Recent Advances on Polypyrrole Electroactuators
Abstract
:1. Introduction
2. Special Doping Counterions for PPy Electroactuators
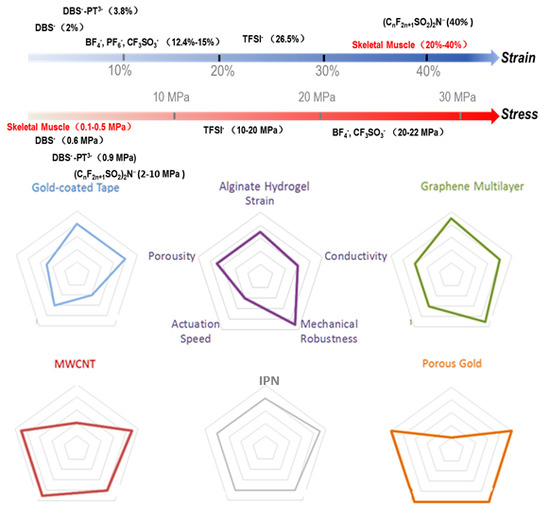
2.1. Immobilized Large Anions as Dopants
2.2. Freely Diffusible Counterions Combined with Organic Solvent Electrolytes
2.3. Conformationally Transformable Anions as Dopants
3. Delamination in Layered PPy Electroactuators
3.1. Mechanism and Models for Cracking and Delamination
3.2. Substrates for Delamination-Free Electroactuators
4. Conclusions
- For monolithic PPy electroactuators designed to work in aqueous solutions, particularly bio-relevant solutions such as PBS, PPy films doped with large counterions especially polyol-borate could provide outstanding strain and electrochemical stability and are thus promising for various biomedical devices. Moreover, alginate hydrogel could serve as an ideal substrate in such electroactuators to further improve its processability and reduce the risks of being broken and torn during fabrication.
- In solutions where supportive anions like BF4−, PF6−, CF3SO3− or TFSI− are accessible, a combination of a graphene or IPN substrate with counterions like DBS−‒PT3−, CF3SO3− or TFSI− may result in delamination-free electroactuators of superb electroactivity. This could also be the first choice for multilayered electroactuators involving ionic gels replenished with required anions.
- For applications requiring fast electroactuation and large output stress, PPy incorporated with ICs or aligned MWCNTs could be a good candidate, though the output strain of such electroactuators is generally limited. It is worth noting that the extremely large porosity and surface area arising from these dopants are highly favored by supercapacitor and photocatalytic applications.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tsai, H.-K.A.; Madou, M. Microfabrication of bilayer polymer actuator valves for controlled drug delivery. JALA 2007, 12, 291–295. [Google Scholar] [CrossRef]
- Entezami, A.A.; Massoumi, B. Artificial muscles, biosensors and drug delivery systems based on conducting polymers: A review. Iran. Polym. J. 2006, 15, 13–30. [Google Scholar]
- Spinks, G.M.; Wallace, G.G. Actuated pins for braille displays. In Biomedical Applications of Electroactive Polymer Actuators; Carpi, F., Smela, E., Eds.; Wiley: Hoboken, NJ, USA, 2009; pp. 265–277. [Google Scholar]
- Fang, Y.; Tan, X. A novel diaphragm micropump actuated by conjugated polymer petals: Fabrication, modeling, and experimental results. Sens. Actuators A 2010, 158, 121–131. [Google Scholar] [CrossRef]
- Jager, E.W.H.; Smela, E.; Inganäs, O. Microfabricating conjugated polymer actuators. Science 2000, 290, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Khaldi, A.; Falk, D.; Maziz, A.; Jager, E.W.H. Fabrication and adhesion of conjugated polymer trilayer structures for soft, flexible micromanipulators. In Electroactive Polymer Actuators and Devices (EAPAD); Bar-Cohen, Y., Ed.; SPIE: Las Vegas, NV, USA, 2016. [Google Scholar]
- Kim, K.J.; Tadokoro, S. Electroactive Polymers for Robotic Applications: Artificial Muscles and Sensors; Springer: New York, NY, USA, 2007; pp. 105–117. [Google Scholar]
- McGovern, S.T.; Abbot, M.; Emery, R.; Alici, G.; Truong, V.T.; Spinks, G.M.; Wallace, G.G. Evaluation of thrust force generated for a robotic fish propelled with polypyrrole actuators. Polym. Int. 2010, 59, 357–364. [Google Scholar] [CrossRef]
- Otero, T.F. Reactive conducting polymers as actuating sensors and tactile muscles. Bioinspir. Biomim. 2008, 3, 035004. [Google Scholar] [CrossRef] [PubMed]
- Otero, T.F.; Broschart, M. Polypyrrole artificial muscles: A new rhombic element. Construction and electrochemomechanical characterization. J. Appl. Electrochem. 2006, 36, 205–214. [Google Scholar] [CrossRef]
- Yamada, M.; Kondo, M.; Mamiya, J.I.; Yu, Y.; Kinoshita, M.; Barrett, C.J.; Ikeda, T. Photomobile polymer materials: Towards light–driven plastic motors. Angew. Chem. Int. Ed. 2008, 47, 4986–4988. [Google Scholar] [CrossRef] [PubMed]
- Alici, G.; Spinks, G.M.; Madden, J.D.W.; Wu, Y.; Wallace, G.G. Response characterization of electroactive polymers as mechanical sensors. IEEE/ASME Trans. Mechatron. 2008, 13, 187–196. [Google Scholar] [CrossRef]
