 1533–1544. [Google Scholar] [CrossRef]
- Smith, D.; Holley, A.C.; McCormick, C.L. RAFT-synthesized copolymers and conjugates designed for therapeutic delivery of siRNA. Polym. Chem. 2011, 2, 1428–1441. [Google Scholar] [CrossRef]
- Chernikova, E.V.; Sivtsov, E.V. Reversible addition-fragmentation chain-transfer polymerization: Fundamentals and use in practice. Polym. Sci. Ser. B 2017, 59, 117–146. [Google Scholar] [CrossRef]
- Boyer, C.; Bulmus, V.; Davis, T.P.; Ladmiral, V.; Liu, J. Bioapplications of RAFT polymerization. Chem. Rev. 2009, 109, 5402–5436. [Google Scholar] [CrossRef] [PubMed]
- Nicolaÿ, R.; Kwak, Y.; Matyjaszewski, K. A green route to well-defined high-molecular-weight (co)polymers using ARGET ATRP with alkyl pseudohalides and copper catalysis. Angew. Chem. Int. Ed. 2009, 49, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wu, J.; Zhang, L.; Cheng, Z.; Zhu, X. Highly active ppm level organic copper catalyzed photo-induced ICAR ATRP of methyl methacrylate. Macromol. Rapid Commun. 2014, 35, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Chmielarz, P.; Fantin, M.; Park, S.; Isse, A.A.; Gennaro, A.; Magenau, A.J.D.; Sobkowiak, A.; Matyjaszewski, K. Progress in polymer science electrochemically mediated atom transfer radical polymerization (eATRP). Prog. Polym. Sci. 2017, 69, 47–78. [Google Scholar] [CrossRef]
- Tonhauser, C.; Natalello, A.; Löwe, H.; Frey, H. Microflow technology in polymer synthesis. Macromolecules 2012, 45, 9551–9570. [Google Scholar] [CrossRef]
