Soil Biochemical Changes Induced by Poultry Litter Application and Conservation Tillage under Cotton Production Systems
Abstract
:1. Introduction
2. Materials and Methods
| No. | Tillage system | Cropping system | N source | N rate kg ha−1 |
|---|---|---|---|---|
| 1 (CTR) | Conventional till | Cotton-rye | None | 0 |
| 2 (CTAN) | Conventional till | Cotton-fallow | Ammonium nitrate | 100 |
| 3 (NTAN) | No-till | Cotton-fallow | Ammonium nitrate | 100 |
| 4 (CTRAN) | Conventional till | Cotton-rye | Ammonium nitrate | 100 |
| 5 (CTRP) | Conventional till | Cotton-rye | Poultry litter | 100 |
| 6 (MTRAN) | Mulch-till | Cotton-rye | Ammonium nitrate | 100 |
| 7 (MTRP) | Mulch-till | Cotton-rye | Poultry litter | 100 |
| 8 (NTRAN) | No-till | Cotton-rye | Ammonium nitrate | 100 |
| 9 (NTRP) | No-till | Cotton-rye | Poultry litter | 100 |
| 10 (NT) | No-till | Cotton-fallow | None | 0 |
| 11 (NTRPP) | No-till | Cotton-rye | Poultry litter | 200 |
| 12 (BF) | None | Bare fallow | None | 0 |
2.1. Soil Sampling and Enzyme Analyses
2.2. Statistical Analyses
3. Results
3.1. Soil Enzyme Activities
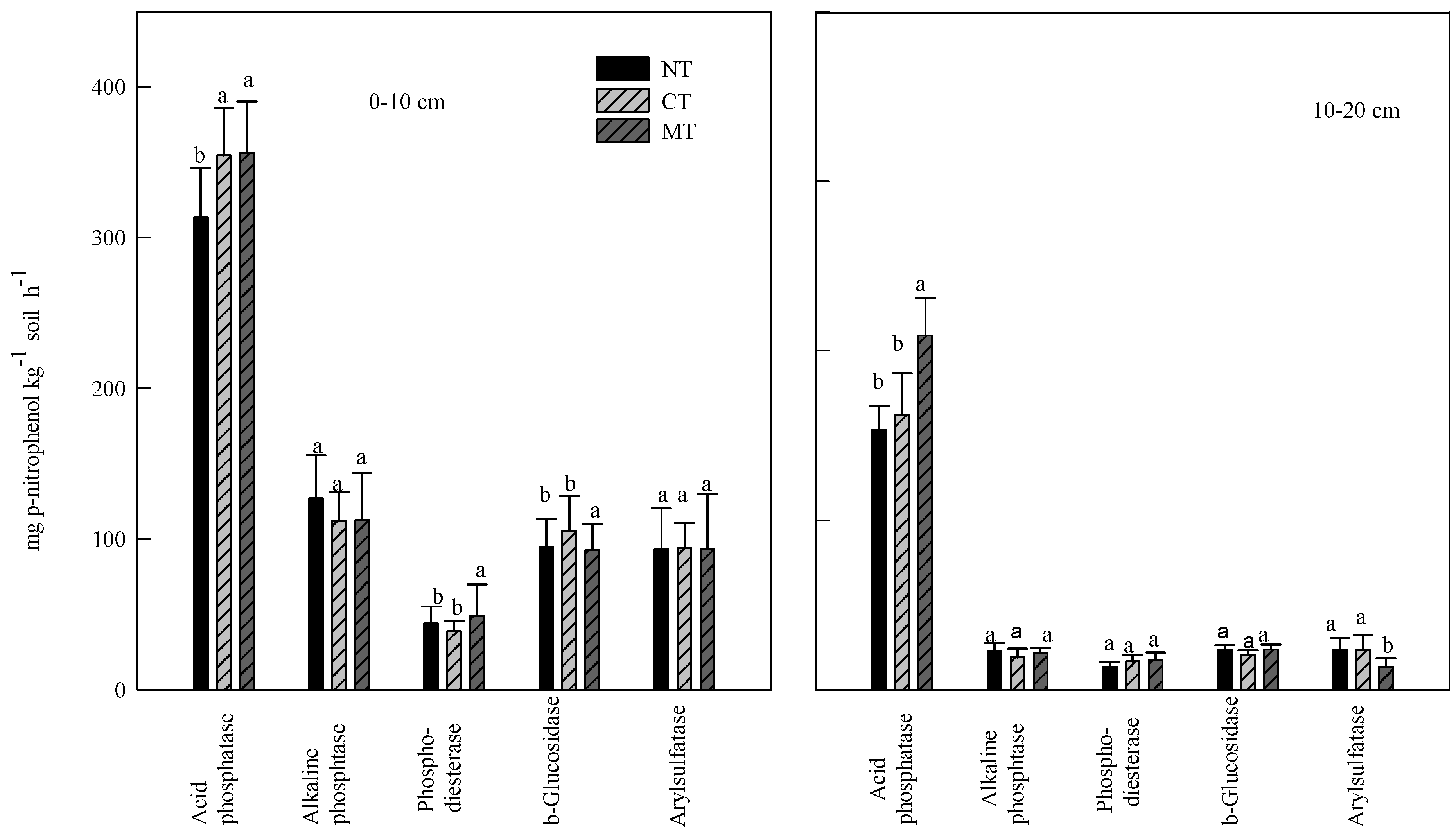
| Depth → | Acid phoshatase | Alkaline phosphatase | Phospho-diesterase | Glucosidase | Arylsulfatase | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | |
| P value | ||||||||||
| Tillage and N source on (Treatment 4, 5, 6, 7, 8, and 9 ‡) | ||||||||||
| Tillage (T) | ns | *** | ns | ns | * | ns | ns | ns | ns | ns |
| N Sources (N) | ns | ns | ** | ns | ns | ns | *** | ns | ** | ns |
| T × N | ns | ** | * | ns | ns | ns | *** | * | ns | ns |