- Lee, K.; Cho, S.; Park, S.H.; Heeger, A.J.; Lee, C.W.; Lee, S.H. Metallic transport in polyaniline. Nature 2006, 441, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Gunbas, G.; Toppare, L. Electrochromic conjugated polyeterocycles and derivatives–highlights from the last decade towards realization of long lived aspirations. Chem. Commun. 2012, 48, 1083–1101. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, K.; Takayanagi, K.; Tominaga, K.; Takashima, W. How to improve electrochemomechanical strain in conducting polymers. In Electroactive Polymer Actuators and Devices (EAPAD); Bar-Cohen, Y., Ed.; SPIE: San Diego, CA, USA, 2012. [Google Scholar]
- Ho, V.; Shimada, M.; Szeto, D.; Casadevall i Solvas, X.; Scott, D.; Dolci, L.S.; Kulinsky, L.; Daunert, S.; Madou, M. Utilization of electroactive polymer actuators in micromixing and in extended-life biosensor applications. Proc. SPIE 2010, 7642, 764235–764238. [Google Scholar]
- Gaihre, B.; Ashraf, S.; Spinks, G.M.; Innis, P.C.; Wallace, G.G. Comparative displacement study of bilayer actuators comprising of conducting polymers, fabricated from polypyrrole, poly(3,4-ethylenedioxythiophene) or poly(3,4-propylenedioxythiophene). Sens. Actuators A 2013, 193, 48–53. [Google Scholar] [CrossRef]
- Naka, Y.; Fuchiwaki, M.; Tanaka, K. A micropump driven by a polypyrrole-based conducting polymer soft actuator. Polym. Int. 2010, 59, 352–356. [Google Scholar] [CrossRef]
- Gao, F.X.; Ning, Z.; Fang, X.D.; Ma, M.M. Bioinspired design of strong, rough and highly conductive polyol-polypyrrole composite for flexible electronics. ACS Appl. Mate. Interfaces 2017, 9, 5692–5698. [Google Scholar] [CrossRef] [PubMed]
- Genovese, M.; Lian, K. Polyoxometalate modified inorganic–organic nanocomposite materials for energy storage applications: A review. Curr. Opin. Solid State Mater. Sci. 2015, 19, 126–137. [Google Scholar] [CrossRef]
- Fedorková, A.; Nacher-Alejos, A.; Gómez‒Romero, P.; Oriňáková, R.; Kaniansky, D. Structural and electrochemical studies of ppy/peg-lifepo4 cathode material for li-ion batteries. Electrochim. Acta 2010, 55, 943–947. [Google Scholar] [CrossRef]
- Madden, J.D.; Cush, R.A.; Kanigan, T.S.; Hunter, I.W. Fast contracting polypyrrole actuators. Synth. Met. 2000, 113, 185–192. [Google Scholar] [CrossRef]
- Madden, J.D.; Cush, R.A.; Kanigan, T.S.; Brenan, C.J.; Hunter, I.W. Encapsulated polypyrrole actuators. Synth. Met. 1999, 105, 61–64. [Google Scholar] [CrossRef]
- Otero, T.F.; Grande, H.; Rodriguez, J. Influence of the counterion size on the rate of electrochemical relaxation in polypyrrole. Synth. Met. 1996, 83, 205–208. [Google Scholar] [CrossRef]
- Johanson, U.; Marandi, M.; Sammelselg, V.; Tamm, J. Electrochemical properties of porphyrin-doped polypyrrole films. J. Electroanal. Chem. 2005, 575, 267–273. [Google Scholar] [CrossRef]
- Otero, T.F. Biomimetic conducting polymers: Synthesis, materials, properties, functions, and devices. Polym. Rev. 2013, 53, 311–351. [Google Scholar] [CrossRef]
- Otero, T.F.; Boyano, I. Comparative study of conducting polymers by the ESCR model. J. Phys. Chem. B 2003, 107, 6730–6738. [Google Scholar] [CrossRef]
- Otero, T.F.; Grande, H.; Rodriguez, J. Reinterpretation of polypyrrole electrochemistry after consideration of conformational relaxation processes. J. Phys. Chem. B 1997, 101, 3688–3697. [Google Scholar] [CrossRef]
- Posey, F.A.; Morozumi, T. Theory of potentiostatic and galvanostatic charging of the double layer in porous electrodes. J. Electrochem. Soc. 1966, 113, 176–184. [Google Scholar] [CrossRef]
- Feldberg, S.W. Reinterpretation of polypyrrole electrochemistry. Consideration of capacitive currents in redox switching of conducting polymers. J. Am. Chem. Soc. 1984, 106, 4671–4674. [Google Scholar] [CrossRef]
- Dvir, T.; Timko, B.P.; Brigham, M.D.; Naik, S.R.; Karajanagi, S.S.; Levy, O.; Jin, H.; Parker, K.K.; Langer, R.; Kohane, D.S. Nanowired three-dimensional cardiac patches. Nat. Nanotechnol. 2011, 6, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Vunjak-Novakovic, G.; Tandon, N.; Godier, A.; Maidhof, R.; Marsano, A.; Martens, T.P.; Radisic, M. Challenges in cardiac tissue engineering. Tissue Eng. Part B 2009, 16, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Hara, S.; Zama, T.; Takashima, W.; Kaneto, K. Polypyrrole–metalcoil composite actuators as artificial muscle fibres. Synth. Met. 2004, 146, 47–55. [Google Scholar] [CrossRef]
