Imidazolium Ionic Liquid Modified Graphene Oxide: As a Reinforcing Filler and Catalyst in Epoxy Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
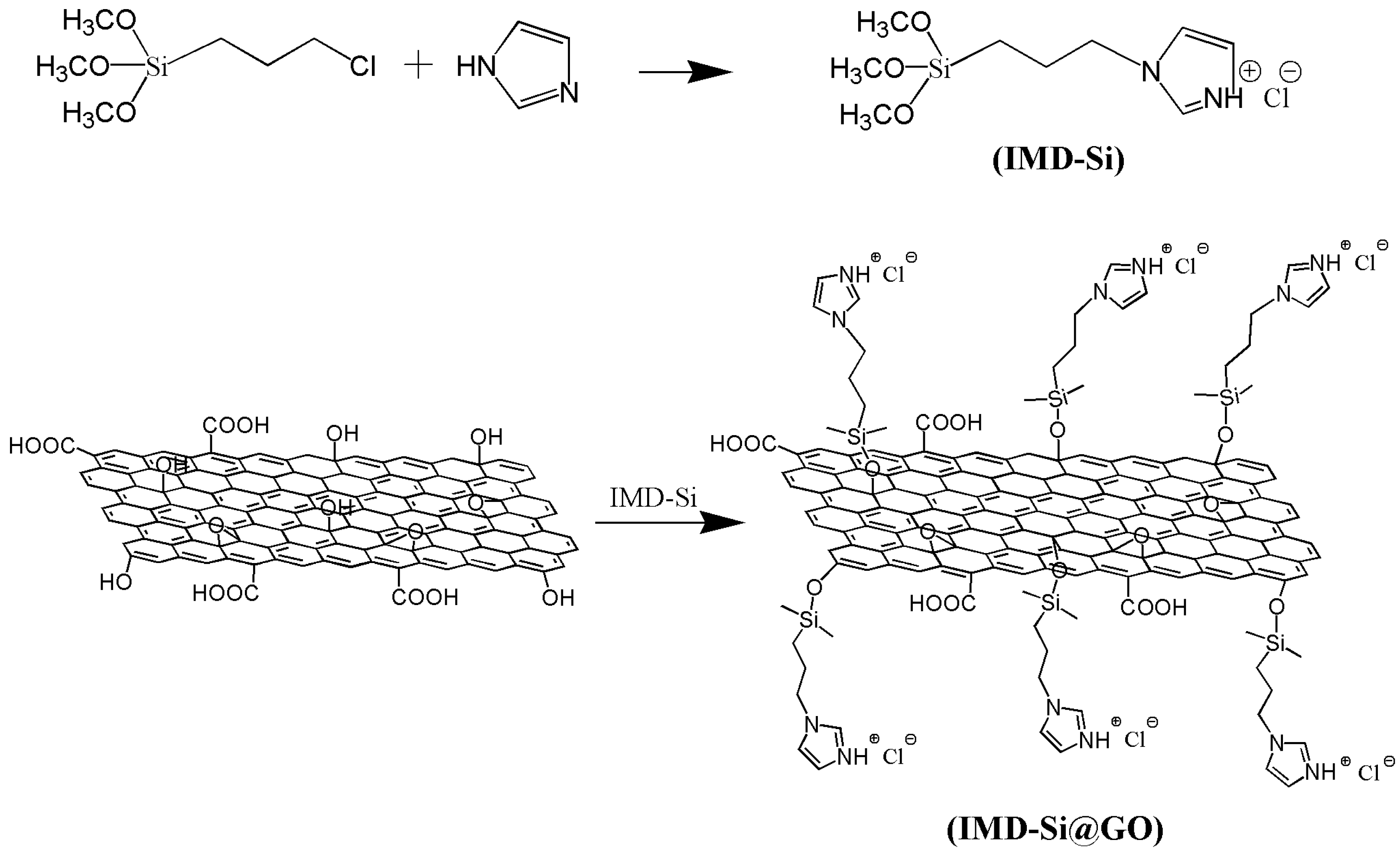
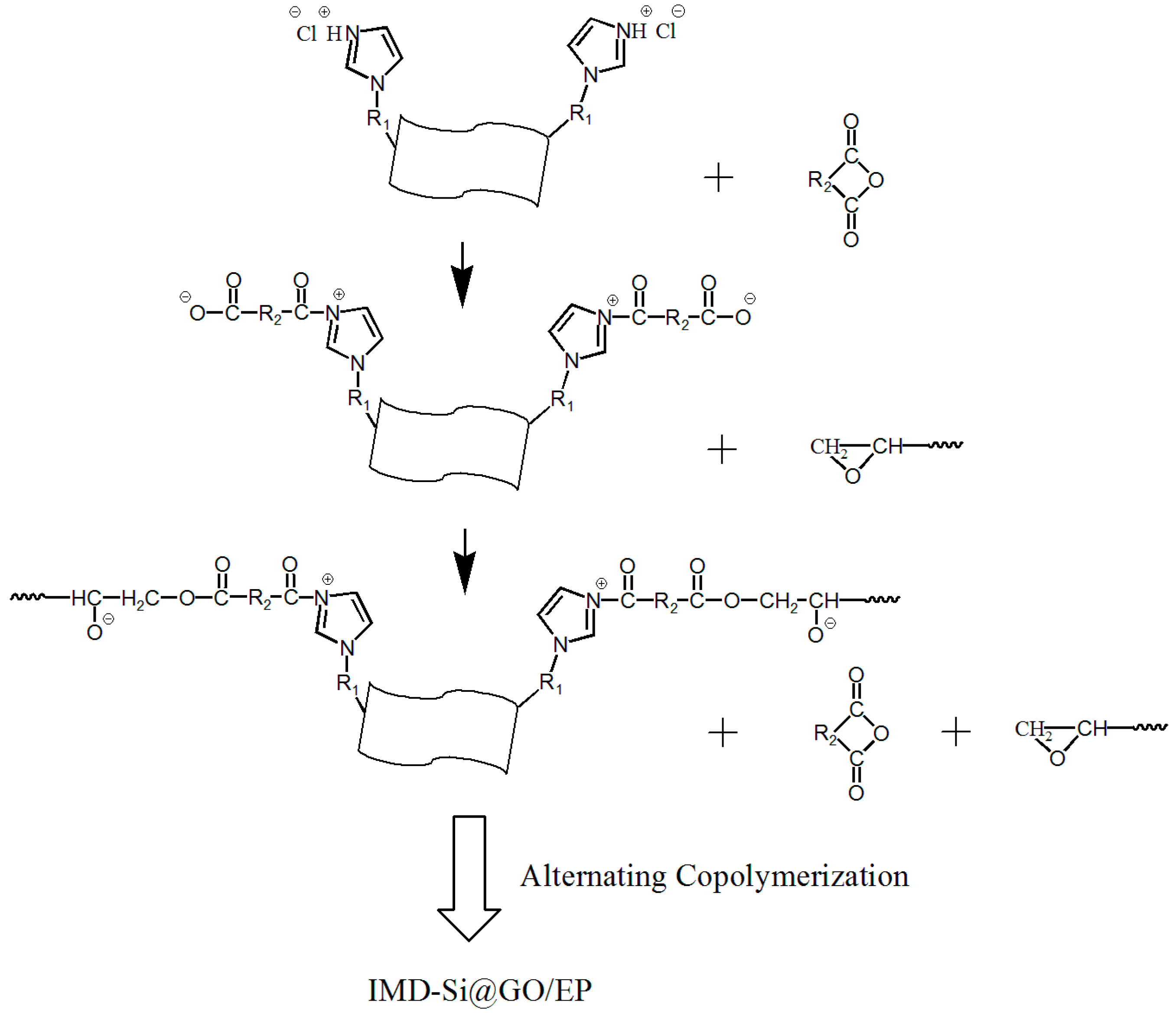
2.2. Synthesis of IMD-Si@GO
2.3. Fabrication of Epoxy Composites
2.4. Characterization
3. Results and Discussion
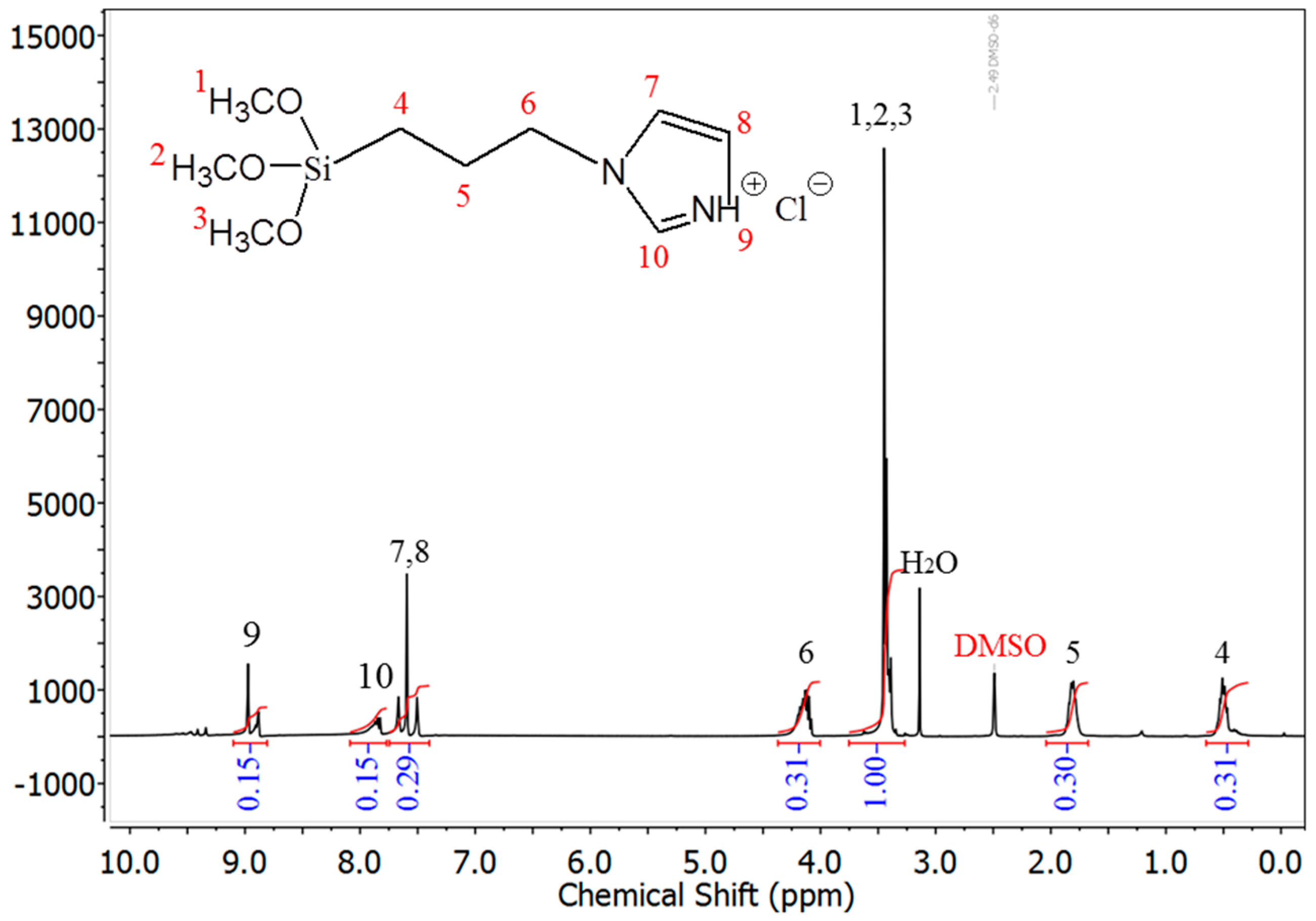
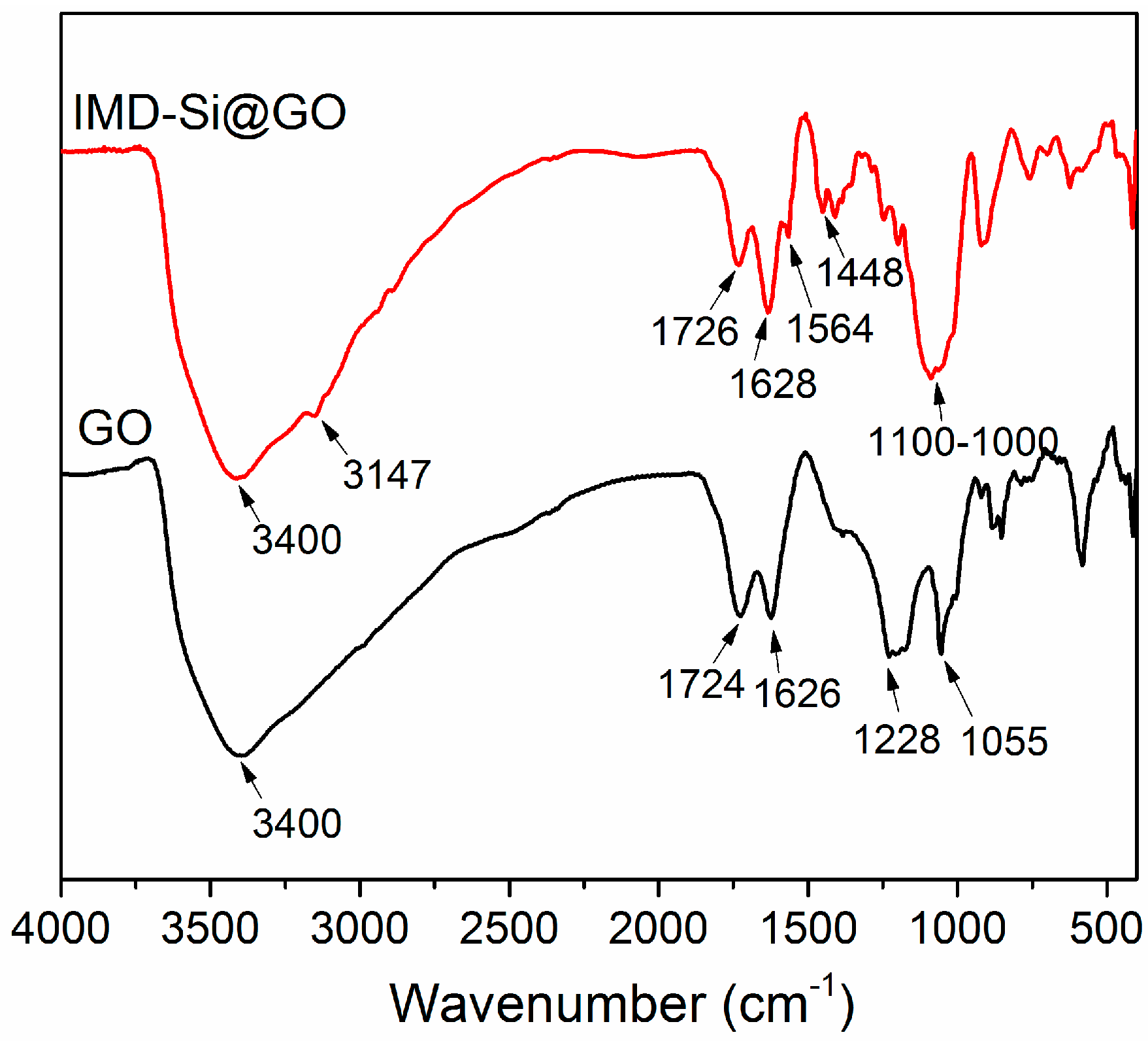
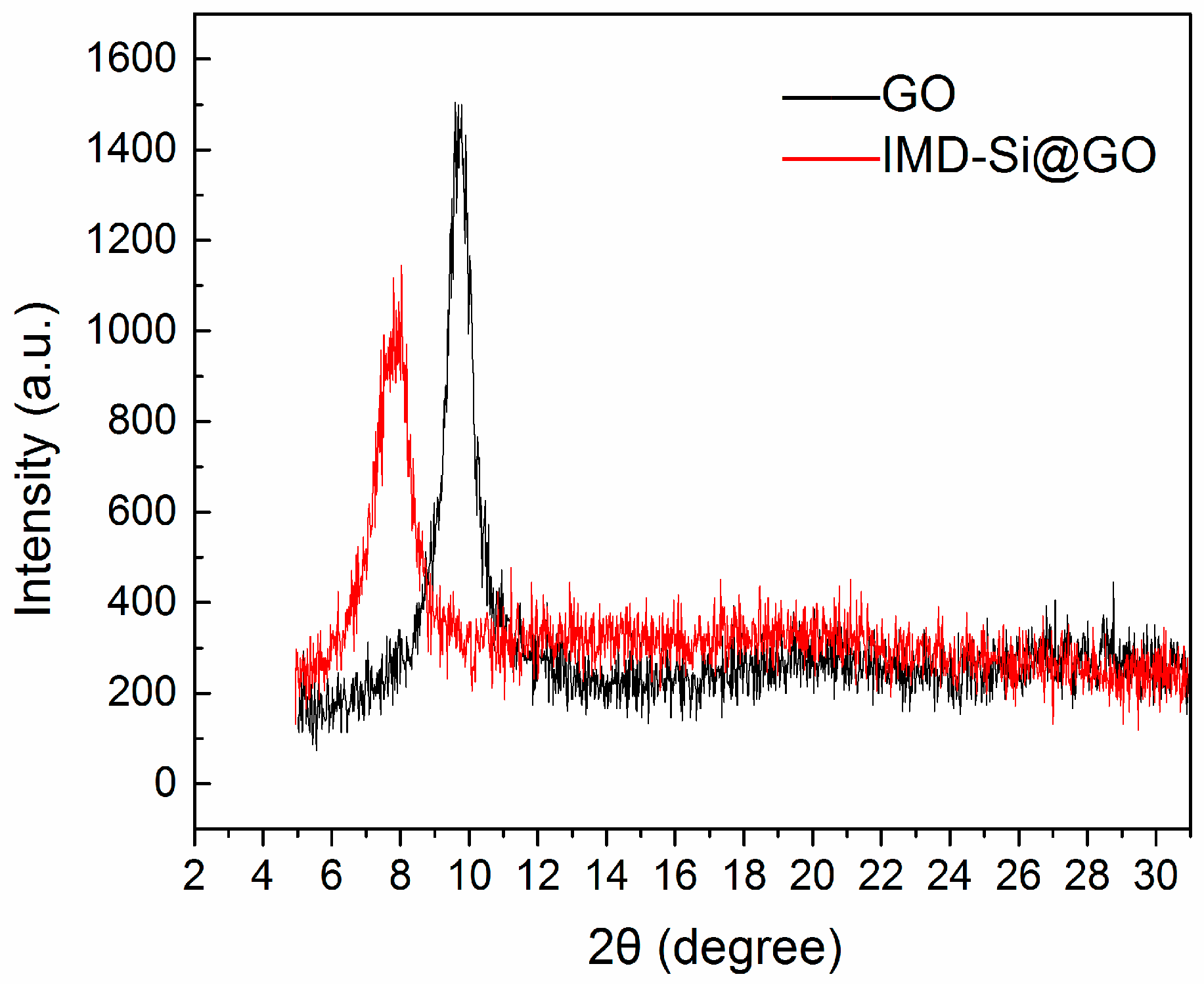
3.1. Characterization of the IMD-Si@GO
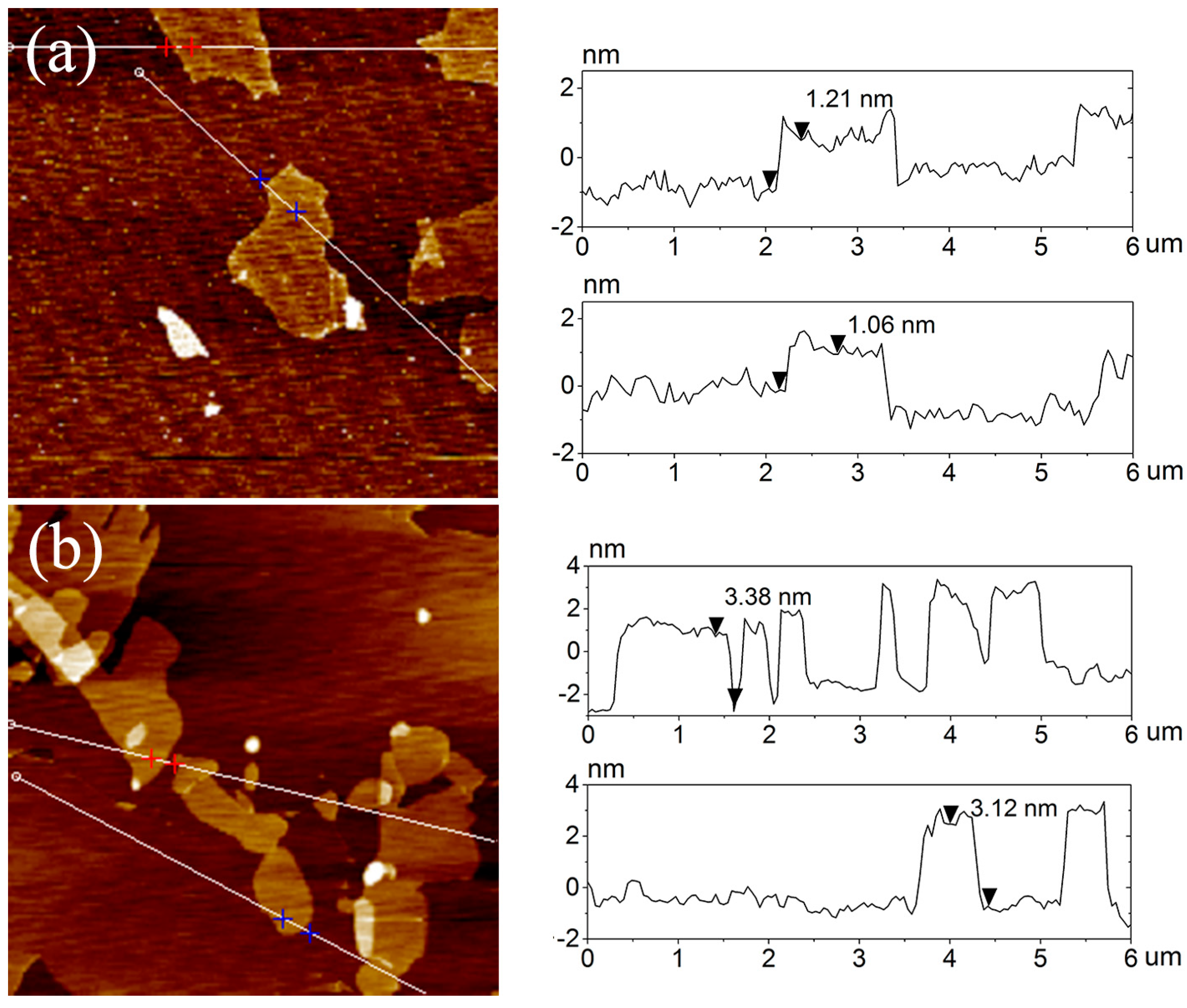
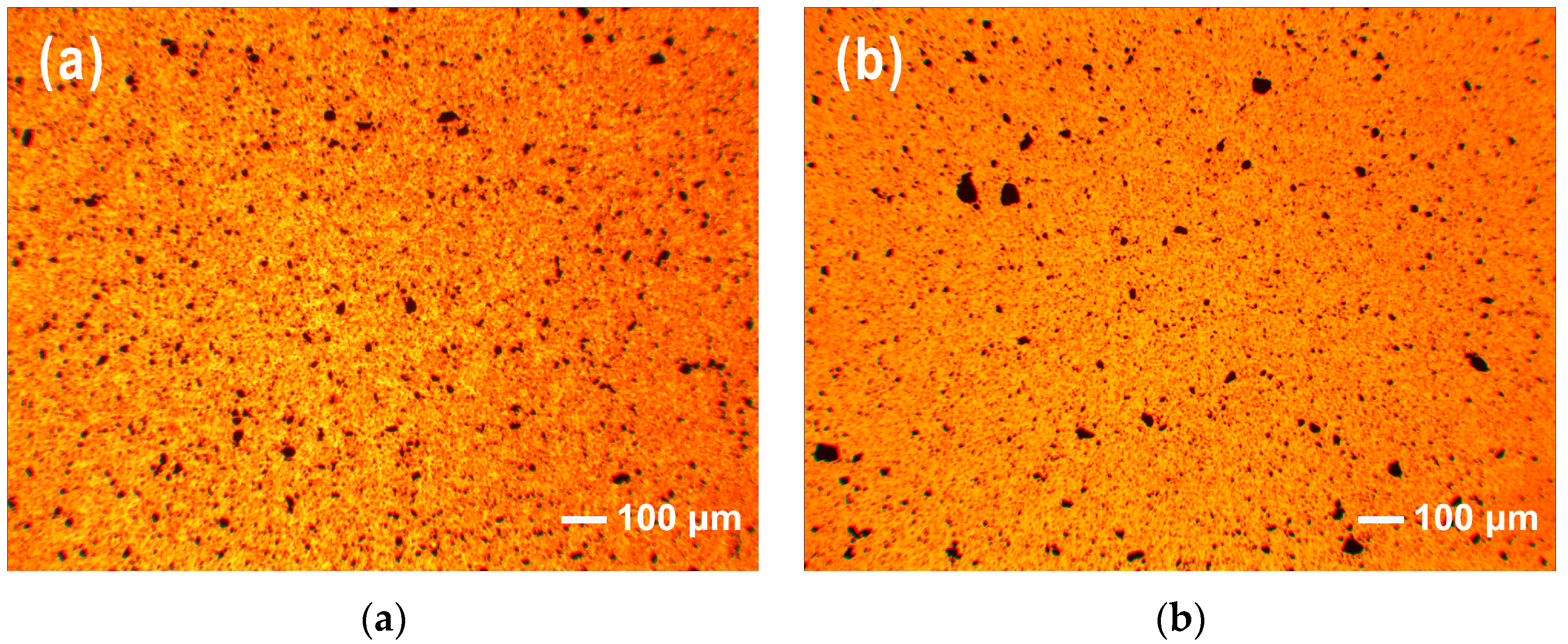
3.2. Dispersion and Exfoliation of IMD-Si@GO
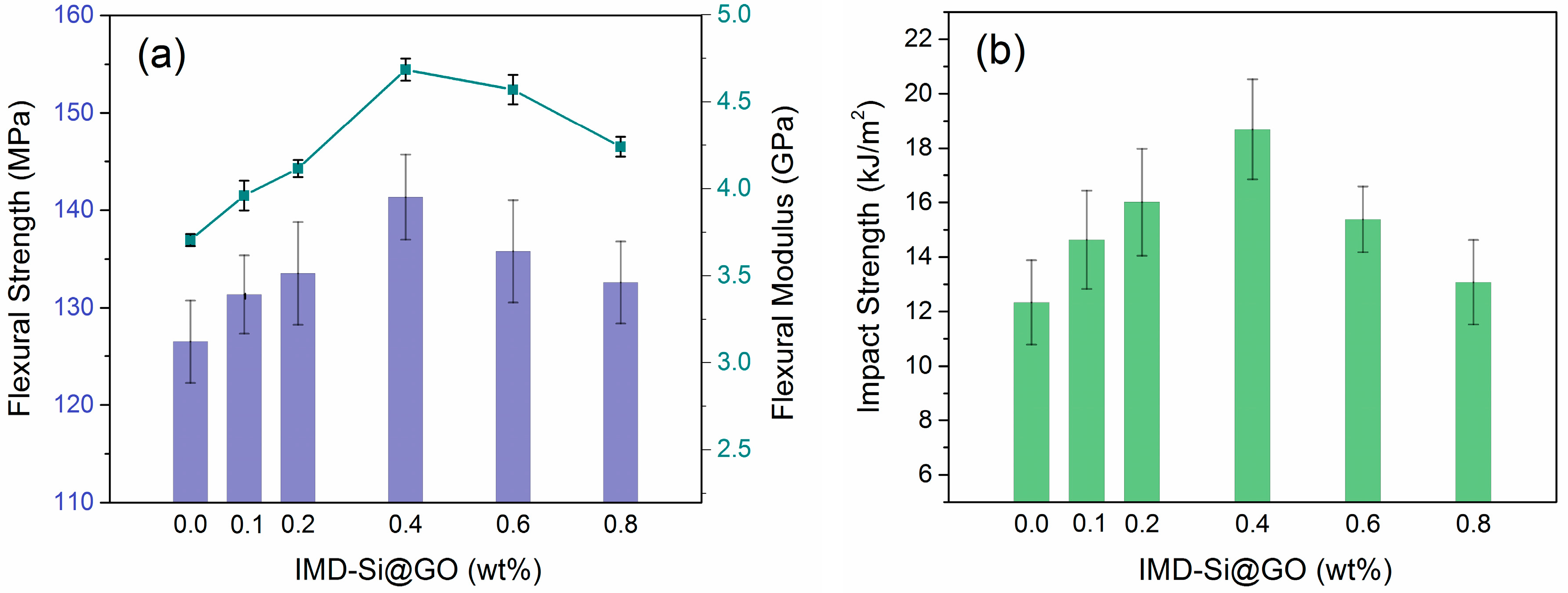
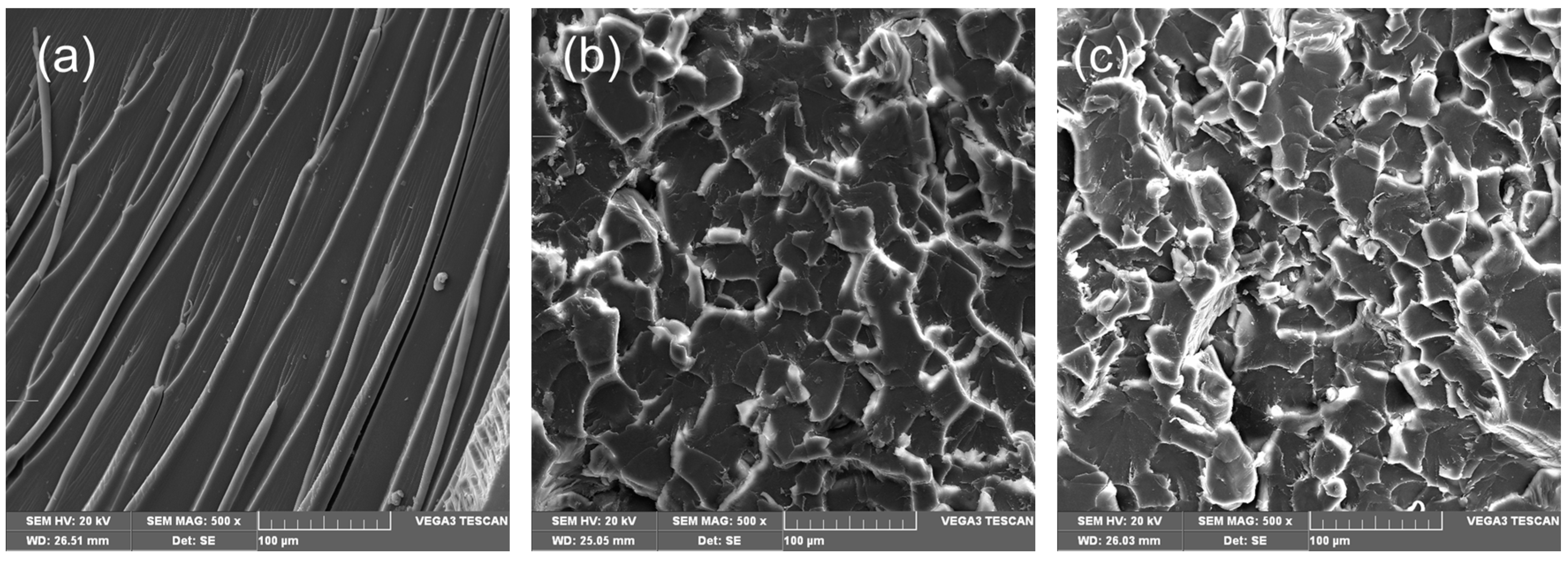
3.3. Mechanical Properties of IMD-Si@GO/EP
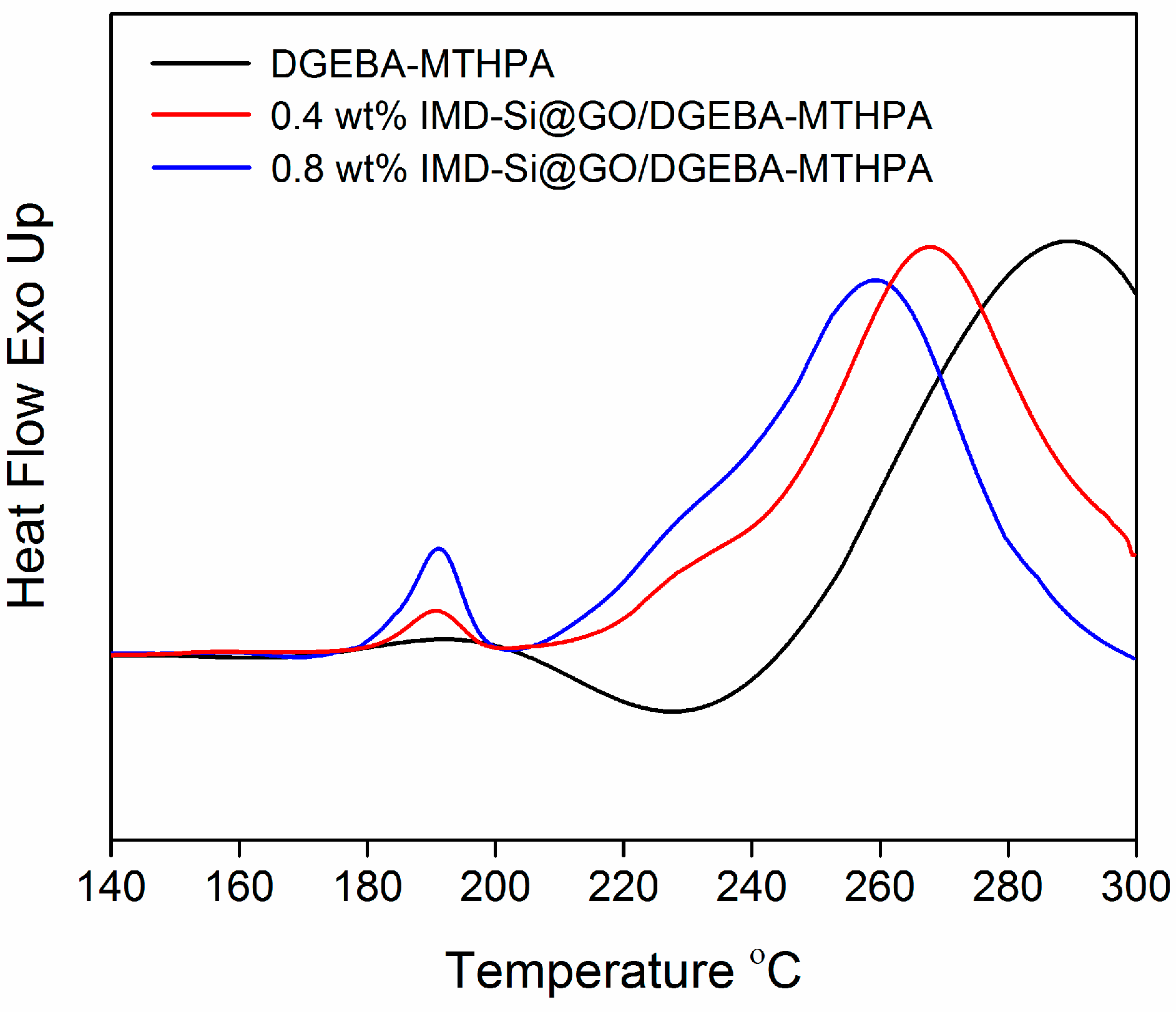
3.4. Catalytic Effect of IMD-Si@GO in Epoxy Resin-Anhydride System
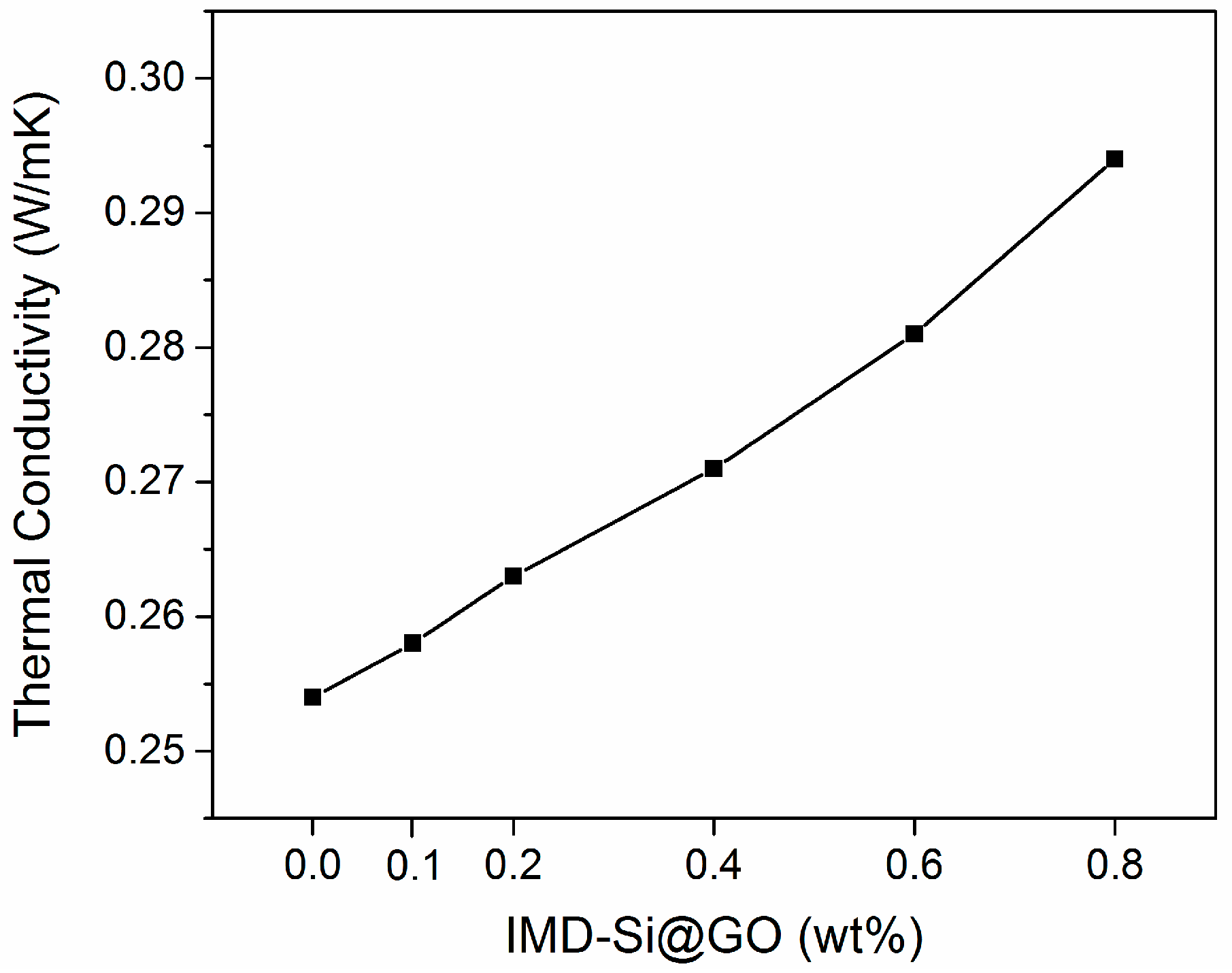
3.5. Thermal Conductivities of IMD-Si@GO/EP
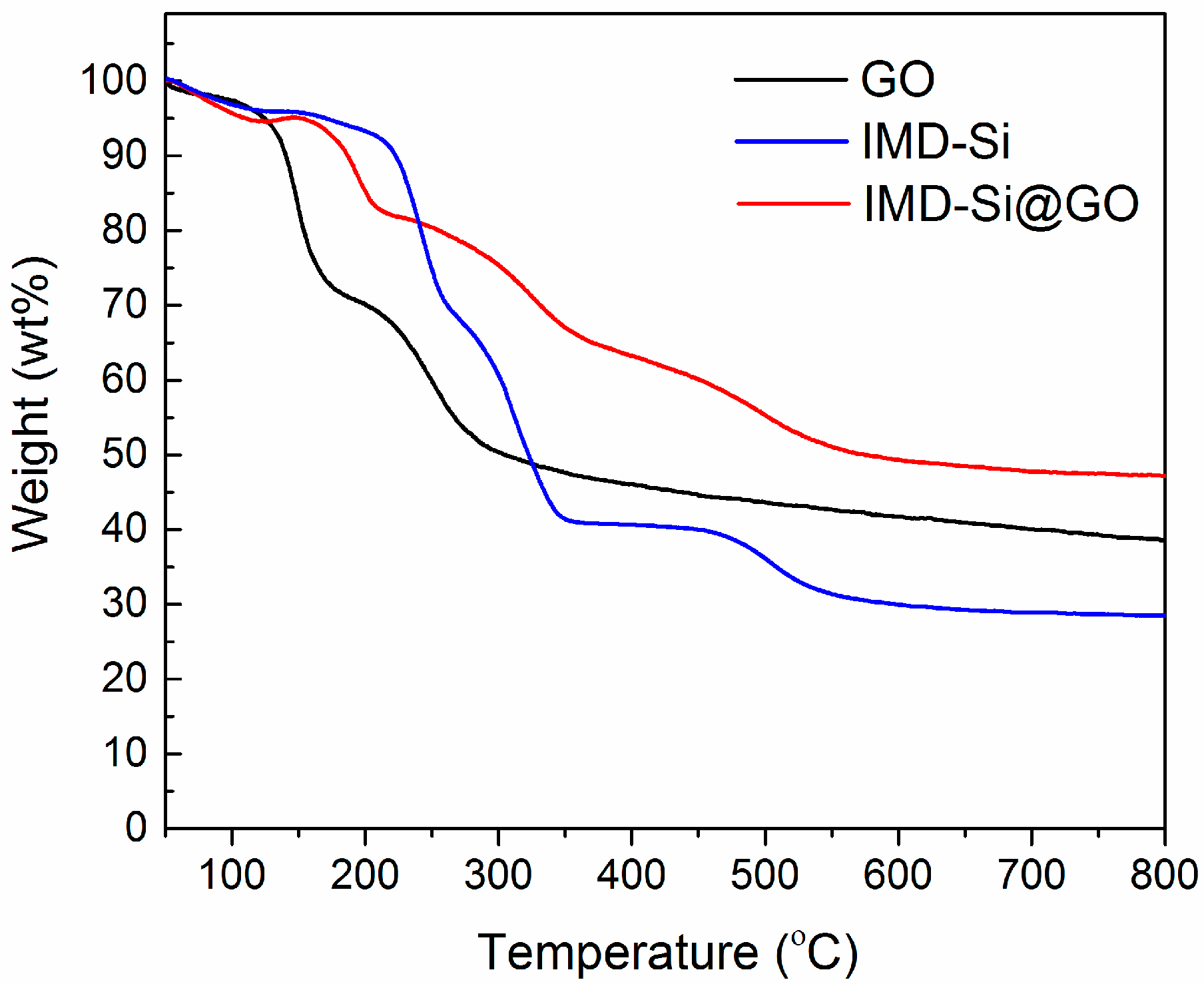
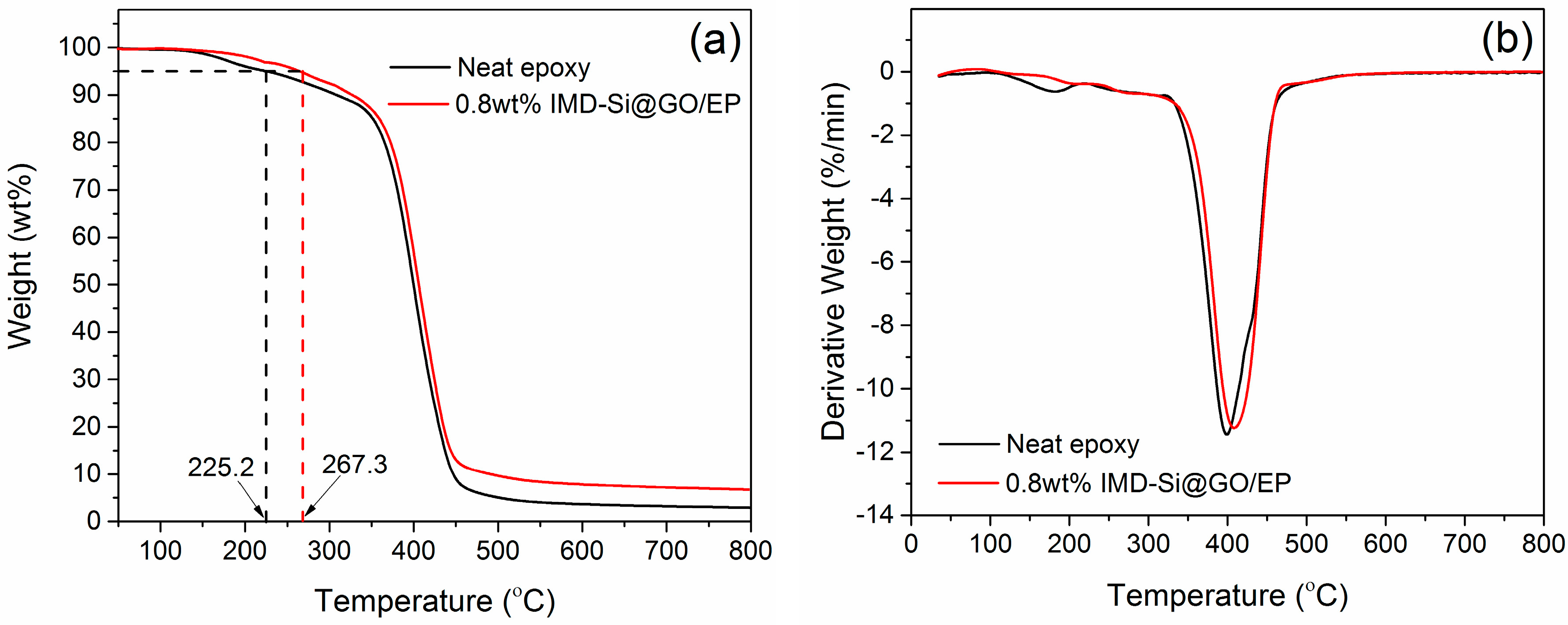
3.6. Thermal Stabilities of IMD-Si@GO/EP
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gao, J.; Li, J.; Benicewicz, B.C.; Zhao, S.; Hillborg, H.; Schadler, L.S. The mechanical properties of epoxy composites filled with rubbery copolymer grafted SiO2. Polymers 2012, 4, 187–210. [Google Scholar] [CrossRef]
- Hu, Y.; Du, G.; Chen, N. A novel approach for Al2O3/epoxy composites with high strength and thermal conductivity. Compos. Sci. Technol. 2016, 124, 36–43. [Google Scholar] [CrossRef]
