Bioconversion of Waste Fiber Sludge to Bacterial Nanocellulose and Use for Reinforcement of CTMP Paper Sheets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Chemicals
2.2. Culture Media
2.3. Preparation of Seed Culture and Inoculation
2.4. Study of Feasibility of Using Fiber Sludge Hydrolysate as a Carbon Source
2.5. Study on Optimization of Cultivation Conditions in STRs
2.6. Analysis of Sugar Concentration
2.7. Measurement of DPv of BNC
2.8. Effects of BNC Addition on the Mechanical Properties of Birch CTMP Paper Sheets
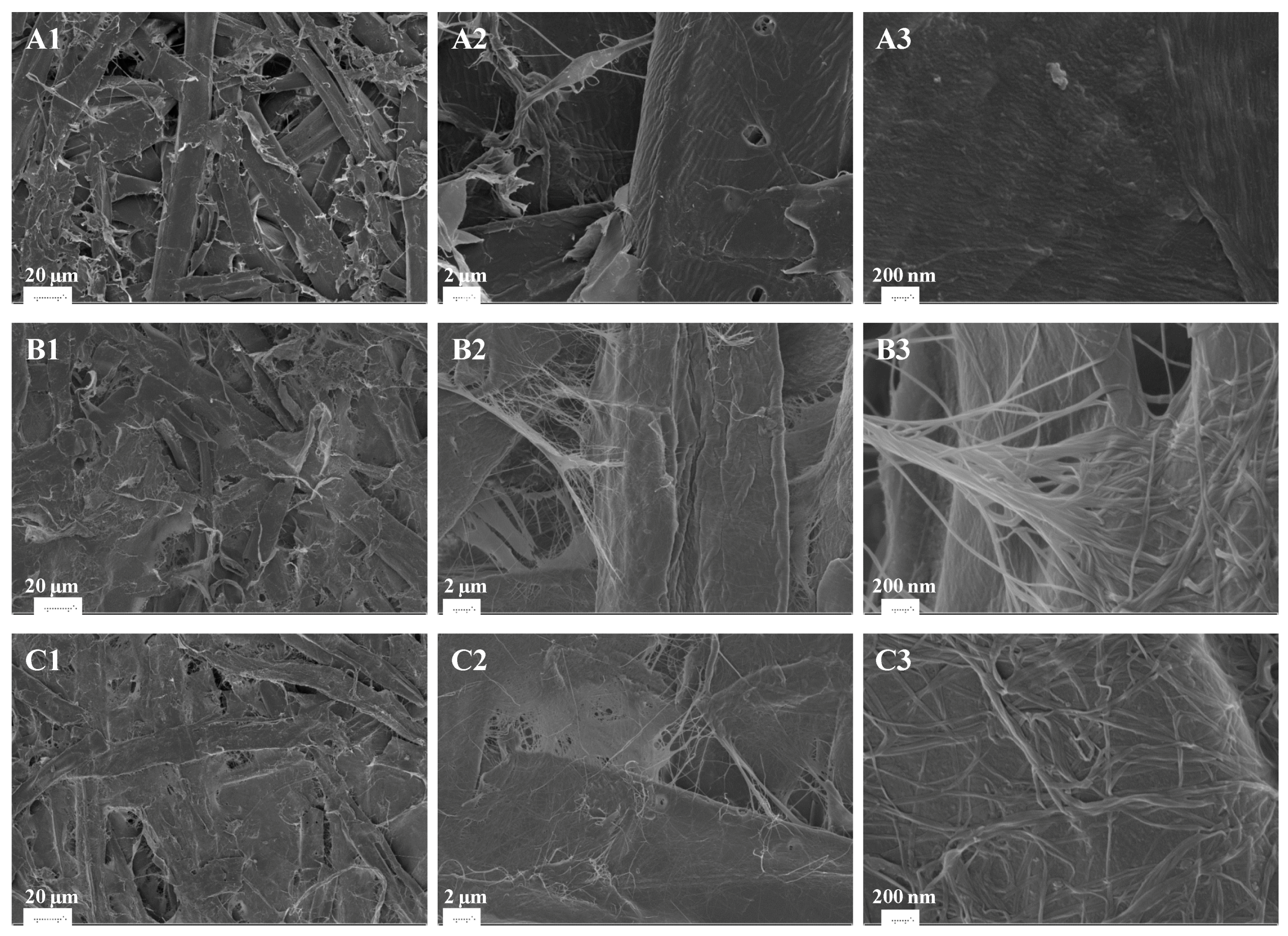
2.9. Scanning Electron Microscopy
3. Results and Discussion
3.1. Feasibility of Using Fiber Sludge Hydrolysate as a Carbon Source in Shake-Flask Cultures
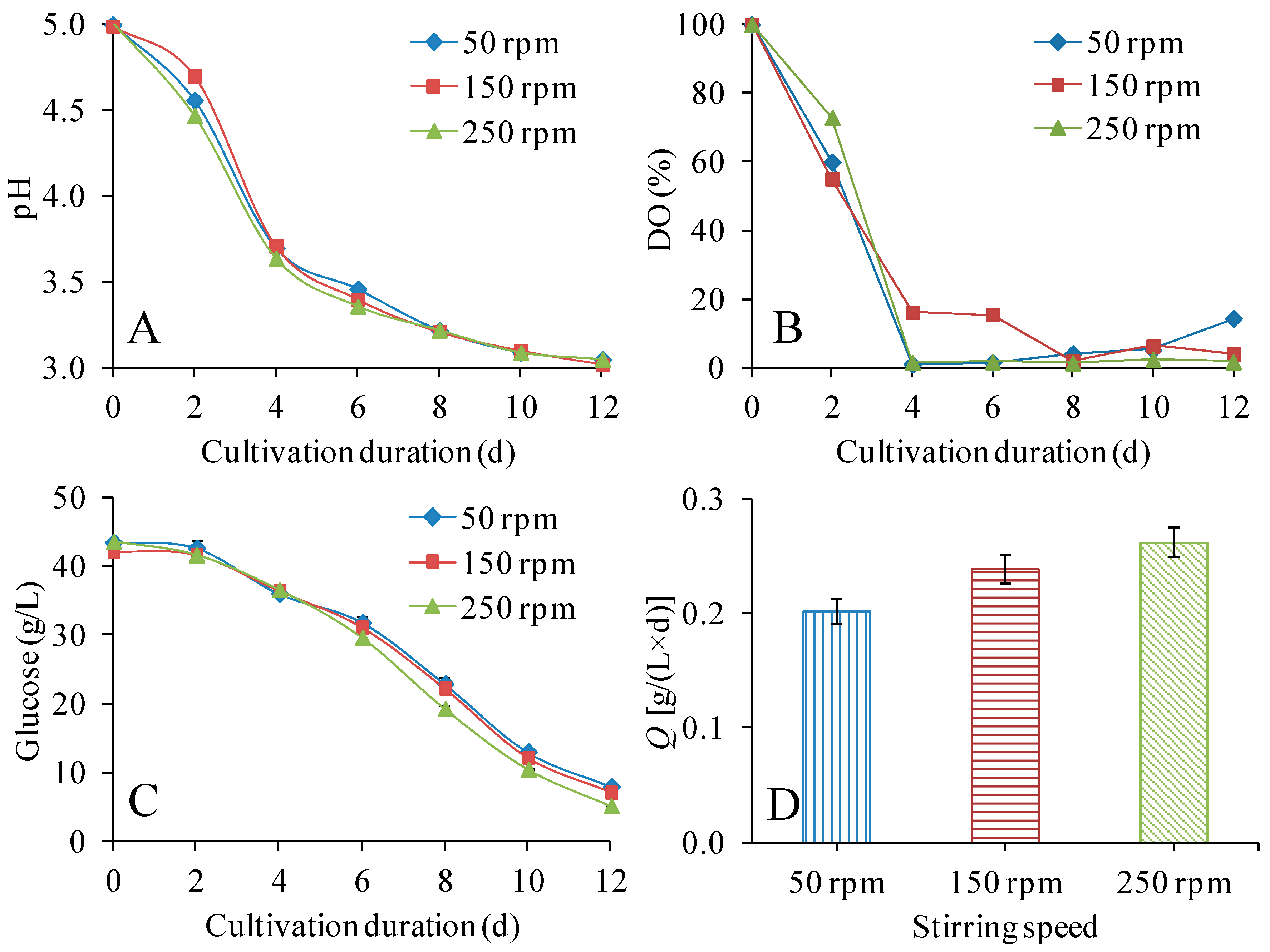
3.2. Effect of Stirring Speed on the Production of BNC in STRs
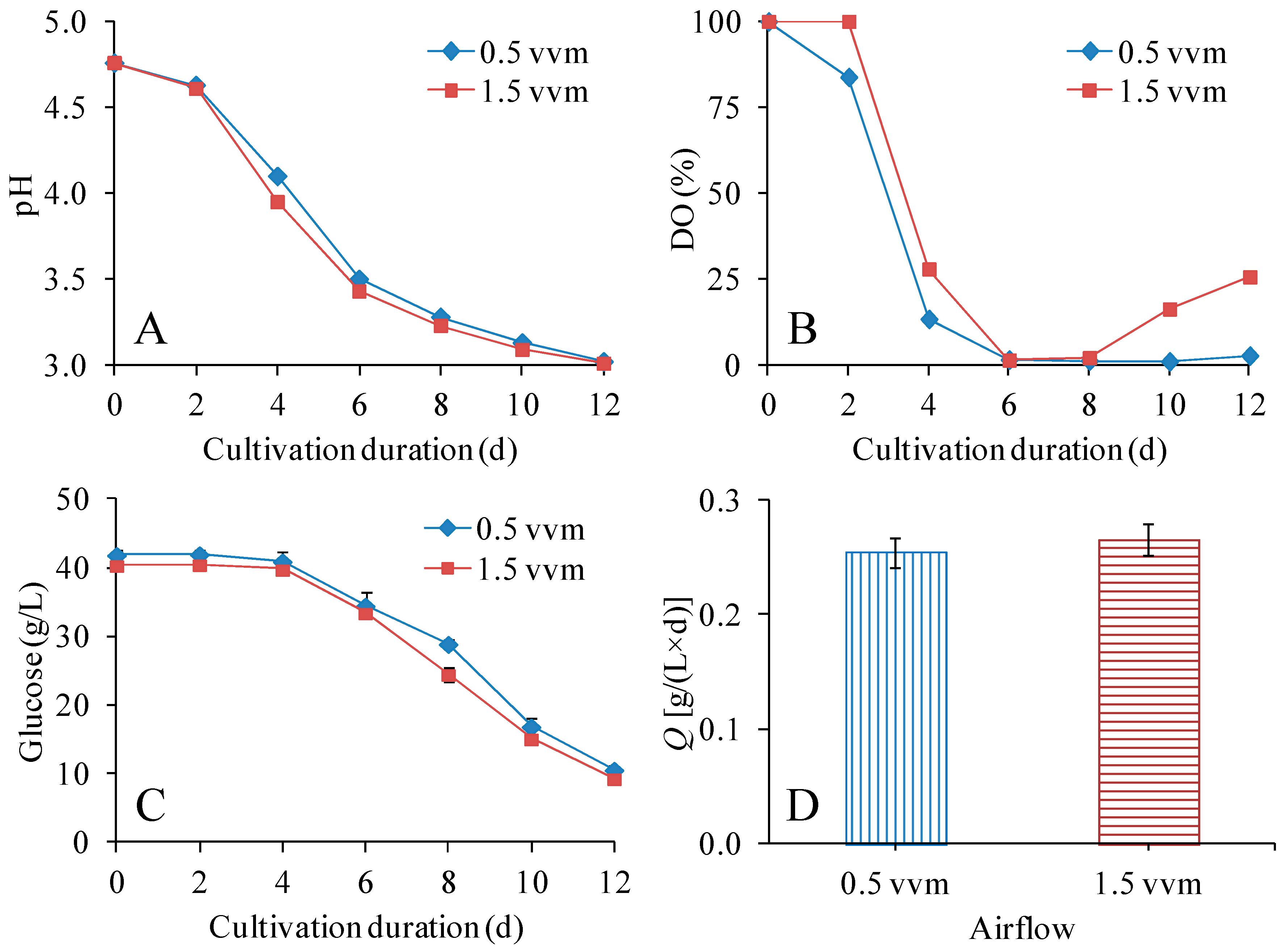
3.3. Effect of Airflow on the Production of BNC in STRs
3.4. Effect of pH Adjustment on the Production of BNC in STRs
3.5. Effects of BNC Addition on the Mechanical Properties of Birch CTMP Paper Sheets
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gama, M.; Gatenholm, P.; Klemm, D. (Eds.) Bacterial Nanocellulose: A Sophisticated Multifunctional Material; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Bielecki, S.; Krystynowicz, A.; Turkiewicz, M.; Kalinowska, H. Bacterial cellulose. In Biopolymers Online; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 5–7. [Google Scholar]