- Vandenbergh, J.; Junkers, T. Use of a continuous-flow microreactor for thiol–ene functionalization of RAFT-derived poly(butyl acrylate). Polym. Chem. 2012, 3, 2739–2742. [Google Scholar] [CrossRef]
- Derboven, P.; Van Steenberge, P.H.M.; Vandenbergh, J.; Reyniers, M.-F.; Junkers, T.; Dagmar, R.D.; Marin, G.B. Improved livingness and control over branching in RAFT polymerization of acrylates: Could microflow synthesis make the difference? Macromol. Rapid Commun. 2015, 36, 2149–2155. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Bai, T.; Xue, X.; Huang, W.; Chen, J.; Qian, X.; Zhang, G.; Jiang, B. A simple route to vinyl-functionalized hyperbranched polymers: Self-condensing anionic copolymerization of allyl methacrylate and hydroxyethyl methacrylate. Polymer 2015, 72, 63–68. [Google Scholar] [CrossRef]
- Liu, X.; Bao, Y.; Tang, X.; Li, Y. Synthesis of hyperbranched polymers via a facile self-condensing vinyl polymerization system e Glycidyl methacrylate/Cp2TiCl2/Zn. Polymer 2010, 51, 2857–2863. [Google Scholar] [CrossRef]
- Tai, H.; Tochwin, A.; Wang, W. Thermoresponsive hyperbranched polymers via in situ RAFT copolymerization of PEG-based monomethacrylate and dimethacrylate monomers. Polym. Chem. 2013, 51, 3751–3761. [Google Scholar] [CrossRef]
- Kennedy, R.; Hassan, W.U.; Tochwin, A.; Zhao, T.; Dong, Y.; Wang, Q.; Tai, H.; Wang, W. In situ formed hybrid hydrogels from PEG-based multifunctional hyperbranched copolymers: A RAFT approach. Polym. Chem. 2014, 5, 1838–1842. [Google Scholar] [CrossRef]