- Gong, J.; Lin, H.; Dunlop, J.W.C.; Yuan, J. Hierarchically arranged helical fiber actuators derived from commercial cloth. Adv. Mater. 2017, 29, 1605103. [Google Scholar] [CrossRef] [PubMed]
- Bay, L.; West, K.; Sommer-Larsen, P.; Skaarup, S.; Benslimane, M. A conducting polymer artificial muscle with 12% linear strain. Adv. Mater. 2003, 15, 310–313. [Google Scholar] [CrossRef]
- Maw, S.; Smela, E.; Yoshida, K.; Stein, R.B. Effects of monomer and electrolyte concentrations on actuation of PPy(DBS) bilayers. Synth. Met. 2005, 155, 18–26. [Google Scholar] [CrossRef]
- Kiefer, R.; Martinez, J.G.; Kesküla, A.; Anbarjafari, G.; Aabloo, A.; Otero, T.F. Polymeric actuators: Solvents tune reaction-driven cation to reaction-driven anion actuator. Sens. Actuators B 2016, 233, 328–336. [Google Scholar] [CrossRef]
- Kivilo, A.; Zondaka, Z.; Kesküla, A.; Rasti, P.; Tamm, T.; Kiefer, R. Electro-chemo-mechanical deformation properties of polypyrrole/dodecylbenzenesulfate linear actuators in aqueous and organic electrolyte. RSC Adv. 2016, 6, 96484–96489. [Google Scholar] [CrossRef]
- Zondaka, Z.; Kesküla, A.; Tamm, T.; Kiefer, R. Polypyrrole linear actuation tuned by phosphotungstic acid. Sens. Actuators B 2017, 247, 742–748. [Google Scholar] [CrossRef]
- Otero, T.F.; Cheng, S.A.; Coronado, E.; Ferrero, E.M.; Gómez‒García, C.J. Functional hybrid materials containing polypyrrole and polyoxometalate clusters: Searching for high conductivities and specific charges. ChemPhysChem 2002, 3, 808–811. [Google Scholar] [CrossRef]
- Cheng, S.; Fernández-Otero, T.; Coronado, E.; Gómez-García, C.J.; Martínez-Ferrero, E.; Giménez-Saiz, C. Hybrid material polypyrrole/[SiCr(H2O)W11O39]5−: Electrogeneration, properties, and stability under cycling. J. Phys. Chem. B 2002, 106, 7585–7591. [Google Scholar] [CrossRef]
- Herrmann, S.; Ritchie, C.; Streb, C. Polyoxometalate–conducting polymer composites for energy conversion, energy storage and nanostructured sensors. Dalton Trans. 2015, 44, 7092–7104. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Lu, M.; Kang, J.; Wang, D.; Brown, J.; Peng, Z. Synthesis and optical properties of conjugated polymers containing polyoxometalate clusters as side-chain pendants. Chem. Mater. 2005, 17, 2841–2851. [Google Scholar] [CrossRef]
- Sen, S.; Palmore, G.T.R. Stimuli-responsive macromolecular composites: Enhanced stress modulation in polypyrrole with redox-active dopants. Macromolecules 2016, 49, 8479–8488. [Google Scholar] [CrossRef]
- Sen, S.; Kim, S.Y.; Palmore, L.R.; Jin, S.; Jadhav, N.; Chason, E.; Palmore, G.T.R. In situ measurement of voltage-induced stress in conducting polymers with redox-active dopants. ACS Appl. Mater. Interfaces 2016, 8, 24168–24176. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Kim, S.; Jin, S.; Palmore, L.R.; Jadhav, N.; Chason, E.; Palmore, G.T.R. In situ measurement of stress evolution and ion dynamics in conducting polymers. ECS Trans. 2013, 45, 15–21. [Google Scholar] [CrossRef]
- Hara, S.; Zama, T.; Takashima, W.; Kaneto, K. TFSI-doped polypyrrole actuator with 26% strain. J. Mater. Chem. 2004, 14, 1516–1517. [Google Scholar] [CrossRef]
- Kadoyama, T.; Yamasaki, J.; Tsumuji, F.; Takamiya, S.; Ogihara, S.; Hoshino, D.; Nishioka, Y. Behaviors of polypyrrole soft actuators in LiTFSI or NaCl electrolyte solutions containing methanol. J. Mater. Sci. Chem. Eng. 2013, 1, 1–7. [Google Scholar] [CrossRef]
- Masurkar, N.; Jamil, K.; Arava, L. Environmental effects on the polypyrrole tri-layer actuator. Actuators 2017, 6, 17. [Google Scholar] [CrossRef]
- Hara, S.; Zama, T.; Takashima, W.; Kaneto, K. Free-standing gel-like polypyrrole actuators doped with bis (perfluoroalkylsulfonyl) imide exhibiting extremely large strain. Smart Mater. Struct. 2005, 14, 1501–1510. [Google Scholar] [CrossRef]
- Yan, B.; Li, B.; Kunecke, F.; Gu, Z.; Guo, L. Polypyrrole-based implantable electroactive pump for controlled drug microinjection. ACS Appl. Mate. Interfaces 2015, 7, 14563–14568. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, B.; Wu, Y.; Guo, L. Hydrogel-reinforced polypyrrole electroactuator. In Proceedings of the 2016 IEEE 38th Annual International Conference on Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; IEEE: New York, NY, USA, 2016. [Google Scholar]