- Pour, Z.S.; Ghaemy, M. Fabrication and characterization of superparamagnetic nanocomposites based on epoxy resin and surface-modified γ-Fe2O3, by epoxide functionalization. J. Mater. Sci. 2014, 49, 4191–4201. [Google Scholar]
- Cha, J.; Jin, S.; Shim, J.H.; Chong, S.P.; Ryu, H.J.; Hong, S.H. Functionalization of carbon nanotubes for fabrication of CNT/epoxy nanocomposites. Mater. Des. 2016, 95, 1–8. [Google Scholar]
- Hu, K.; Kulkarni, D.D.; Choi, I.; Tsukruk, V.V. Graphene-polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 2014, 39, 1934–1972. [Google Scholar] [CrossRef]
- Atif, R.; Shyha, I.; Inam, F. Mechanical, thermal, and electrical properties of graphene-epoxy nanocomposites-a review. Polymers 2016, 8, 281. [Google Scholar] [CrossRef]
- Zhu, Y.; James, D.K.; Tour, J.M. ChemInform abstract: new routes to graphene, graphene oxide and their related applications. Adv. Mater. 2012, 24, 4924–4955. [Google Scholar] [CrossRef] [PubMed]
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A.; Bagherzadeh, M.R. Distinctive roles of silane coupling agents on the corrosion inhibition performance of graphene oxide in epoxy coatings. Prog. Org. Coat. 2017, 111, 47–56. [Google Scholar] [CrossRef]
- Wan, Y.; Gong, L.; Tang, L.; Wu, L.; Jiang, J. Mechanical properties of epoxy composites filled with silane-functionalized graphene oxide. Compos. Part A 2014, 64, 79–89. [Google Scholar] [CrossRef]