- Tai, H.; Wang, W.; Vermonden, T.; Heath, F.; Hennink, W.E.; Alexander, C.; Shakesheff, K.M.; Howdle, S.M. Thermoresponsive and photocrosslinkable PEGMEMA–PPGMA–EGDMA copolymers from a one-step ATRP synthesis. Biomacromolecules 2009, 10, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.; Howard, D.; Takae, S.; Wang, W.; Vermonden, T.; Hennink, W.E.; Stayton, P.S.; Hoffman, A.S.; Endruweit, A.; Alexander, C.; et al. Photo-cross-linked hydrogels from thermoresponsive PEGMEMA–PPGMA–EGDMA copolymers containing multiple methacrylate groups: Mechanical property, swelling, protein release, and cytotoxicity. Biomacromolecules 2009, 10, 2895–2903. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Gunning, P.; Cao, H.; Mathew, A.; Newland, B.; Saeed, O.; Magnusson, J.P.; Alexander, C.; Tai, H.; Wang, W.; et al. Dual stimuli responsive PEG based hyperbranched polymers. Polym. Chem. 2010, 1, 827–830. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, H.; Newland, B.; Aied, A.; Zhou, D.; Wang, W. Significance of branching for transfection: Synthesis of highly branched degradable functional poly(dimethylaminoethyl methacrylate) by vinyl oligomer combination. Angew. Chem. Int. Ed. 2014, 53, 6095–6100. [Google Scholar] [CrossRef] [PubMed]
- Cremlyn, R.J. An Introduction to Organosulfur Chemistry; Wiley: Chicester, UK, 1996. [Google Scholar]
- Aliyar, H.A.; Hamilton, P.D.; Ravi, N. Refilling of ocular lens capsule with copolymeric hydrogel containing reversible disulfide. Biomacromolecules 2005, 6, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Tsarevsky, N.V.; Matyjaszewski, K. Reversible redox cleavage/coupling of polystyrene with disulfide or thiol groups prepared by atom transfer radical polymerization. Macromolecules 2002, 35, 9009–9014. [Google Scholar] [CrossRef]