- Mohite, B.V.; Patil, S.V. A novel biomaterial: Bacterial cellulose and its new era applications. Biotechnol. Appl. Biochem. 2014, 61, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-Y.; Buldum, G.; Mantalaris, A.; Bismarck, A. More than meets the eye in bacterial cellulose: Biosynthesis, bioprocessing, and applications in advanced fiber composites. Macromol. Biosci. 2014, 14, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Toyosaki, H.; Naritomi, T.; Seto, A.; Matsuoka, M.; Tsuchida, T.; Yoshinaga, F. Screening of bacterial cellulose-producing Acetobacter strains suitable for agitated culture. Biosci. Biotechnol. Biochem. 1995, 59, 1498–1502. [Google Scholar] [CrossRef]
- White, D.G.; Brown, R.M., Jr. Prospects for the commercialization of the biosynthesis of microbial cellulose. In Cellulose and Wood-Chemistry and Technology; Wiley: Hoboken, NJ, USA, 1989; Volume 573, pp. 573–590. [Google Scholar]
- Cacicedo, M.L.; Castro, M.C.; Servetas, I.; Bosnea, L.; Boura, K.; Tsafrakidou, P.; Dima, A.; Terpou, A.; Koutinas, A.; Castro, G.R. Progress in bacterial cellulose matrices for biotechnological applications. Bioresour. Technol. 2016, 213, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Qiu, K. An alternative carbon source from konjac powder for enhancing production of bacterial cellulose in static cultures by a model strain Acetobacter aceti subsp. xylinus ATCC 23770. Carbohydr. Polym. 2008, 72, 545–549. [Google Scholar] [CrossRef]
- Hong, F.; Zhu, Y.X.; Yang, G.; Yang, X.X. Wheat straw acid hydrolysate as a potential cost-effective feedstock for production of bacterial cellulose. J. Chem. Technol. Biotechnol. 2011, 86, 675–680. [Google Scholar] [CrossRef]
- Chen, L.; Hong, F.; Yang, X.-X.; Han, S.-F. Biotransformation of wheat straw to bacterial cellulose and its mechanism. Bioresour. Technol. 2013, 135, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Cavka, A.; Guo, X.; Tang, S.