- Sharif, F.; Gudarzi, M.M. Enhancement of dispersion and bonding of graphene-polymer through wet transfer of functionalized graphene oxide. Express. Polym. Lett. 2012, 6, 1017–1031. [Google Scholar] [Green Version]
- Jiang, T.; Kuila, T.; Kim, N.H.; Lee, J.H. Effects of surface-modified silica nanoparticles attached graphene oxide using isocyanate-terminated flexible polymer chains on the mechanical properties of epoxy composites. J. Mater. Chem. A 2014, 2, 10557–10567. [Google Scholar] [CrossRef]
- Wan, Y.; Tang, L.; Gong, L.; Yan, D.; Li, Y.; Wu, L.; Jiang, J.; Lai, G. Grafting of epoxy chains onto graphene oxide for epoxy composites with improved mechanical and thermal properties. Carbon 2014, 69, 467–480. [Google Scholar] [CrossRef]
- Ryu, S.H.; Sin, J.H.; Shanmugharaj, A.M. Study on the effect of hexamethylene diamine functionalized graphene oxide on the curing kinetics of epoxy nanocomposites. Eur. Polym. J. 2014, 52, 88–97. [Google Scholar] [CrossRef]
- Dupont, J.; Souza, R.F.; Suarez, P.A. Ionic liquid (molten salt) phase organometallic catalysis. Chem. Rev. 2002, 102, 3667–3692. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Luo, F.; Wu, H.; Liu, Y.; Zhang, C.; Chen, J. One-step ionic-liquid-assisted electrochemical synthesis of ionic-liquid-functionalized graphene sheets directly from graphite. Adv. Funct. Mater. 2008, 18, 1518–1525. [Google Scholar] [CrossRef]