- Li, Y.; Armes, S.P. Synthesis and chemical degradation of branched vinyl polymers prepared via ATRP: Use of a cleavable disulfide-based branching agent. Macromolecules 2005, 38, 8155–8162. [Google Scholar] [CrossRef]
- Plunkett, K.N.; Berkowski, K.L.; Moore, J.S. Chymotrypsin responsive hydrogel: Application of a disulfide exchange protocol for the preparation of methacrylamide containing peptides. Biomacromolecules 2005, 6, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lim, Y.T.; Park, O.O.; Kim, J.K.; Yu, J.; Kim, Y.C. Polymer/gold nanoparticle nanocomposite light-emitting diodes: Enhancement of electroluminescence stability and quantum efficiency of blue-light-emitting polymers. Chem. Mater. 2004, 16, 688–692. [Google Scholar] [CrossRef]
- Ting, S.R.S.; Min, E.H.; Zetterlund, P.B.; Stenzel, M.H. Controlled/living ab initio emulsion polymerization via a glucose RAFT stab: Degradable cross-linked glyco-particles for concanavalin A/Fim H Conjugations to cluster E. coli bacteria. Macromolecules 2010, 43, 5211–5221. [Google Scholar] [CrossRef]
- Benaglia, M.; Rizzardo, E.; Alberti, A.; Guerra, M. Searching for more effective agents and conditions for the RAFT polymerization of MMA: Influence of dithioester substituents, solvent, and temperature. Macromolecules 2005, 38, 3129–3140. [Google Scholar] [CrossRef]