-J.; Winestrand, S.; Jönsson, L.J.; Hong, F. Production of bacterial cellulose and enzyme from waste fiber sludge. Biotechnol. Biofuels 2013, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cavka, A.; Jönsson, L.J.; Hong, F. Comparison of methods for detoxification of spruce hydrolysate for bacterial cellulose production. Microb. Cell Fact. 2013, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Guo, X.; Zhang, S.; Han, S.-F.; Yang, G.; Jönsson, L.J. Bacterial cellulose production from cotton-based waste textiles: Enzymatic saccharification enhanced by ionic liquid pretreatment. Bioresour. Technol. 2012, 104, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, L.; Tang, J.; Jönsson, L.J.; Hong, F.F. Production of bacterial nanocellulose and enzyme from [Amim]Cl-pretreated waste cotton fabrics: Effects of dyes on enzymatic saccharification and nanocellulose production. J. Chem. Technol. Biotechnol. 2016, 91, 1413–1421. [Google Scholar] [CrossRef]
- Zhang, S.; Winestrand, S.; Guo, X.; Chen, L.; Hong, F.; Jönsson, L.J. Effects of aromatic compounds on the production of bacterial nanocellulose by Gluconacetobacter xylinus. Microb. Cell Fact. 2014, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Winestrand, S.; Chen, L.; Li, D.; Jönsson, L.J.; Hong, F. Tolerance of the nanocellulose-producing bacterium Gluconacetobacter xylinus to lignocellulose-derived acids and aldehydes. J. Agric. Food Chem. 2014, 62, 9792–9799. [Google Scholar] [CrossRef] [PubMed]
- Kouda, T.; Yano, H.; Yoshinaga, F. Effect of agitator configuration on bacterial cellulose productivity in aerated and agitated culture. J. Biosci. Bioeng. 1997, 83, 371–376. [Google Scholar] [CrossRef]