- Yang, H.; Shan, C.; Li, F.; Han, D.; Zhang, Q.; Niu, L. Covalent functionalization of polydisperse chemically-converted graphene sheets with amine-terminated ionic liquid. Chem. Commun. 2009, 45, 3880–3882. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wang, H.; Bi, S.; Xue, Y.; Xue, Z.; Zhou, X.; Xie, X.; Mai, Y. High performance composite polymer electrolyte using polymeric ionic liquid-functionalized graphene molecular brushes. J. Mater. Chem. A 2015, 3, 18064–18073. [Google Scholar] [CrossRef]
- Wu, W.; Wang, J.; Liu, J.; Chen, P.; Zhang, H.; Huang, J. Intercalating ionic liquid in graphene oxide to create efficient and stable anhydrous proton transfer highways for polymer electrolyte membrane. Int. J. Hydrog. Energy 2017, 42, 11400–11410. [Google Scholar] [CrossRef]
- Bouillon, N.; Pascault, J.P.; Lan, T. Influence of different imidazole catalysts on epoxy-anhydride copolymerization and on their network properties. J. Appl. Polym. Sci. 2010, 38, 2103–2113. [Google Scholar] [CrossRef]
- Pour, Z.S.; Ghaemy, M. Polymer grafted graphene oxide: For improved dispersion in epoxy resin and enhancement of mechanical properties of nanocomposite. Compos. Sci. Technol. 2016, 136, 145–157. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, M.; Qu, H.; Zhang, X.; Wei, H.; Luo, Z.; Colorado, H.A.; Wei, S.; Guo, Z. Interfacial polymerized polyaniline/graphite oxide nanocomposites toward electrochemical energy storage. Polymers 2012, 53, 5953–5964. [Google Scholar] [CrossRef]