- Milovanovic, M.B.; Avaramovic, M.; Katsiksa, L.; Popovic, I.G. Simplification of the synthesis of the reversible addition–fragmentation chain transfer agent 2-(2-cyanopropyl)-dithiobenzoate. J. Serbian Chem. Soc. 2010, 75, 1711–1719. [Google Scholar] [CrossRef]
- Lutz, J.; Weichenhan, K.; Akdemir, O.; Hoth, A. About the phase transitions in aqueous solutions of thermoresponsive copolymers and hydrogels based on 2-(2-methoxyethoxy)ethyl methacrylate and oligo(ethylene glycol) methacrylate. Macromolecules 2007, 40, 2503–2508. [Google Scholar] [CrossRef]
- Saeki, S.; Kuwahara, N.; Mitsuo, N.; Kaneko, M. Upper and lower critical solution temperatures in poly(ethylene glycol)solutions. Polymer 1976, 17, 685–689. [Google Scholar] [CrossRef]
- Potta, T.; Chun, C.; Song, S. Dual cross-linking systems of functionally photo-cross-linkable and thermoresponsive polyphosphazene hydrogels for biomedical applications. Biomacromolecules 2010, 11, 1741–1753. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Kashyap, N.; Kumar, N.; Kumar, M. Hydrogels for pharmaceutical and biomedical applications. Crit. Rev. Ther. Drug Carr. Syst. 2005, 22, 107–149. [Google Scholar] [CrossRef]
- Slaughter, B.B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.K.; Lee, D.S. Injectable biodegradable hydrogels. Macromol. Biosci. 2010, 10, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Vunjak-Novakovic, G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng. A 2009, 15, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Azagarsamy, M.A.; Anseth, K.S. Bioorthogonal click chemistry: An indispensable tool to create multifaceted cell culture scaffolds. ACS Macro Lett. 2012, 2, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Mather, B.D.; Viswanathan, K.; Miller, K.M.; Long, T.E. Michael addition reactions in macromolecular design for emerging technologies. Prog. Polym. Sci. 2006, 31, 487–531. [Google Scholar] [CrossRef]
- Witczak, Z.J.; Lorchak, D.; Nguyen, N. A click chemistry approach to glycomimetics: Michael addition of 2,3,4,6-tetra-O-acetyl-1-thio-beta-d-glucopyranose to 4-deoxy-1,2-O-isopropylidene-l-glycero-pent-4-enopyranos-3-ulose—A convenient route to novel 4-deoxy-(1−>5)-5-C-thiodisaccharides. Carbohydr. Res. 2007, 342, 1929–1933. [Google Scholar] [CrossRef] [PubMed]
- Dix, L.R.; Ebdon, J.R.; Hodge, P. Chain extension and crosslinking of telechelic oligomers. II: Michael additions of bisthiols to bismaleimides, bismaleates and bis(acetylene ketone)s to give linear and crosslinked polymers. Eur. Polym. J. 1995, 31, 653–658. [Google Scholar] [CrossRef]
- Yu, Y.; Deng, C.; Meng, F.; Shi, Q.; Feijen, J.; Zhong, Z.J. Novel injectable biodegradable glycol chitosan-based hydrogels crosslinked by Michael-type addition reaction with oligo(acryloyl carbonate)-β-poly(ethylene glycol)-β-oligo(acryloyl carbonate) copolymers. Biomed. Mater. Res. A 2011, 99A, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Roth, P.J.; Boyer, C.; Lowe, A.B.; Davis, T.P. RAFT polymerization and thiol chemistry: A complementary pairing for implementing modern macromolecular design. Macromol. Rapid Commun. 2011, 32, 1123–1143. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Chan, J.W.; Hoyle, C.E.; Lowe, A.B. Sequential thiol-ene/thiol-ene and thiol-ene/thiol-yne reactions as a route to well-defined mono and bis end-functionalized poly(N-isopropylacrylamide). J. Polym. Sci. A 2009, 47, 3544–3557. [Google Scholar] [CrossRef]