- Yoshinaga, F.; Tonouchi, N.; Watanabe, K. Research progress in production of bacterial cellulose by aeration and agitation culture and its application as a new industrial material. Biosci. Biotechnol. Biochem. 1997, 61, 219–224. [Google Scholar] [CrossRef]
- Bae, S.; Shoda, M. Statistical optimization of culture conditions for bacterial cellulose production using Box-Behnken design. Biotechnol. Bioeng. 2005, 90, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.W.; Yang, Y.K.; Hwang, J.K.; Pyun, Y.R.; Kim, Y.S. Effects of pH and dissolved oxygen on cellulose production by Acetobacter xylinum BRC5 in agitated culture. J. Biosci. Bioeng. 1999, 88, 183–188. [Google Scholar] [CrossRef]
- Kouda, T.; Naritomi, T.; Yano, H.; Yoshinaga, F. Effects of oxygen and carbon dioxide pressures on bacterial cellulose production by Acetobacter in aerated and agitated culture. J. Ferment. Bioeng. 1997, 84, 124–127. [Google Scholar] [CrossRef]
- Jung, J.Y.; Khan, T.; Park, J.K.; Chang, H.N. Production of bacterial cellulose by Gluconacetobacter hansenii using a novel bioreactor equipped with a spin filter. Korean J. Chem. Eng. 2007, 24, 265–271. [Google Scholar] [CrossRef]
- Yamanaka, S.; Watanabe, K.; Kitamura, N.; Iguchi, M.; Mitsuhashi, S.; Nishi, Y.; Uryu, M. The structure and mechanical properties of sheets prepared from bacterial cellulose. J. Mater. Sci. 1989, 24, 3141–3145. [Google Scholar] [CrossRef]
- Gao, W.-H.; Chen, K.-F.; Yang, R.-D.; Yang, F.; Han, W.-J. Properties of bacterial cellulose and its influence on the physical properties of paper. BioResources 2010, 6, 144–153. [Google Scholar]
- Santos, S.M.; Carbajo, J.M.; Gómez, N.; Quintana, E.; Ladero, M.; Sánchez, A.; Chinga-Carrasco, G.; Villar, J.C. Use of bacterial cellulose in degraded paper restoration. Part II: Application on real samples. J. Mater. Sci. 2016, 51, 1553–1561. [Google Scholar] [CrossRef]