- Kooti, M.; Afshari, M. Phosphotungstic acid supported on magnetic nanoparticles as an efficient reusable catalyst for epoxidation of alkenes. Mater. Res. Bull. 2012, 47, 3473–3478. [Google Scholar] [CrossRef]
- Xu, J.; Xu, M.; Wu, J.; Wu, H.; Zhang, W.; Li, Y. Graphene oxide immobilized with ionic liquids: Facile preparation and efficient catalysis for solvent-free cycloaddition of CO2 to propylene carbonate. RSC Adv. 2015, 5, 72361–72368. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascón, J.M.D. Graphene oxide dispersions in organic solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, Y.; Dai, J.; Lang, M.; Huang, X. An efficient way to functionalize graphene sheets with presynthesized polymer via ATNRC chemistry. J. Polym. Sci. Polym. Chem. 2015, 49, 1582–1590. [Google Scholar] [CrossRef]
- Acik, M.; Dreyer, D.R.; Bielawski, C.W.; Chabal, Y.J. Impact of ionic liquids on the exfoliation of graphite Oxide. J. Phys. Chem. C 2012, 116, 7867–7873. [Google Scholar] [CrossRef]
- Tasviri, M.; Ghasemi, S.; Ghourchian, H.; Gholami, M.R. Ionic liquid/graphene oxide as a nanocomposite for improving the direct electrochemistry and electrocatalytic activity of glucose oxidase. J. Solid State Electrochem. 2013, 17, 183–189. [Google Scholar] [CrossRef]
- Layek, R.K.; Das, A.K.; Min, J.P.; Kim, N.H.; Lee, J.H. Enhancement of physical, mechanical, and gas barrier properties in noncovalently functionalized graphene oxide/poly(vinylidene fluoride) composites. Carbon 2015, 81, 329–338. [Google Scholar] [CrossRef]