- Xi, W.; Wang, C.; Kloxin, C.J.; Bowman, C.N. Nitrogen-centered nucleophile catalyzed thiol-vinylsulfone addition, another thiol-ene “Click” reaction. ACS Macro Lett. 2012, 1, 811–814. [Google Scholar] [CrossRef]
- Chan, J.W.; Hoyle, C.E.; Lowe, A.B. Sequential phosphine-catalyzed, nucleophilic thiol-ene/radical-mediated thiol-yne reactions and the facile orthogonal synthesis of polyfunctional materials. J. Am. Chem. Soc. 2009, 131, 5751–5753. [Google Scholar] [CrossRef] [PubMed]
- Dondoni, A. The emergence of thiol-ene coupling as a click process for materials and bioorganic chemistry. Angew. Chem. Int. Ed. 2008, 47, 8995–8997. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Hassan, W.; Zheng, Y. Thermoresponsive hyperbranched copolymer with multi acrylate functionality for in situ cross-linkable hyaluronic acid composite semi-IPN hydrogel. J. Mater. Sci. Med. 2012, 23, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, C.E.; Bowman, C.N. Thiol-ene click chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef] [PubMed]
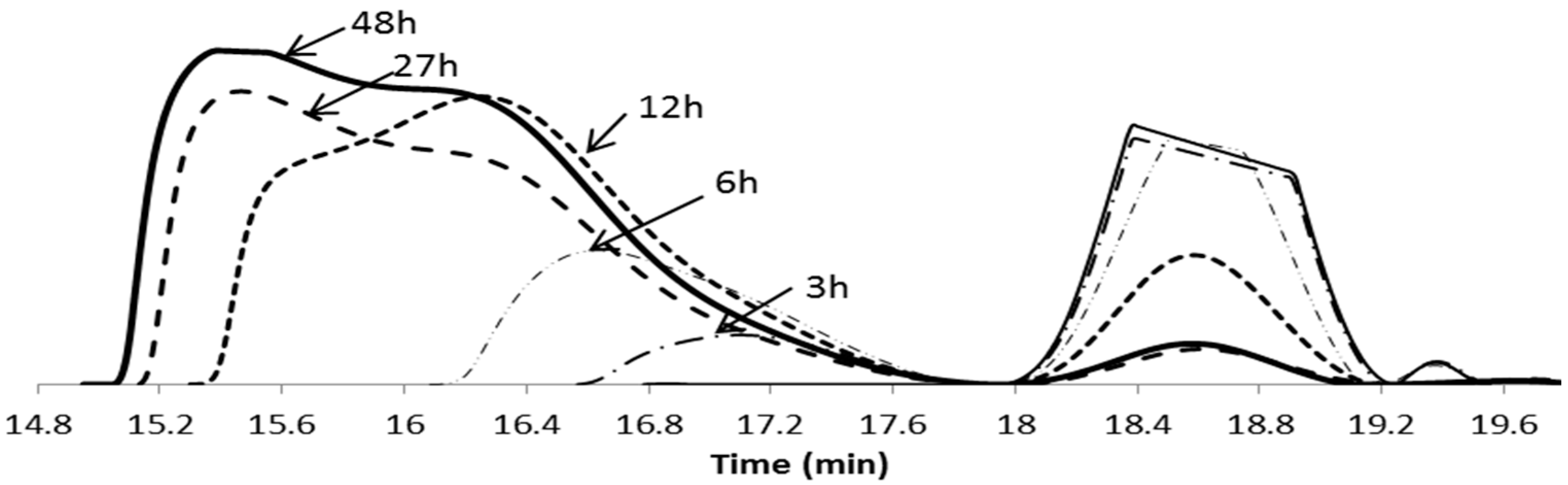
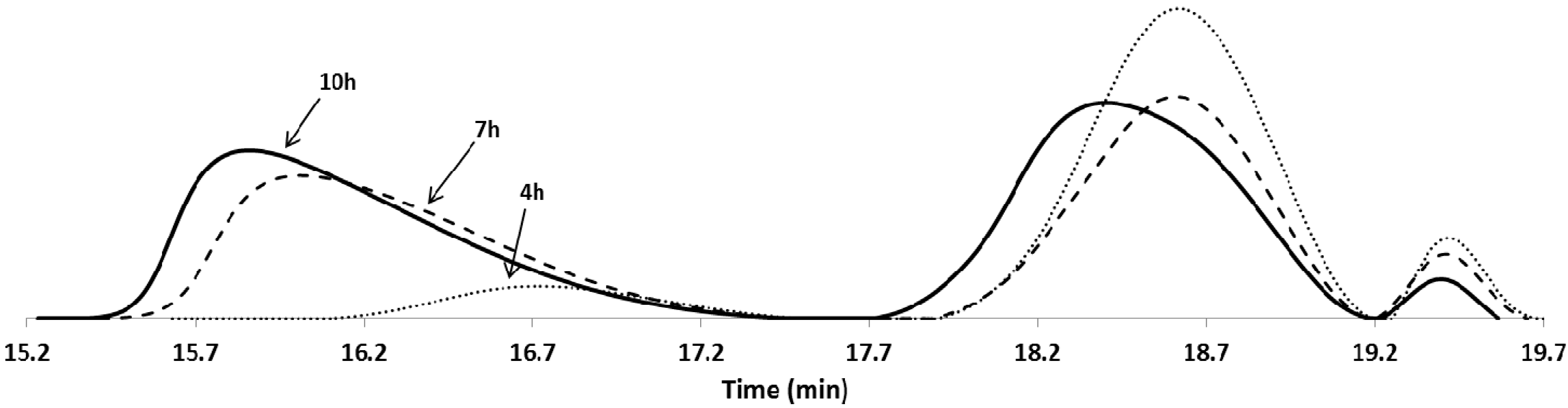
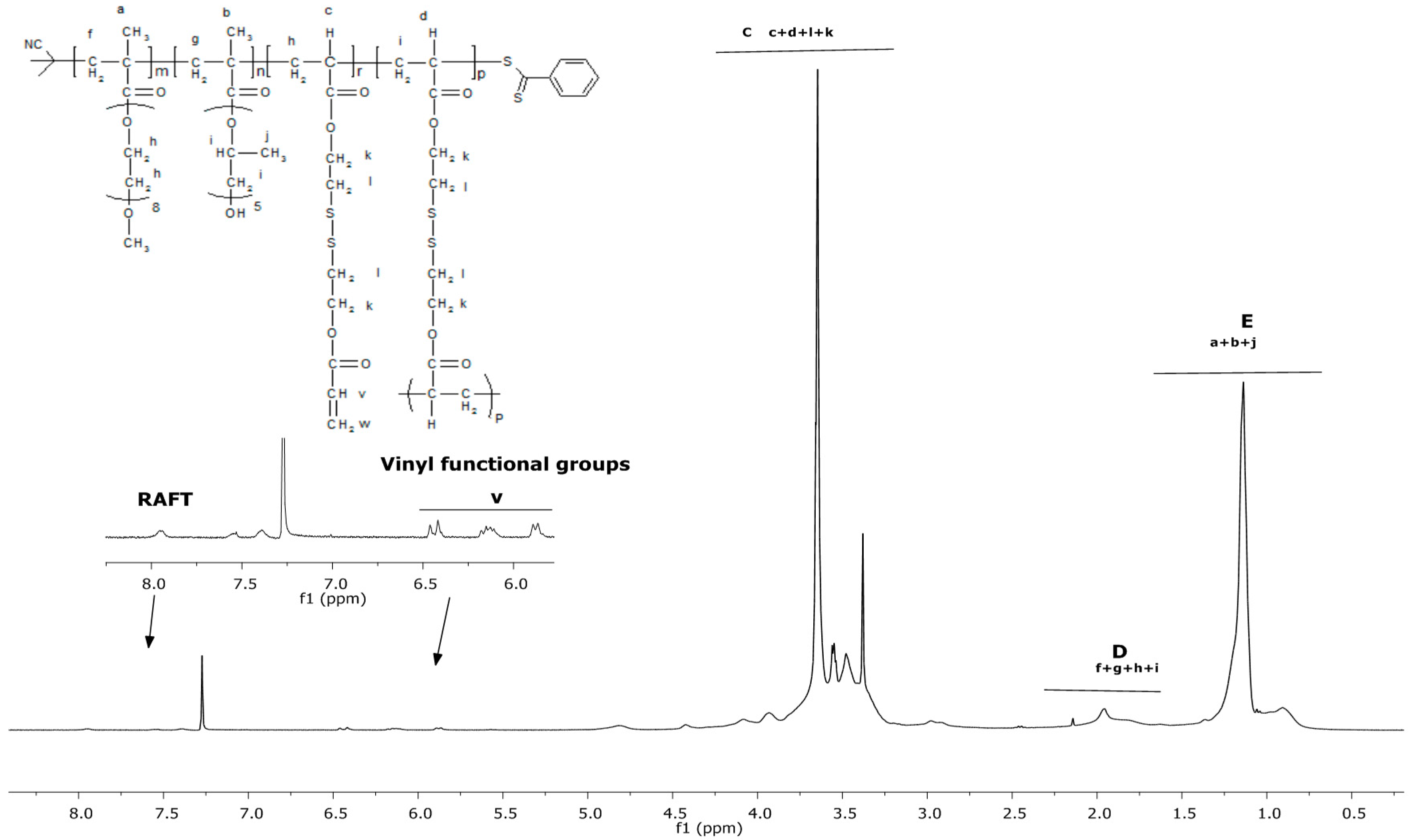
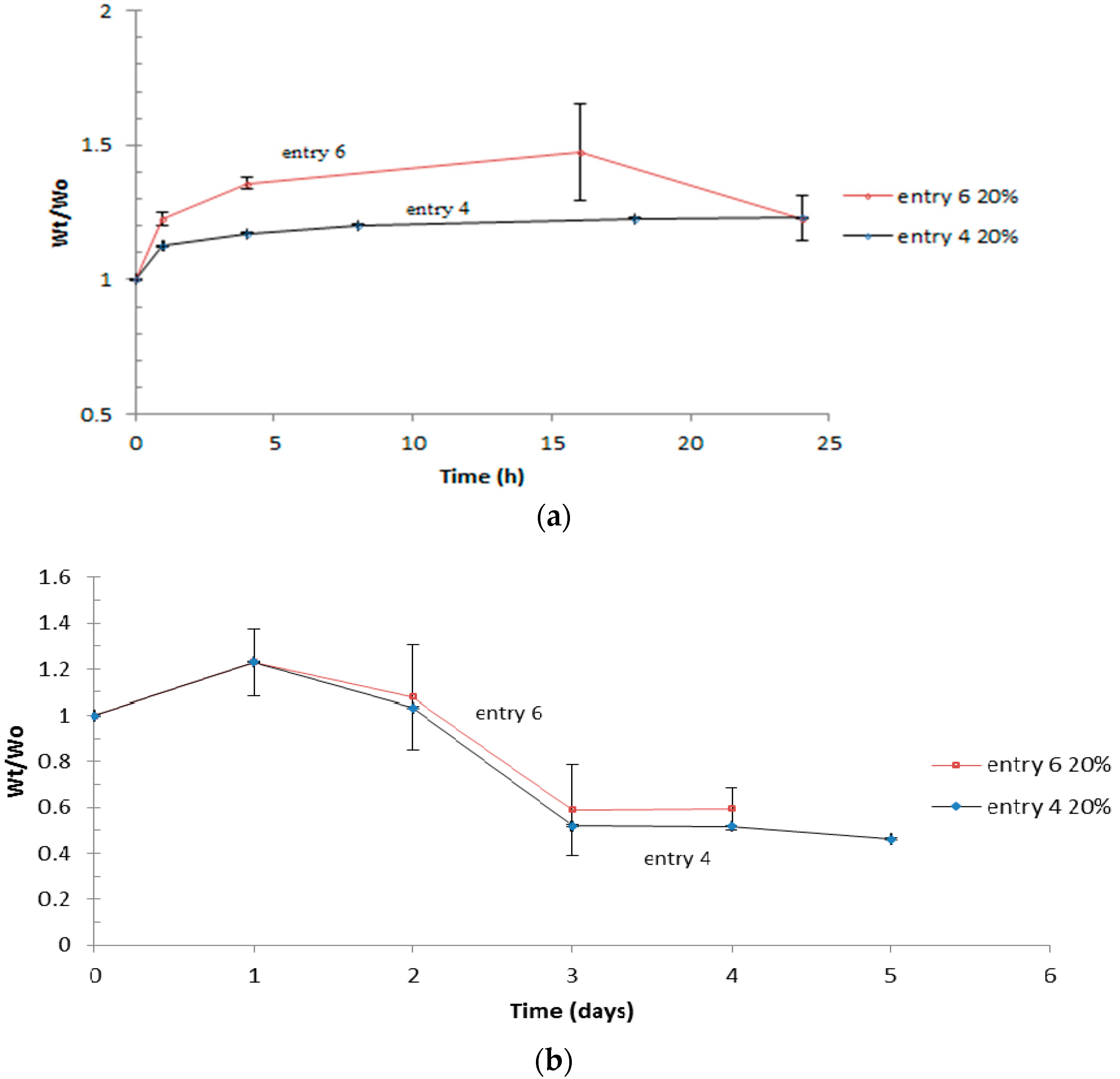
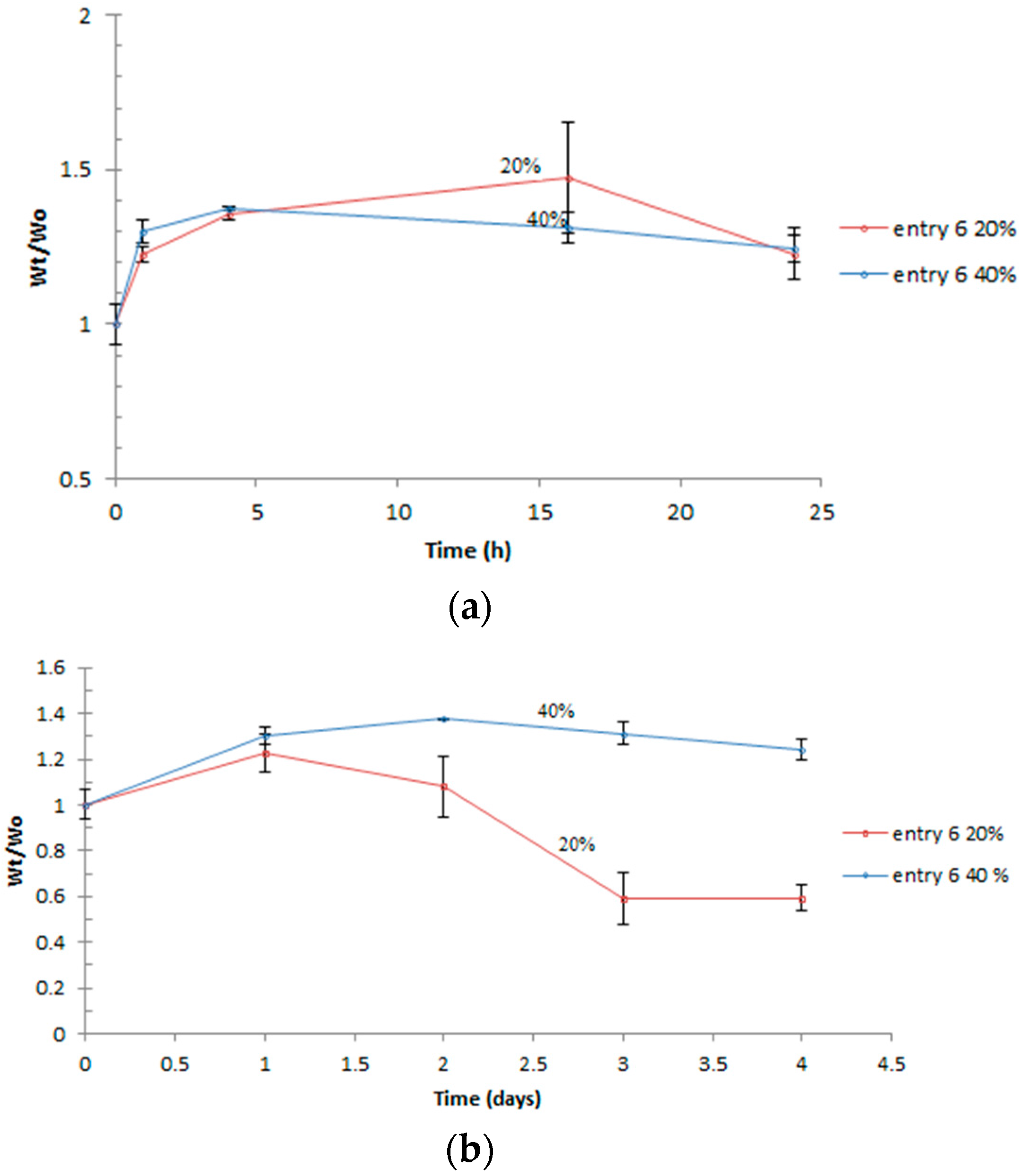
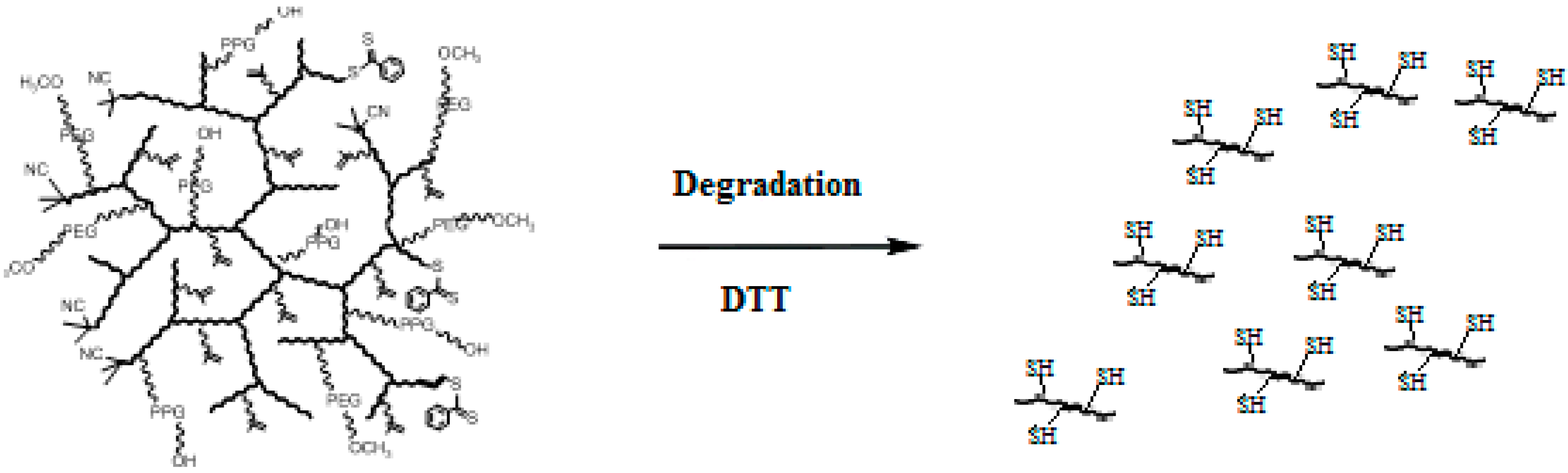
| Entry | Polymer samples obtained | f a | RT b (h) | Cov c % | GPC RI | LCST °C | |||