| Tillage and cropping systems on (Treatment (2, 3, 4, and 8) | ||||||||||
| Tillage (T) | ns | *** | ns | ns | * | ns | ns | ns | ns | ns |
| Cropping (C) | ns | ns | ns | ns | * | ns | *** | * | *** | * |
| T × C | ns | ns | ns | ns | ** | * | *** | ns | * | * |
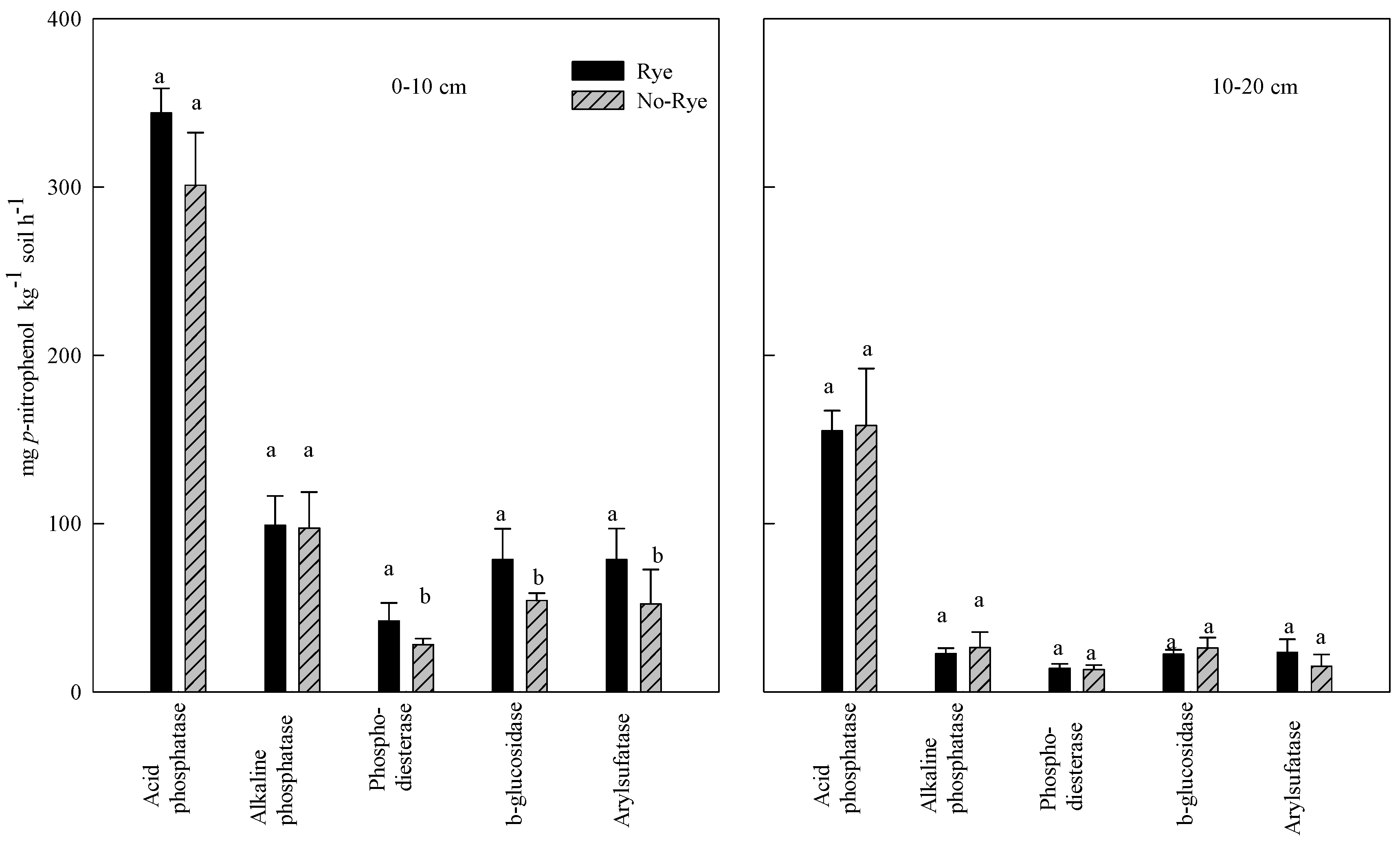
3.2. Effect of Rye Cover Crops on Soil Enzyme Activities
3.3. Effect of Poultry Litter and NH4NO3 Application on Soil Enzyme Activities
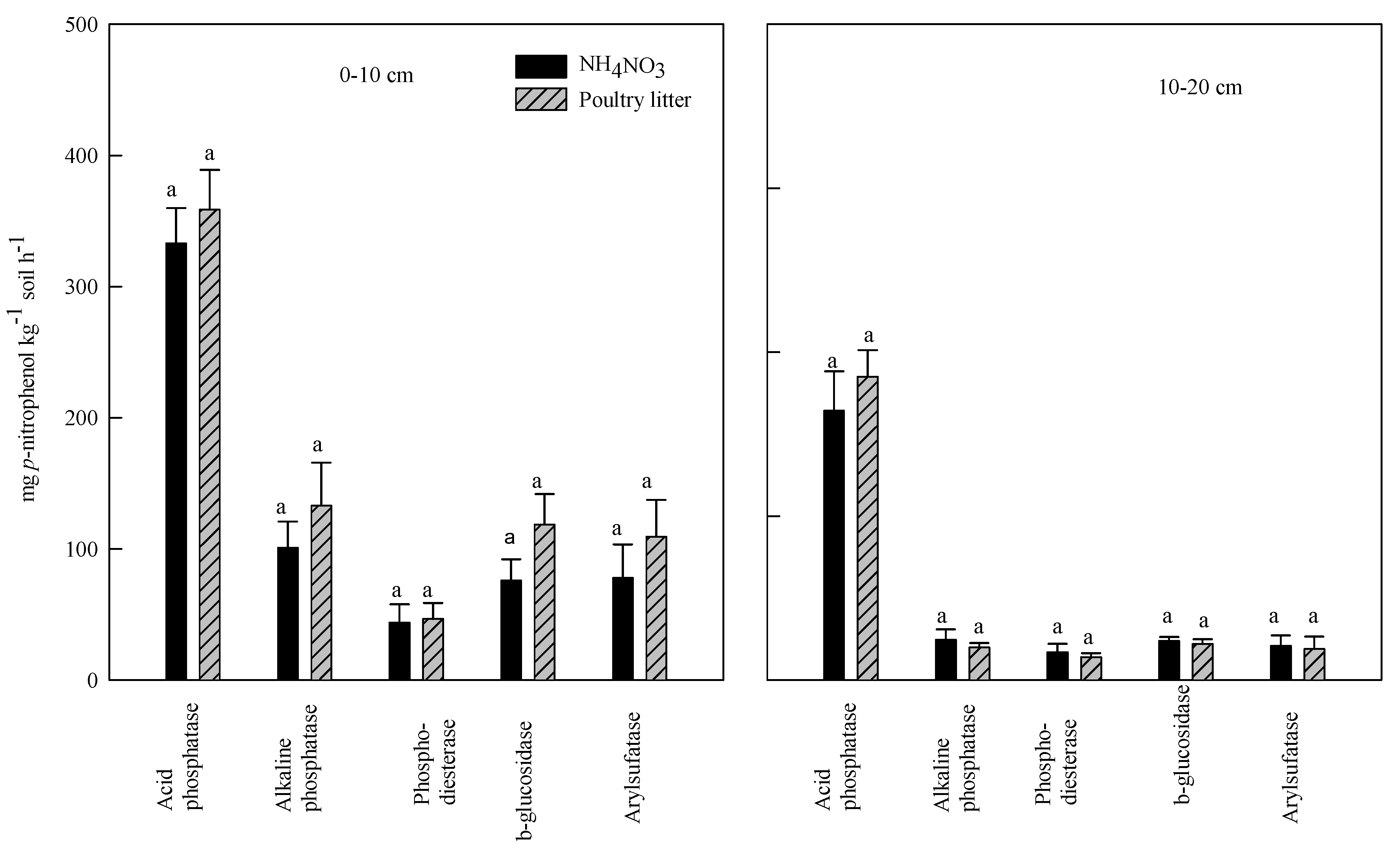
3.4. Correlation between Soil Enzyme Activities and Organic Carbon, and Total Nitrogen
| Depth → | Acid Phosphatase | Alkaline Phosphatase | Phospho-Diesterase | Arylsulfatase | Organic C | Total N | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | |
| β-glucosidase | 0.30 * | 0.10 | 0.55 *** | 0.30 * | 0.46 ** | 0.04 | 0.75 *** | −0.003 | 0.58 *** | 0.16 | 0.39 ** | 0.15 |
| Acid Phosphatase | −0.009 | −0.04 | 0.06 | 0.01 | 0.21 | −0.25 | 0.19 | 0.17 | 0.10 | 0.30 * | ||
| Alkaline Phosphatase | 0.59 *** | 0.08 | 0.68 *** | 0.06 | 0.58 *** | 0.23 | 0.47 ** | 0.16 | ||||
| Phospho-Diesterase | 0.62 *** | 0.21 | 0.50 *** | 0.36 ** | 0.46 ** | 0.03 | ||||||
| Arylsulfatase | 0.66 *** | 0.23 | 0.48 *** | 0.26 | ||||||||
4. Discussion and Conclusions
References
- Nyakatawa, E.Z.; Reddy, K.C.; Sistani, K.R. Tillage, cover cropping, and poultry litter effects on selected soil chemistry properties. Soil Tillage Res. 2001, 58, 69–74. [Google Scholar] [CrossRef]