- Hara, S.; Zama, T.; Takashima, W.; Kaneto, K. Free-standing polypyrrole actuators with response rate of 10.8 %/s. Synth. Met. 2005, 149, 199–201. [Google Scholar] [CrossRef]
- Bay, L.; Jacobsen, T.; Skaarup, S.; West, K. Mechanism of actuation in conducting polymers: Osmotic expansion. J. Phys. Chem. B 2001, 105, 8492–8497. [Google Scholar] [CrossRef]
- Guo, L. Conducting Polymers as Smart Materials for Tissue Engineering. In Smart Materials for Tissue Engineering: Fundamental Principles; Wang, Q., Ed.; Royal Society of Chemistry: Cambridge, UK, 2016; pp. 239–268. [Google Scholar]
- Pugal, D.; Kim, K.J.; Punning, A.; Kasemägi, H.; Kruusmaa, M.; Aabloo, A. A self-oscillating ionic polymer-metal composite bending actuator. J. Appl. Phys. 2008, 103, 084908. [Google Scholar] [CrossRef]
- Dove, P.M.; Nix, C.J. The influence of the alkaline earth cations, magnesium, calcium, and barium on the dissolution kinetics of quartz. Geochim. Cosmochim. Acta 1997, 61, 3329–3340. [Google Scholar] [CrossRef]
- Naoi, K.; Lien, M.; Smyrl, W.H. Quartz crystal microbalance study: Ionic motion across conducting polymers. J. Electrochem. Soc. 1991, 138, 440–445. [Google Scholar] [CrossRef]
- Maia, G.; Torresi, R.M.; Ticianelli, E.A.; Nart, F.C. Charge compensation dynamics in the redox processes of polypyrrole-modified electrodes. J. Phys. Chem. 1996, 100, 15910–15916. [Google Scholar] [CrossRef]
- Skaarup, S.; Bay, L.; West, K. Polypyrrole actuators working at 2–30 Hz. Synth. Met. 2007, 157, 323–326. [Google Scholar] [CrossRef]
- Suárez, M.F.; Compton, R.G. In situ atomic force microscopy study of polypyrrole synthesis and the volume changes induced by oxidation and reduction of the polymer. J. Electroanal. Chem. 1999, 462, 211–221. [Google Scholar] [CrossRef]
- Torop, J.; Aabloo, A.; Jager, E.W.H. Novel actuators based on polypyrrole/carbide-derived carbon hybrid materials. Carbon 2014, 80, 387–395. [Google Scholar] [CrossRef]
- Kiefer, R.; Aydemir, N.; Torop, J.; Tamm, T.; Temmer, R.; Travas-Sejdic, J.; Must, I.; Kaasik, F.; Aabloo, A. Carbide-derived carbon as active interlayer of polypyrrole tri-layer linear actuator. Sens. Actuators B 2014, 201, 100–106. [Google Scholar] [CrossRef]
- Kang, H.C.; Geckeler, K.E. Enhanced electrical conductivity of polypyrrole prepared by chemical oxidative polymerization: Effect of the preparation technique and polymer additive. Polymer 2000, 41, 6931–6934. [Google Scholar] [CrossRef]
- Yee, L.M.; Mahmud, H.N.M.E.; Kassim, A.; Yunus, W.M.M. Polypyrrole-polyethylene glycol conducting polymer composite films: Preparation and characterization. Synth. Met. 2007, 157, 386–389. [Google Scholar] [CrossRef] [Green Version]
- Popescu, S.; Pirvu, C.; Mindroiu, M.; Manole, C.; Demetrescu, I. Electrochemical synthesis and characterization of Ti modified electrodes with polypyrrole-polyethelene glycol hybrid coating. Rev. Chim. 2010, 61, 245–248. [Google Scholar]
- Pirvu, C.; Mindroiu, M.; Stancu, R.; Bojin, D.; Demetrescu, I. Scanning electronic microscopy in supporting electrochemical deposition and characterization of hybrid polymeric composite. Key Eng. Mater. 2009, 415, 69–72. [Google Scholar] [CrossRef]
- Pirvu, C.; Manole, C.C.; Stoian, A.B.; Demetrescu, I. Understanding of electrochemical and structural changes of polypyrrole/polyethylene glycol composite films in aqueous solution. Electrochim. Acta 2011, 56, 9893–9903. [Google Scholar] [CrossRef]
- Baker, G.A.; Jordan, J.D.; Bright, F.V. Effects of poly(ethylene glycol) doping on the behavior of pyrene, rhodamine 6G, and acrylodan-labeled bovine serum albumin sequestered within tetramethylorthosilane-derived sol-gel-processed composites. J. Sol-Gel Sci. Technol. 1998, 11, 43–54. [Google Scholar] [CrossRef]
- Sasahara, K.; Sakurai, M.; Nitta, K. Volume and compressibility changes for short poly(ethylene glycol)—water system at various temperatures. Colloid Polym. Sci. 1998, 276, 643–647. [Google Scholar] [CrossRef]