- Santos, S.M.; Carbajo, J.M.; Gómez, N.; Quintana, E.; Ladero, M.; Sánchez, A.; Chinga-Carrasco, G.; Villar, J.C. Use of bacterial cellulose in degraded paper restoration. Part I: Application on model papers. J. Mater. Sci. 2016, 51, 1541–1552. [Google Scholar] [CrossRef]
- Nishi, Y.; Uryu, M.; Yamanaka, S.; Watanabe, K.; Kitamura, N.; Iguchi, M.; Mitsuhashi, S. The structure and mechanical properties of sheets prepared from bacterial cellulose Part 2 Improvement of the mechanical properties of sheets and their applicability to diaphragms of electroacoustic transducers. J. Mater. Sci. 1990, 25, 2997–3001. [Google Scholar] [CrossRef]
- Shah, J.; Malcolm Brown, R. Towards electronic paper displays made from microbial cellulose. Appl. Microbiol. Biotechnol. 2005, 66, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Winslow, A. Bacterial cellulose has potential application as new paper coating. Pulp Pap. 1990, 64, 105–107. [Google Scholar]
- Johnson, D.C.; LeBlanc, H.A.; Neogi, A.N. Bacterial cellulose as surface treatment for fibrous web. U.S. Patent 4,861,427A, 29 August 1988. [Google Scholar]
- Watanabe, K.; Tabuchi, M.; Morinaga, Y.; Yoshinaga, F. Structural features and properties of bacterial cellulose produced in agitated culture. Cellulose 1998, 5, 187–200. [Google Scholar] [CrossRef]
- Evans, R.; Wallis, A.F.A. Cellulose molecular weights determined by viscometry. J. Appl. Polym. Sci. 1989, 37, 2331–2340. [Google Scholar] [CrossRef]
- ASTM D4243-99, Standard Test Method for Measurement of Average Viscometric Degree of Polymerization of New and Aged Electrical Papers and Boards; ASTM International: West Conshohocken, PA, USA, 1999.