- Mikanova, O.; Javurek, M.; Simon, T.; Friedlova, M.; Vach, M. The effect of tillage systems on some microbial characteristics. Soil Tillage Res. 2009, 105, 72–76. [Google Scholar] [CrossRef]
- Acosta-Martinez, V.; Mikha, M.M.; Vigil, M.F. Microbial communities and enzyme activities in soils under alternative crop rotations compared to wheat–fallow for the central great plains. Appl. Soil Ecol. 2007, 37, 41–52. [Google Scholar] [CrossRef]
- Liu, B.; Tu, C.; Hu, S.; Gumpertz, M.; Ristaino, J.B. Effect of organic, sustainable, and conventional management strategies in grower fields on soil physical, chemical, and biological factors and the incidence of Southern blight. Appl. Soil Ecol. 2007, 37, 202–214. [Google Scholar] [CrossRef]
- Alvear, N.; Rosas, A.; Rouanet, J.L.; Borie, F. Effect of three soil tillage systems on some biological activities in an ultisols from southern Chile. Soil Tillage Res. 2005, 8, 195–202. [Google Scholar]
- Omidi, H.; Tahmasebi, Z.; Torabi, H.; Miransari, M. Soil enzymatic activities and available P and Zn as affected by tillage practices, canola (Brassica napus L.) cultivars and planting dates. Eur. J. Soil Biol. 2008, 44, 443–450. [Google Scholar]
- Balota, E.L.; Colozzi-Filho, A.; Andrade, D.S.; Dick, R.P. Long-term tillage and crop rotation effects on microbial biomass and C and N mineralization in a Brazilian Oxisol. Soil Tillage Res. 2004, 77, 137–145. [Google Scholar] [CrossRef]
- Ingram, L.J.; Stahl, P.D.; Schuman, G.E.; Buyer, J.S.; Vance, G.F.; Ganjegunte, G.K.; Welker, J.M.; Derner, J.D. Grazing impacts on soil carbon and microbial communities in a mixed-grass ecosystem. Soil Sci. Soc. Am. J. 2008, 72, 939–948. [Google Scholar] [CrossRef]