- Branca, C.; Magazù, S.; Maisano, G.; Migliardo, F.; Migliardo, P.; Romeo, G. Hydration study of peg/water mixtures by quasi elastic light scattering, acoustic and rheological measurements. J. Phys. Chem. B 2002, 106, 10272–10276. [Google Scholar] [CrossRef]
- Farajollahi, M.; Usgaocar, A.; Dobashi, Y.; Woehling, V.; Plesse, C.; Vidal, F.; Sassani, F.; Madden, J.D.W. Nonlinear two-dimensional transmission line models for electrochemically driven conducting polymer actuators. IEEE/ASME Trans. Mechatron. 2017, 22, 705–716. [Google Scholar] [CrossRef]
- Ma, M.M.; Guo, L.; Anderson, D.G.; Langer, R. Bio-inspired polymer composite actuator and generator driven by water gradients. Science 2013, 339, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ma, M.M.; Zhang, N.; Langer, R.; Anderson, D.G. Stretchable polymeric multielectrode array for conformal neural interfacing. Adv. Mater. 2014, 26, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Okuzaki, H.; Kuwabara, T.; Funasaka, K.; Saido, T. Humidity-sensitive polypyrrole films for electro-active polymer actuators. Adv. Funct. Mater. 2013, 23, 4400–4407. [Google Scholar] [CrossRef]
- Wang, X.; Gu, X.; Yuan, C.; Chen, S.; Zhang, P.; Zhang, T.; Yao, J.; Chen, F.; Chen, G. Evaluation of biocompatibility of polypyrrole in vitro and in vivo. J. Biomed. Mater. Res. A 2004, 68, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.M.; Wu, Z.; Huang, X.; Kapsa, R.M.I.; Wallace, G.G. Use of conducting polymers to facilitate neurite branching in schizophrenia-related neuronal development. Biomater. Sci. 2016, 4, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Guimard, N.K.; Gomez, N.; Schmidt, C.E. Conducting polymers in biomedical engineering. Prog. Polym. Sci. 2007, 32, 876–921. [Google Scholar] [CrossRef]
- Svennersten, K.; Berggrren, M.; Richter-Dahlfors, A.; Jager, E.W.H. Mechanical stimulation of epithelial cells using polypyrrole microactuators. Lab Chip 2011, 11, 3287–3293. [Google Scholar] [CrossRef] [PubMed]
- Gelmi, A.; Ljunggren, M.K.; Rafat, M.; Jager, E.W.H. Influence of conductive polymer doping on the viability of cardiac progenitor cells. J. Mater. Chem. B 2014, 2, 3860–3867. [Google Scholar] [CrossRef]
- Gilmore, K.J.; Kita, M.; Han, Y.; Gelmi, A.; Higgins, M.J.; Moulton, S.E.; Clark, G.M.; Kapsa, R.; Wallace, G.G. Skeletal muscle cell proliferation and differentiation on polypyrrole substrates doped with extracellular matrix components. Biomaterials 2009, 30, 5292–5304. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Bashur, C.A.; Goldstein, A.S.; Schmidt, C.E. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials 2009, 30, 4325–4335. [Google Scholar] [CrossRef] [PubMed]
- Vaitkuviene, A.; Ratautaite, V.; Mikoliunaite, L.; Kaseta, V.; Ramanauskaite, G.; Biziuleviciene, G.; Ramanaviciene, A.; Ramanavicius, A. Some biocompatibility aspects of conducting polymer polypyrrole evaluated with bone marrow-derived stem cells. Colloids Surf. A 2014, 442, 152–156. [Google Scholar] [CrossRef]
- Mao, C.; Zhu, A.; Wu, Q.; Chen, X.; Kim, J.; Shen, J. New biocompatible polypyrrole-based films with good blood compatibility and high electrical conductivity. Colloids Surf. B 2008, 67, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Giglio, E.D.; Guascito, M.R.; Sabbatini, L.; Zambonin, G. Electropolymerization of pyrrole on titanium substrates for the future development of new biocompatible surfaces. Biomaterials 2001, 22, 2609–2616. [Google Scholar] [CrossRef]
- Jaouhari, A.E.; Laabd, M.; Bazzaoui, E.A.; Albourine, A.; Martins, J.I.; Wang, R.; Nagy, G.; Bazzaoui, M. Electrochemical and spectroscopical studies of polypyrrole synthesized on carbon steel from aqueous medium. Synth. Met. 2015, 209, 11–18. [Google Scholar] [CrossRef]