- Afshari, M.; Gorjizadeh, M.; Nazari, S.; Naseh, M. Cobalt salophen complex supported on imidazole functionalized magnetic nanoparticles as a recoverable catalyst for oxidation of alkenes. J. Magn. Magn. Mater. 2014, 363, 13–17. [Google Scholar] [CrossRef]
- Ahmadi-Moghadam, B.; Sharafimasooleh, M.; Shadlou, S.; Taheri, F. Effect of functionalization of graphene nanoplatelets on the mechanical response of graphene/epoxy composites. Mater. Des. 2015, 66, 142–149. [Google Scholar] [CrossRef]
- Kleinschmidt, A.C.; Donato, R.K.; Perchacz, M.; Benes, H.; Stengl, V.; Amico, S.C.; Schrekker, H.S. “Unrolling” multi-walled carbon nanotubes with ionic liquids: Application as fillers in epoxy-based nanocomposites. RSC Adv. 2014, 4, 43436–43443. [Google Scholar] [CrossRef]
- Marra, F.; D’Aloia, A.G.; Tamburrano, A.; Ochando, I.M.; Bellis, G.D.; Ellis, G.; Sarto, M.S. Electromagnetic and dynamic mechanical properties of epoxy and vinylester-based composites filled with graphene nanoplatelets. Polymers 2016, 8, 272. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, H.; Liu, T.; Niu, S. Nanosheets of MoS2 and reduced graphene oxide as hybrid fillers improved the mechanical and tribological properties of bismaleimide composites. Compos. Sci. Technol. 2016, 125, 47–54. [Google Scholar] [CrossRef]