|---|---|---|---|---|---|---|---|---|---|
| Mw d | Mn e | PDI f | LCST g | LCST h | |||||
| kg/mol | kg/mol | ||||||||
| 1 | R1 | 70:20:10:5:1 | 48 | 95 | 12.7 | 9.0 | 1.40 | - | - |
| 2 | R2 | 50:40:10:2:0.4 | 24 | 80 | 7.3 | 6.6 | 1.12 | 55 | 56 |
| 3 | R3 | 50:40:10:1:0.2 | 24 | 64 | 11.3 | 9.8 | 1.15 | 57 | 57 |
| 4 | R4 | 20:70:10:1:0.2 | 29 | 54 | 12.4 | 10.1 | 1.22 | 28 | 28 |
| 5 | R5 | 15:65:20:1:0.2 | 25 | 58 | 11.2 | 8.9 | 1.26 | 22 | 23 |
| 6 | R6 | 20:70:10:1:0.2 | 17 | 65 | 13.4 | 11.4 | 1.17 | 28 | 30 |
| 7 | R7 | 20:70:10:0.5:0.1 | 11 | 46 | 10.2 | 8.7 | 1.16 | 18 | 17 |
| Polymer samples | f a PEGMEMA:PPGMA:DSDA | f b PEGMEMA:PPGMA:DSDA | Double bond (mol %) | Branching degree (mol %) |
|---|---|---|---|---|
| R4 | 20:70:10 | 24:49:27 | 0.5 | 45.9 |
| R7 | 20:70:10 | 22:45:33 | 1 | 61.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tochwin, A.; El-Betany, A.; Tai, H.; Chan, K.Y.; Blackburn, C.; Wang, W. Thermoresponsive and Reducible Hyperbranched Polymers Synthesized by RAFT Polymerisation. Polymers 2017, 9, 443. https://doi.org/10.3390/polym9090443
Tochwin A, El-Betany A, Tai H, Chan KY, Blackburn C, Wang W. Thermoresponsive and Reducible Hyperbranched Polymers Synthesized by RAFT Polymerisation. Polymers. 2017; 9(9):443. https://doi.org/10.3390/polym9090443
Chicago/Turabian StyleTochwin, Anna, Alaa El-Betany, Hongyun Tai, Kai Yu Chan, Chester Blackburn, and Wenxin Wang. 2017. "Thermoresponsive and Reducible Hyperbranched Polymers Synthesized by RAFT Polymerisation" Polymers 9, no. 9: 443. https://doi.org/10.3390/polym9090443