- Khan, W.; Kapoor, M.; Kumar, N. Covalent attachment of proteins to functionalized polypyrrole-coated metallic surfaces for improved biocompatibility. Acta Biomater. 2007, 3, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.W.; Hsu, Y.T.; Cheng, Y.C.; Li, C.; Ruaan, R.C.; Chien, C.C.; Chuang, C.A.; Tsao, C.W. Electrical simulation to promote osteogenesis using conductive polypyrrole films. Mater. Sci. Eng. C 2014, 37, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Forciniti, L.; Ybarra, J., 3rd; Zaman, M.H.; Schmidt, C.E. Schwann cell response on polypyrrole substrates upon electrical stimulation. Acta Biomater. 2014, 10, 2423–2433. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Wang, Y.; Ma, T.; Zhu, S.; Zeng, W.; Hu, X.; Liu, Z.; Huang, J.; Luo, Z. Electrical regulation of olfactory ensheathing cells using conductive polypyrrole/chitosan polymers. Biomaterials 2013, 34, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Fahlgren, A.; Bratengeier, C.; Gelmi, A.; Semeins, C.M.; Klein-Nulend, J.; Jager, E.W.H.; Bakker, A.D. Biocompatibility of polypyrrole with human primary osteoblasts and the effect of dopants. PLoS ONE 2015, 10, e0134023. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.; Kobayashi, N.R.; Higgins, M.J.; Quigley, A.F.; Jamali, S.; Moulton, S.E.; Kapsa, R.M.I.; Wallace, G.C.; Crook, J.M. Electrical stimulation using conductive polymer polypyrrole promotes differentiation of human neural stem cells: A biocompatible platform for translational neural tissue engineering. Tissue Eng. Part C 2015, 21, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Fonner, J.M.; Forciniti, L.; Nguyen, H.; Byrne, J.D.; Kou, Y.; Syeda-Nawaz, J.; Schmidt, C.E. Biocompatibility implications of polypyrrole synthesis techniques. Biomed. Mater. 2008, 3, 034124. [Google Scholar] [CrossRef] [PubMed]
- Ateh, D.D.; Navsaria, H.A.; Vadgama, P. Polypyrrole-based conducting polymers and interactions with biological tissues. J. R. Soc. Interface 2006, 3, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.B.; Yin, G.F.; Liao, X.M.; Gu, J.W. Conducting polypyrrole in tissue engineering applications. Front. Mater. Sci. 2014, 8, 39–45. [Google Scholar] [CrossRef]
- Hara, S.; Zama, T.; Takashima, W.; Kaneto, K. Gel-like polypyrrole based artificial muscles with extremely large strain. Polym. J. 2004, 36, 933–936. [Google Scholar] [CrossRef]
- Garcia-Cordova, F.; Valero, L.; Ismail, Y.A.; Otero, T.F. Biomimetic polypyrrole based all three-in-one triple layer sensing actuators exchanging cations. J. Mater. Chem. 2011, 21, 17265–17272. [Google Scholar] [CrossRef]
- Kiefer, R.; Kilmartin, P.A.; Bowmaker, G.A.; Cooney, R.P.; Travas-Sejdic, J. Actuation of polypyrrole films in propylene carbonate electrolytes. Sens. Actuators B 2007, 125, 628–634. [Google Scholar] [CrossRef]
- Okuzaki, H.; Funasaka, K. Electrically driven polypyrrole film actuator working in air. J. Intell. Mater. Syst. Struct. 1999, 10, 465–469. [Google Scholar] [CrossRef]
- Khalili, N.; Naguib, H.E.; Kwon, R.H. Transmission line circuit model of a PPy based trilayer mechanical sensor. Proc. SPIE. 2015, 9430, 94302. [Google Scholar]
- Yamato, K.; Kaneto, K. Tubular linear actuators using conducting polymer, polypyrrole. Anal. Chim. Acta 2006, 568, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Otero, T.F.; Schumacher, J.; Pascual, V.H. Construction and coulodynamic characterization of PPy-DBS-MWCNT/tape bilayer artificial muscles. RSC Adv. 2016, 6, 68538–68544. [Google Scholar] [CrossRef]
- Zheng, W.; Alici, G.; Clingan, P.R.; Munro, B.J.; Spinks, G.M.; Steele, J.R.; Wallace, G.G. Polypyrrole stretchable actuators. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 57–63. [Google Scholar] [CrossRef]
- Küttel, C.; Stemmer, A.; Wei, X. Strain response of polypyrrole actuators induced by redox agents in solution. Sens. Actuators B 2009, 141, 478–484. [Google Scholar] [CrossRef]
- Naficy, S.; Stoboi, N.; Whitten, P.G.; Spinks, G.M.; Wallace, G.G. Evaluation of encapsulating coatings on the performance of polypyrrole actuators. Smart Mater. Struct. 2013, 22, 075005. [Google Scholar] [CrossRef]
- Christophersen, M.; Shapiro, B.; Smela, E. Characterization and modeling of PPy bilayer microactuators: Part 1. Curvature. Sens. Actuators B 2006, 115, 596–609. [Google Scholar] [CrossRef]
- Hutchinson, J.W.; Thouless, M.D.; Liniger, E.G. Growth and configurational stability of circular, buckling-driven film delaminations. Acta Metall. Mater. 1992, 40, 295–308. [Google Scholar] [CrossRef]
- Ho, V.; Narenji, A.G.; Kulinsky, L.; Madou, M. The detachment process and release efficiency of polypyrrole/gold bilayer actuators. J. Microelectromech. Syst. 2015, 24, 1616–1621. [Google Scholar] [CrossRef]