- Umboh, M.K.; Adachi, T.; Nemoto, T.; Higuchi, M.; Major, Z. Non-stoichiometric curing effect on fracture toughness of nanosilica particulate-reinforced epoxy composites. J. Mater. Sci. 2014, 49, 7454–7461. [Google Scholar] [CrossRef]
- Liu, C.; Yan, H.; Lv, Q.; Li, S.; Niu, S. Enhanced tribological properties of aligned reduced graphene oxide-Fe3O4@polyphosphazene/bismaleimides composites. Carbon 2016, 102, 145–153. [Google Scholar] [CrossRef]
- Monteserín, C.; Blanco, M.; Aranzabe, E.; Aranzabe, A.; Vilas, J.L. Effects of graphene oxide and chemically reduced graphene oxide on the curing kinetics of epoxy amine composites. J. Appl. Polym. Sci. 2017, 134, 44803–44815. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.S. Curing behavior and physical properties of an epoxy nanocomposite with amine-functionalized graphene nanoplatelets. Compos. Interfaces 2016, 23, 1–13. [Google Scholar] [CrossRef]
- Park, W.H.; Lee, J.K.; Kwon, K.J. Cure behavior of an epoxy-anhydride-imidazole system. Polym. J. 1996, 28, 407–411. [Google Scholar] [CrossRef]
- Gu, J.; Yang, X.; Lv, Z.; Li, N.; Liang, C.; Zhang, Q. Functionalized graphite nanoplatelets/epoxy resin nanocomposites with high thermal conductivity. Int. J. Heat. Mass Transf. 2016, 92, 15–22. [Google Scholar] [CrossRef]
- Wang, R.; Zhuo, D.; Weng, Z.; Wu, L.; Cheng, X.; Zhou, Y.; Wang, J.; Xuan, B. A novel nanosilica/graphene oxide hybrid and its flame retarding epoxy resin with simultaneously improved mechanical, thermal conductivity, and dielectric properties. J. Mater. Chem. A 2015, 3, 9826–9836. [Google Scholar] [CrossRef]