- Dick, R.P.; Doran, J.W.; Coleman, D.C.; Bezdicek, D.F.; Stewart, B.A. Soil Enzyme Activities as Indicators of Soil Quality. In Defining Soil Quality for a Sustainable Environment; Soil Science Society of America (SSSA): Madison, WI, USA, 1994; pp. 107–124. [Google Scholar]
- Dodd, J.C.; Burton, C.C.; Burns, R.G.; Jeffries, P. Phosphatase activity associated with the roots and the rhizosphere of plants infected with vesicular-arbuscular mycorrhizal fungi. New Phytol. 1987, 107, 163–172. [Google Scholar] [CrossRef]
- Tazisong, I.A.; Senwo, Z.N.; Taylor, R.W.; He, Z. Hydrolysis of organic phosphates by commercially available phytases: Biocatalytic potentials and effects of ions on their enzymatic activities. J. Food Agric. Environ. 2008, 6, 500–505. [Google Scholar]
- Makoi, J.H.; Chimphango, S.B.M.; Dakora, F.D. Elevated levels of acid and alkaline phosphatase activity in roots and rhizosphere of cowpeas (Vigna unguiculata L.Walp) genotypes grown in mixed culture and at different densities with sorghum (Sorghum bicolor L.). Crop Pasture Sci. 2010, 6, 279–289. [Google Scholar]
- Mudge, S.R.; Rac, A.L.; Diatloff, E.; Smith, F.W. Expression analysis suggests novel roles for members of Pht1 family of phosphate transporters in Arbidopsis. Plant J. 2002, 31, 341–353. [Google Scholar] [CrossRef]
- Richardson, A.E.; Hadobas, P.A.; Hayes, J.E. Phosphomonoesterase and phytase activities of wheat (Triticum aestivum L.) roots and utilization of organic phosphorus substrates by seedlings grown in sterile conditions. Plant Cell Environ. 2000, 23, 397–405. [Google Scholar]