- Kassim, A.; Basar, Z.B.; Mahmud, H.N.M.E. Effects of preparation temperature on the conductivity of polypyrrole conducting polymer. J. Chem. Sci. 2002, 114, 155–162. [Google Scholar] [CrossRef]
- Wang, J.; Botelho, S.J.; Naguib, H.E.; Bazylak, A. Development of a novel active polypyrrole trilayer membrane. ACS Sustain. Chem. Eng. 2013, 1, 226–231. [Google Scholar] [CrossRef]
- Rice, J.R.; Liebowitz, H. Mathematical analysis in the mechanics of fracture. In Fracture: An Advanced Treatise; Liebowitz, H., Ed.; Academic: New York, NY, USA, 1968; Volume 2, pp. 191–311. [Google Scholar]
- Alben, S.; Balakrisnan, B.; Smela, E. Edge effects determine the direction of bilayer bending. Nano Lett. 2011, 11, 2280–2285. [Google Scholar] [CrossRef] [PubMed]
- Pyo, M.; Bohn, C.C.; Smela, E.; Reynolds, J.R.; Brennan, A.B. Direct strain measurement of polypyrrole actuators controlled by the polymer/gold interface. Chem. Mater. 2003, 15, 916–922. [Google Scholar] [CrossRef]
- Liu, Y.; Gan, Q.; Baig, S.; Smela, E. Improving PPy adhesion by surface roughening. J. Phys. Chem. C 2007, 111, 11329–11338. [Google Scholar] [CrossRef]
- Plesse, C.; Khaldi, A.; Soyer, C.; Cattan, E.; Teyssié, D.; Chevrot, C.; Vidal, F. Pedot based conducting IPN actuators: Effects of electrolyte on actuation. Adv. Sci. Technol. 2013, 79, 53–62. [Google Scholar] [CrossRef]
- Sun, X.; Sun, H.; Li, H.; Peng, H. Developing polymer composite materials: Carbon nanotubes or graphene? Adv. Mater. 2013, 25, 5153–5176. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Stenner, C.; Weissmüller, J. A nanoporous gold-polypyrrole hybrid nanomaterial for actuation. Sens. Actuators B 2017, 248, 622–629. [Google Scholar] [CrossRef]
- Kramer, D.; Viswanath, R.N.; Weissmüller, J. Surface-stress induced macroscopic bending of nanoporous gold cantilevers. Nano Lett. 2004, 4, 793–796. [Google Scholar] [CrossRef]
- Wang, K.; Kobler, A.; Kubel, C.; Jelitto, H.; Schneider, G.; Weissmüller, J. Nanoporous-gold-based composites: Toward tensile ductility. NPG Asia Mater. 2015, 7, e187. [Google Scholar] [CrossRef]
- Biener, J.; Wittstock, A.; Zepeda-Ruiz, L.A.; Biener, M.M.; Zielasek, V.; Kramer, D. Surface-chemistry-driven actuation in nanoporous gold. Nat. Mater. 2009, 8, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Zondaka, Z.; Valner, R.; Tamm, T.; Aabloo, A.; Kiefer, R. Carbide-derived carbon in polypyrrole changing the elastic modulus with a huge impact on actuation. RSC Adv. 2016, 6, 26380–26385. [Google Scholar] [CrossRef]
- Zheng, W.; Razal, J.M.; Whitten, P.G.; Ovalle-Robles, R.; Wallace, G.G.; Baughman, R.H.; Spinks, G.M. Artificial muscles based on polypyrrole/carbon nanotube laminates. Adv. Mater. 2011, 23, 2966–2970. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Jin, H.; Zhang, L.; Zhang, H.; Chen, Z.; Gao, F.; Zhang, Z. Low-voltage and high-performance electrothermal actuator based on multi-walled carbon nanotube/polymer composites. Carbon 2015, 84, 327–334. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F.; Ng, B.C. Directional alignment of carbon nanotubes in polymer matrices: Contemporary approaches and future advances. Compos. Part A 2014, 56, 103–126. [Google Scholar] [CrossRef]
- Schnoor, T.I.W.; Vainio, U.; Shao, L.-H.; Lilleodden, E.T.; Müller, M.; Schreyer, A.; Schulte, K.; Fiedler, B. Nanostructured mwcnt/polypyrrole actuators with anisotropic strain response. Adv. Eng. Mater. 2016, 18, 597–607. [Google Scholar] [CrossRef]
- Khaldi, A.; Plesse, C.; Soyer, C.; Cattan, E.; Vidal, F.; Legrand, C.; Teyssié, D. Conducting interpenetrating polymer network sized to fabricate microactuators. Appl. Phys. Lett. 2011, 98, 164101. [Google Scholar] [CrossRef]
- Wu, Y.; Alici, G.; Spinks, G.M.; Wallace, G.G. Fast trilayer polypyrrole bending actuators for high speed applications. Synth. Met. 2006, 156, 1017–1022. [Google Scholar] [CrossRef]
- Ramasamy, M.S.; Mahapatra, S.S.; Yoo, H.J.; Kim, Y.A.; Cho, J.W. Soluble conducting polymer-functionalized graphene oxide for air-operable actuator fabrication. J. Mater. Chem. A 2014, 2, 4788–4794. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Zhao, Y.; Cheng, H.; Hu, C.; Jiang, L.; Qu, L. Three-dimensional grapheme-polypyrrole hybrid electrochemical actuator. Nanoscale 2012, 4, 7563–7568. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bian, K.; Hu, C.; Zhang, Z.; Chen, N.; Zhang, H.; Qu, L. Flexible and wearable graphene/polypyrrole fibers towards multifunctional actuator applications. Electrochem. Commun. 2013, 35, 49–52. [Google Scholar] [CrossRef]