- Teng, C.; Ma, C.M.; Lu, C.; Yang, S.; Lee, S.; Hsiao, M.; Yen, M.; Chiou, K.; Lee, T. Thermal conductivity and structure of non-covalent functionalized graphene/epoxy composites. Carbon 2011, 49, 5107–5116. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, W.; Yu, S.; Sun, R.; Wong, C.; Liao, W. Covalent polymer functionalization of graphene for improved dielectric properties and thermal stability of epoxy composites. Compos. Sci. Technol. 2016, 122, 27–35. [Google Scholar] [CrossRef]



| Element name | C | H | N |
|---|---|---|---|
| IMD-Si@GO (wt %) | 43.64 | 3.07 | 2.87 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, Q.; Yan, H.; Li, L.; Chen, Z.; Yao, H.; Nie, Y. Imidazolium Ionic Liquid Modified Graphene Oxide: As a Reinforcing Filler and Catalyst in Epoxy Resin. Polymers 2017, 9, 447. https://doi.org/10.3390/polym9090447
Lyu Q, Yan H, Li L, Chen Z, Yao H, Nie Y. Imidazolium Ionic Liquid Modified Graphene Oxide: As a Reinforcing Filler and Catalyst in Epoxy Resin. Polymers. 2017; 9(9):447. https://doi.org/10.3390/polym9090447
Chicago/Turabian StyleLyu, Qing, Hongxia Yan, Lin Li, Zhengyan Chen, Huanhuan Yao, and Yufeng Nie. 2017. "Imidazolium Ionic Liquid Modified Graphene Oxide: As a Reinforcing Filler and Catalyst in Epoxy Resin" Polymers 9, no. 9: 447. https://doi.org/10.3390/polym9090447