- ISO 5263-3:2004, Pulps—Laboratory Wet Disintegration—Part 3: Disintegration of Mechanical Pulps at >85 °C; International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 5269-2:2004, Pulps—Preparation of Laboratory Sheets for Physical Testing—Part 2: Rapid-Köthen Method; International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 187:1990, Paper, Board and Pulps—Standard Atmosphere for Conditioning and Testing and Procedure for Monitoring the Atmosphere and Conditioning of Samples; International Organization for Standardization: Geneva, Switzerland, 1990.
- ISO 536:2012, Paper and board—Determination of Grammage; International Organization for Standardization: Geneva, Switzerland, 2012.
- ISO 1924-3:2005, Paper and Board—Determination of Tensile Properties—Part 3: Constant Rate of Elongation Method (100 mm/min); International Organization for Standardization: Geneva, Switzerland, 2005.
- ISO 1974:2012, Paper—Determination of Tearing Resistance—Elmendorf Method; International Organization for Standardization: Geneva, Switzerland, 2012.
- Kuo, C.-H.; Chen, J.-H.; Liou, B.-K.; Lee, C.-K. Utilization of acetate buffer to improve bacterial cellulose production by Gluconacetobacter xylinus. Food Hydrocolloids 2016, 53, 98–103. [Google Scholar] [CrossRef]
- Shibazaki, H.; Kuga, S.; Okano, T. Mercerization and acid hydrolysis of bacterial cellulose. Cellulose 1997, 4, 75–87. [Google Scholar] [CrossRef]
- Immergut, E.H.; Ranby, B.G.; Mark, H.F. Recent work on molecular weight of cellulose. Ind. Eng. Chem. 1953, 45, 2483–2490. [Google Scholar] [CrossRef]