- Bergstorm, D.W.; Monreal, C.M.; King, D.J. Sensitivity of soil enzyme activities to conservation practices. Soil Sci. Soc. Am. J. 1998, 62, 1286–1295. [Google Scholar] [CrossRef]
- Bunemann, E.K.; Steinebrunner, F.; Frossard, P.C.; Oberson, A. Phosphorus dynamics in a highly weathered soil as revealed by isotopic labeling techniques. Soil Sci. Soc. Am. J. 2004, 68, 1645–1655. [Google Scholar] [CrossRef]
- Sharpley, A.N.; Rekolainen, S. Phosphorus in Agriculture and Its Environmental Implications. In Phosphorus Loss from Soil to Water; Tunney, H., Carton, O.T., Brookes, P.C., Johnston, A.E., Eds.; Commonwealth Agricultural Bureaux (CABI): Wallingford, UK, 1997; pp. 1–54. [Google Scholar]
- Jakobsen, I.; Leggett, M.E.; Richardson, A.E. Rhizosphere Microorganisms and Plant Phosphorus Uptake. In Phosphorus. Agriculture and the Environment; Sims, J.T., Sharpley, A.N., Eds.; American Society of Agronomy (ASA) Crop Science Society of America (CSSA) Soil Science Society of America(SSSA): Madison, WI, USA, 2005; pp. 437–492. [Google Scholar]
- Martinez, C.E.; Tabatabai, M.A. Decomposition of biotechnology by products in soils. J. Environ. Qual. 1997, 26, 625–632. [Google Scholar]
- Acosta-Martinez, V.; Upchurch, D.R.; Schubert, A.M.; Porter, D.; Wheeler, T. Early impacts of cotton and peanut cropping systems on selected soil chemical, physical, microbiological and biochemical properties. Biol. Fertil. Soils 2004, 40, 44–54. [Google Scholar] [CrossRef]