- Wang, T.; Huang, J.; Yang, Y.; Zhang, E.; Sun, W.; Tong, Z. Bioinspired smart actuator based on graphene oxide-polymer hybrid hydrogels. ACS Appl. Mater. Interfaces 2015, 7, 23423–23430. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Z.; Xie, X.; Cheng, H.; Zhao, Y.; Qu, L. A rationally-designed synergetic polypyrrole/graphene bilayer actuator. J. Mater. Chem. 2012, 22, 4015–4020. [Google Scholar] [CrossRef]
- Liu, A.; Yuan, W.; Shi, G. Electrochemical actuator based on polypyrrole/sulfonated graphene/graphene tri-layer film. Thin Solid Films 2012, 520, 6307–6312. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, C.; Cheng, H.; Li, C.; Xu, T.; Zhao, Y.; Shao, H.; Qu, L. Spontaneous, straightforward fabrication of partially reduced graphene oxide–polypyrrole composite films for versatile actuators. ACS Nano 2016, 10, 4735–4741. [Google Scholar] [CrossRef] [PubMed]
- Maziz, A.; Khaldi, A.; Persson, N.-K.; Jager, E.W.H. Soft linear electroactive polymer actuators based on polypyrrole. In Proceedings of the Electroactive Polymer Actuators and Devices (EAPAD) 2015, San Diego, CA, USA, 8–12 March 2015; SPIE: Bellingham, WA, USA, 2015; Volume 9430. [Google Scholar]
- Valero, L.; Martinez, J.G.; Otero, T.F. Creeping and structural effects in faradaic artificial muscles. J. Solid State Electrochem. 2015, 19, 2683–2689. [Google Scholar] [CrossRef]
- Anquetil, P.A.; Rinderknecht, D.; Vandesteeg, N.A.; Madden, J.D.; Hunter, I.W. Large strain actuation in polypyrrole actuators. Proc. SPIE 2004, 5385, 380–387. [Google Scholar]
- Grote, F.; Lei, Y. A complete three-dimensionally nanostructured asymmetric supercapacitor with high operating voltage window based on PPy and MnO2. Nano Energy 2014, 10, 63–70. [Google Scholar] [CrossRef]
- De Oliveira, H.P.; Sydlik, S.A.; Swager, T.M. Supercapacitors from free-standing polypyrrole/graphene nanocomposites. J. Phys. Chem. C. 2013, 20, 10270–10276. [Google Scholar] [CrossRef]
- Li, P.; Shi, E.; Yang, Y.; Shang, Y.; Peng, Q.; Wu, S.; Wei, J.; Wang, K.; Zhu, H.; Yuan, Q.; et al. Carbon nanotube-polypyrrole core-shell sponge and its application as highly compressible supercapacitor electrode. Nano Res. 2014, 7, 209–218. [Google Scholar] [CrossRef]
- Sun, J.; Huang, Y.; Fu, C.; Wang, Z.; Huang, Y.; Zhu, M.; Zhi, C.; Hu, H. High-performance stretchable yarn supercapacitor based on PPy@CNTs@urethane elastic fiber core spun yarn. Nano Energy 2016, 27, 230–237. [Google Scholar] [CrossRef]
- Suppes, G.M.; Deore, B.A.; Freund, M.S. Porous conducting polymer/heteropolyoxometalate hybrid material for electrochemical supercapacitor applications. Langmuir 2008, 24, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Pan, L.; Liu, B.; Wang, Y.; Cui, Y.; Bao, Z.; Yu, G. Nanostructured conducting polypyrrole hydrogels as high-performance, flexible supercapacitor electrodes. J. Mater. Chem. A 2014, 2, 6086–6091. [Google Scholar] [CrossRef]








| DBS | DBS-PT | IC | TFSI | Polyol-Borate | |
|---|---|---|---|---|---|
| Testing Solution | NaDBS, aq. | LiTFSI, aq. | LiCl, aq. | LiTFSI, org. | Saline, aq. |
| Strain | 2% (L), 10.4%(B) | 3.8% (L) | 22.9‒34% (V) | 118% (B) | |
| Stress | 0.62 MPa | 0.86 MPa | 16 MPa | 6.7‒10.5 MPa | |
| Actuation Speed | 0.05 Hz | 0.05 Hz | 0.02 Hz | 0.01 Hz | 0.04 Hz |
| Efficiency | 0.6%/mC | 1.9%/mC | |||
| Conductivity | 0.5 S/cm | 8.5 S/cm | 129 S/cm | 92.2 S/cm | |
| Tensile Tolerance | 15 MPa | 27 MPa | 13.5 MPa | ||
| Reference | [35,36,37,38] | [39,40,41,42,43] | [44,45,46] | [33,47,48,49,50] | [51,52] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, B.; Wu, Y.; Guo, L. Recent Advances on Polypyrrole Electroactuators. Polymers 2017, 9, 446. https://doi.org/10.3390/polym9090446
Yan B, Wu Y, Guo L. Recent Advances on Polypyrrole Electroactuators. Polymers. 2017; 9(9):446. https://doi.org/10.3390/polym9090446
Chicago/Turabian StyleYan, Bingxi, Yu Wu, and Liang Guo. 2017. "Recent Advances on Polypyrrole Electroactuators" Polymers 9, no. 9: 446. https://doi.org/10.3390/polym9090446