- Zahan, K.A.; Pa’e, N.; Muhamad, I.I. Process parameters for fermentation in a rotary disc reactor for optimum microbial cellulose production using response surface methodology. BioResources 2014, 9, 1858–1872. [Google Scholar] [CrossRef]
- Tahara, N.; Tabuchi, M.; Watanabe, K.; Yano, H.; Morinaga, Y.; Yoshinaga, F. Degree of polymerization of cellulose from Acetobacter xylinum BPR2001 decreased by cellulase produced by the strain. Biosci. Biotechnol. Biochem. 1997, 61, 1862–1865. [Google Scholar] [CrossRef] [PubMed]
- Surma-Ślusarska, B.; Danielewicz, D.; Presler, S. Properties of composites of unbeaten birch and pine sulphate pulps with bacterial cellulose. Fibers Text. East Eur. 2008, 127–129. [Google Scholar]
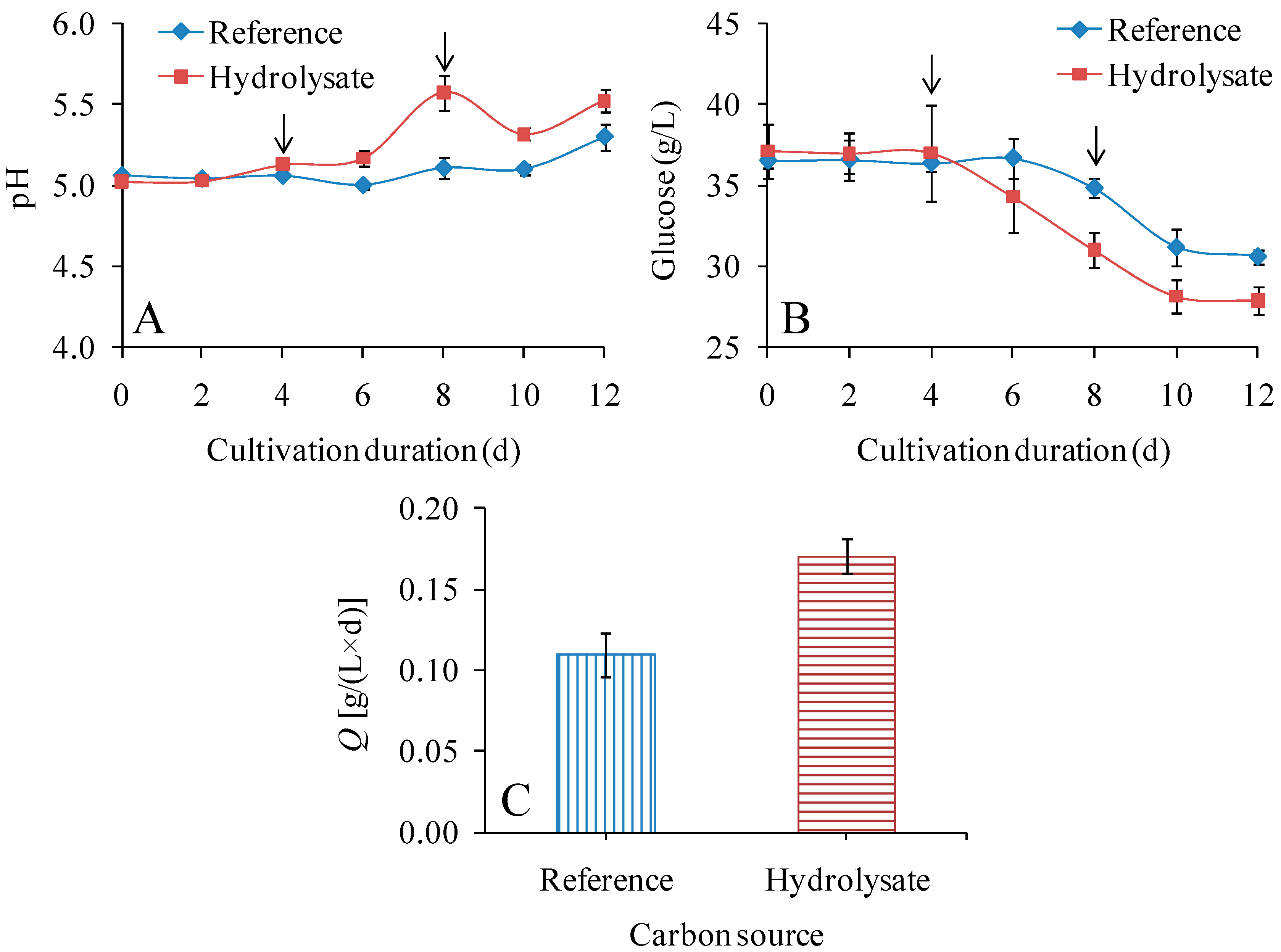

| Medium | Glucose Consumption Rate [g/(L × d)] | Q [g/(L × d)] | YP/initial G (g/g) | YP/consumed G (g/g) | DPv |
|---|---|---|---|---|---|
| Reference | 0.47 ± 0.04 | 0.110 ± 0.013 | 0.036 ± 0.003 | 0.235 ± 0.010 | 6760 ± 110 |
| Hydrolysate | 0.77 ± 0.07 | 0.171 ± 0.011 | 0.055 ± 0.001 | 0.223 ± 0.010 | 6050 ± 80 |
| Variable | Conditions | Glucose Consumption Rate [g/(L × d)] | Q [g/(L × d)] | YP/initial G (g/g) | YP/consumed G (g/g) | DPv |
|---|---|---|---|---|---|---|
| Stirring speed (1.0 vvm) | 50 rpm | 2.96 ± 0.07 | 0.202 ± 0.010 | 0.056 ± 0.002 | 0.068 ± 0.006 | 6390 ± 90 |
| 150 rpm | 2.93 ± 0.05 | 0.239 ± 0.011 | 0.068 ± 0.002 | 0.082 ± 0.003 | 6480 ± 70 | |
| 250 rpm | 3.21 ± 0.04 | 0.262 ± 0.013 | 0.072 ± 0.002 | 0.082 ± 0.003 | 6210 ± 30 | |
| Airflow (250 rpm) | 0.5 vvm | 2.61 ± 0.11 | 0.255 ± 0.013 | 0.073 ± 0.004 | 0.098 ± 0.007 | 6650 ± 70 |
| 1.5 vvm | 2.58 ± 0.04 | 0.266 ± 0.013 | 0.079 ± 0.004 | 0.103 ± 0.007 | 6200 ± 60 | |