- Deng, S.P.; Tabatabai, M.A. Effect of tillage and residue management on enzyme activities in soils: I. amidohydrolases. Biol. Fertil. Soils 1996, 22, 202–207. [Google Scholar]
- Deng, S.P.; Tabatabai, M.A. Effect of tillage and residue management on enzyme activities in soils phosphatase and arysulfatase. Biol. Fertil. Soils 1997, 24, 141–146. [Google Scholar] [CrossRef]
- Acosta-Martinez, V.; Tabatabai, M.A. Enzyme activities of a lime agricultural soil. Biol. Fertil. Soils 2000, 31, 85–91. [Google Scholar] [CrossRef]
- Sainju, U.M.; Senwo, Z.N.; Nyakatawa, E.Z.; Tazisong, I.A.; Reddy, C.K. Soil carbon and nitrogen sequestration as affected by long-term tillage cropping systems, and nitrogen fertilizer sources. Agric. Ecosyst. Environ. 2008, 127, 234–240. [Google Scholar] [CrossRef]
- Nyakatawa, E.Z.; Reddy, K.C.; Mays, D.A. Tillage, cover cropping, and poultry litter effects on cotton: II. Growth and yield parameters. Agron. J. 2000, 92, 1000–1007. [Google Scholar] [CrossRef]
- Adams, M.A. Phosphatase activity and phosphorus fractions in Karri (Eucalyptus diversicolor F. Muell.) forest soils. Biol. Fertil. Soils 1992, 14, 200–204. [Google Scholar]
- Tabatabai, M.A. Soil Enzymes. In Methods of Soil Analysis: Microbiological and Biochemical Properties; Weaver, R.W., Ed.; Soil Science Society of America (SSSA): Madison, WI, USA, 1994; pp. 775–833, SSSA Book Series. No.5. [Google Scholar]
- Eivazi, F.; Tabatabai, M.A. Glucosidase and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- SAS/STAT User’s Guide, Version 9.1; Statistical Analysis System (SAS) Institute: Cary, NC, USA, 2005.
- Zhao, Y.; Wang, P.; Li, J.; Chen, Y.; Ying, X.; Liu, S. The effects of two organic manures on soil properties and crops on a temperate calcareous soil under a wheat-maize cropping system. Eur. J. Agron. 2009, 31, 36–42. [Google Scholar] [CrossRef]
- Green, V.S.; Scott, D.E.; Cruz, J.C.; Curi, N. Tillage impacts on soil biological activity and aggregation in a Brazilian Cerrado Oxisol. Soil Tillage Res. 2007, 92, 114–121. [Google Scholar] [CrossRef]
- McCallister, D.L.; Bahadir, M.A.; Blumenthal, J.M. Phosphorus partitioning and phosphatase activity in semi-arid region soils under increasing crop growth intensity. Soil Sci. 2002, 167, 616–624. [Google Scholar]
- Juma, N.G.; Tabatabai, M.A. Distribution of phosphomonoesterases in soils. Soil Sci. 1978, 126, 101–108. [Google Scholar]
- Harrison, A.F. Relationship between intensity of phosphatase activity and physico-chemical properties in woodland soils. Soil Biol. Biochem. 1983, 15, 93–99. [Google Scholar] [CrossRef]
- Turner, B.L.; Haygarth, P.M. Phosphatase activity in temperate pasture soils: Potential regulation of labile organic phosphorus turnover by phosphodiesterase activity. Sci. Total Environ. 2005, 344, 27–36. [Google Scholar] [CrossRef]
- Sun, R.L.; Zhao, B.Q.; Zhu, L.; Xu, J.; Zhang, F.D. Effects of long-term fertilization on soil enzyme activities and its role in adjusting-controlling soil fertility. Plant Nutr. Fertil. 2003, 9, 406–410. [Google Scholar]
- Eivazi, F.; Tabatabai, M.A. Factors affecting glucosidase and galactosidase activities in soils. Soil Biol. Biochem. 1990, 22, 891–897. [Google Scholar] [CrossRef]
- Benitez, E.; Nogales, R.; Campos, M.; Ruano, F. Biochemical variability of olive-orchard soils under different management systems. Appl. Soil Ecol. 2005, 32, 221–231. [Google Scholar]
- Biederbeck, V.O.; Campbell, C.A.; Rassiah, V.; Zentner, R.P.; Guang, W. Soil quality attributes as influenced by annual legumes used as green manure. Soil Biol. Biochem. 1998, 30, 1177–1185. [Google Scholar] [CrossRef]
- Acosta-Martinez, V.; Harmel, R.D. Soil microbi Soil Science Society of America al communities and enzyme activities under various poultry litter application rates. J. Environ. Qual. 2006, 35, 1309–1318. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mankolo, R.; Reddy, C.; Senwo, Z.; Nyakatawa, E.; Sajjala, S. Soil Biochemical Changes Induced by Poultry Litter Application and Conservation Tillage under Cotton Production Systems. Agronomy 2012, 2, 187-198. https://doi.org/10.3390/agronomy2030187
Mankolo R, Reddy C, Senwo Z, Nyakatawa E, Sajjala S. Soil Biochemical Changes Induced by Poultry Litter Application and Conservation Tillage under Cotton Production Systems. Agronomy. 2012; 2(3):187-198. https://doi.org/10.3390/agronomy2030187
Chicago/Turabian StyleMankolo, Regine, Chandra Reddy, Zachary Senwo, Ermson Nyakatawa, and Seshadri Sajjala. 2012. "Soil Biochemical Changes Induced by Poultry Litter Application and Conservation Tillage under Cotton Production Systems" Agronomy 2, no. 3: 187-198. https://doi.org/10.3390/agronomy2030187