| pH adjustment (250 rpm, 1.0 vvm) | pH adjustment | 2.72 ± 0.03 | 0.312 ± 0.016 | 0.095 ± 0.005 | 0.115 ± 0.006 | 6080 ± 50 |
| No adjustment | 2.59 ± 0.03 | 0.240 ± 0.012 | 0.072 ± 0.004 | 0.092 ± 0.005 | 6310 ± 90 |
| Fraction of BNC (w/w %) | 0 | 5 | 10 |
|---|---|---|---|
| Grammage, paper (g/m2) | 57.1 ± 2.6 | 59.0 ± 1.4 | 58.7 ± 3.2 |
| Thickness (mm) | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.15 ± 0.01 |
| Density (kg/m3) | 354 ± 20 | 374 ± 7 | 389 ± 8 |
| Tensile Index (kNm/kg) a | 16.6 ± 1.7 | 19.3 ± 1.5 | 24.7 ± 2.5 |
| Tensile stiffness index (MNm/kg) b | 3.45 ± 0.30 | 4.05 ± 0.11 | 4.97 ± 0.43 |
| Tensile energy absorption index (J/kg) c | 56.7 ± 9.2 | 69.3 ± 10.9 | 87.7 ± 15.7 |
| Strain at break (%) | 0.601 ± 0.032 | 0.624 ± 0.061 | 0.626 ± 0.075 |
| E-modulus (GPa) | 1.22 ± 0.08 | 1.51 ± 0.05 | 1.89 ± 0.20 |
| Tear Index (mNm2/g) d | 1.35 ± 0.28 | 2.24 ± 0.07 | 3.20 ± 0.20 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Wu, G.; Alriksson, B.; Wang, W.; Hong, F.F.; Jönsson, L.J. Bioconversion of Waste Fiber Sludge to Bacterial Nanocellulose and Use for Reinforcement of CTMP Paper Sheets. Polymers 2017, 9, 458. https://doi.org/10.3390/polym9090458
Chen G, Wu G, Alriksson B, Wang W, Hong FF, Jönsson LJ. Bioconversion of Waste Fiber Sludge to Bacterial Nanocellulose and Use for Reinforcement of CTMP Paper Sheets. Polymers. 2017; 9(9):458. https://doi.org/10.3390/polym9090458
Chicago/Turabian StyleChen, Genqiang, Guochao Wu, Björn Alriksson, Wei Wang, Feng F. Hong, and Leif J. Jönsson. 2017. "Bioconversion of Waste Fiber Sludge to Bacterial Nanocellulose and Use for Reinforcement of CTMP Paper Sheets" Polymers 9, no. 9: 458. https://doi.org/10.3390/polym9090458







