Understanding Lolium rigidum Seeds: The Key to Managing a Problem Weed?
Abstract
:1. Introduction
2. The Problem with Lolium rigidum
3. Persistence and Dormancy of L. rigidum Seeds
3.1. Persistence of L. rigidum Seeds in the Soil Seed Bank
3.2. Development of Dormancy in Maturing L. rigidum Seeds
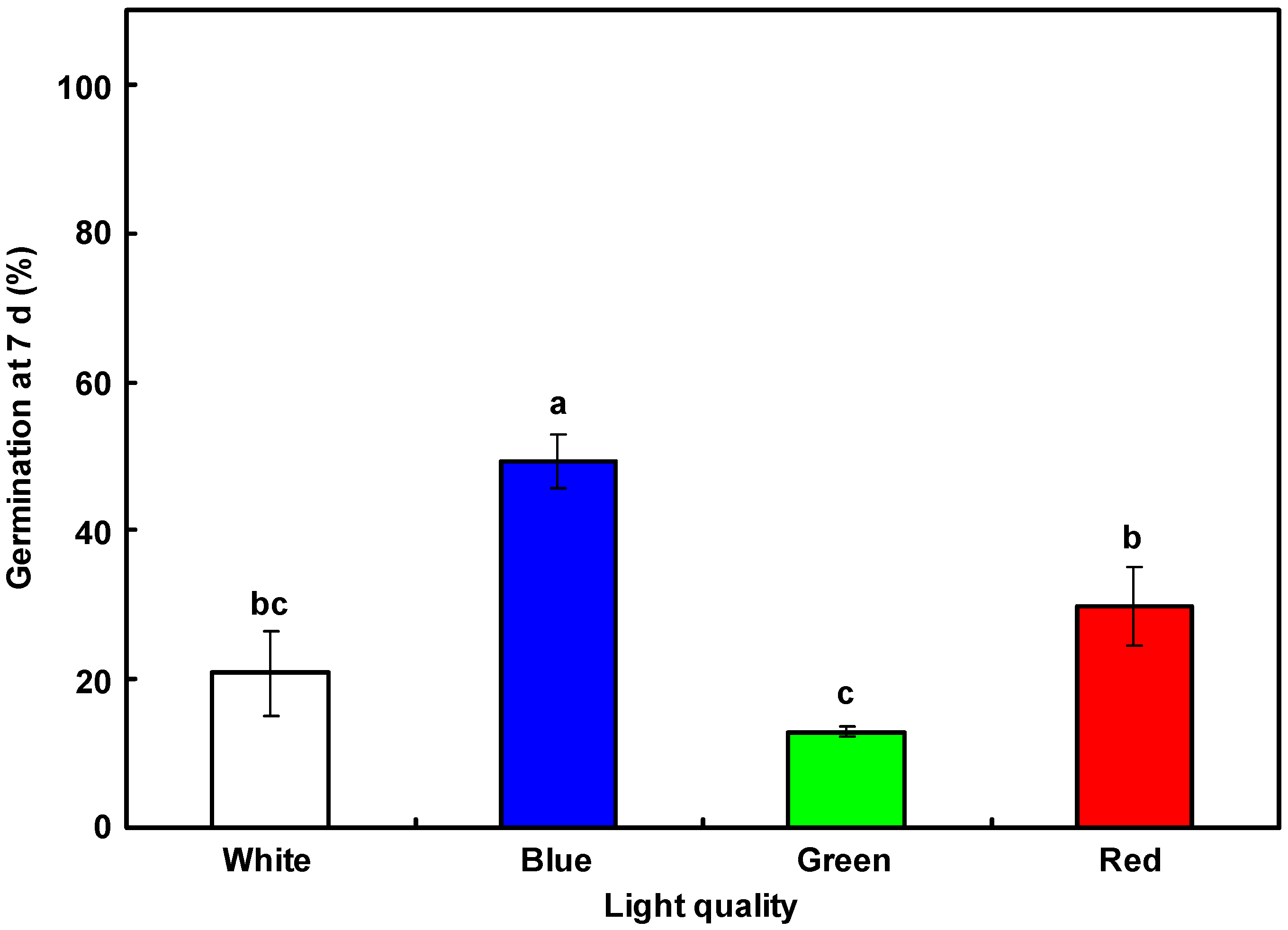
3.3. Dormancy Release in Mature L. rigidum Seeds
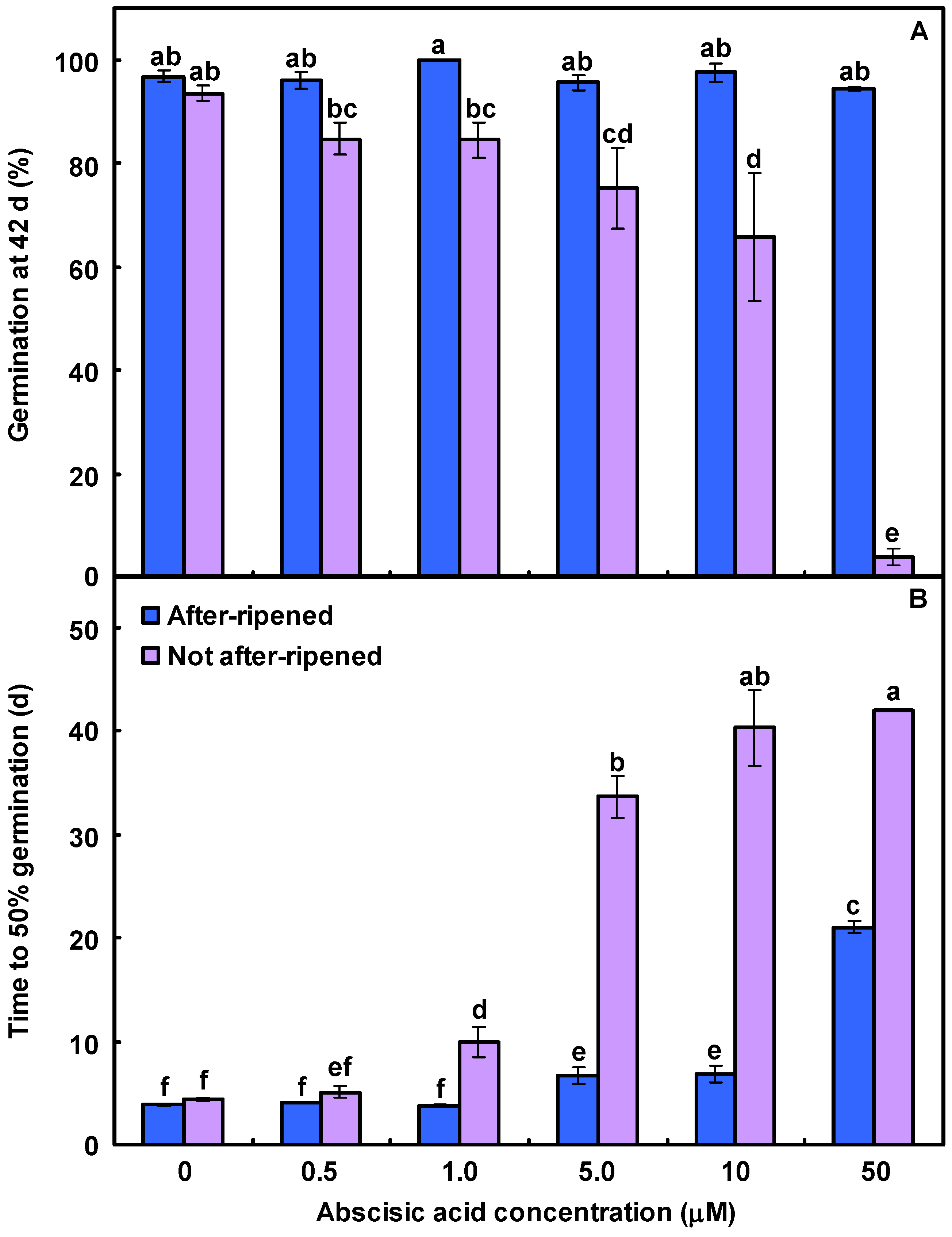
3.4. Interaction of Lolium Seed Germination and Agronomic Practices
4. Depletion of the L. rigidum Seed Bank
4.1. Prevention of Seed Set
4.2. Removal of Flower Spikes before Seed Shedding
4.3. Killing or Removing Mature Seeds
4.4. Manipulating Seed Germination
5. Modelling L. rigidum Seedling Emergence

6. Conclusions
Acknowledgements
References
- Henzell, T. Australian Agriculture: Its History and Challenges; CSIRO Publishing: Collingwood, Australia, 2007. [Google Scholar]
- Kloot, P.M. The genus Lolium in Australia. Aust. J. Bot. 1983, 31, 421–435. [Google Scholar] [CrossRef]
- Appleby, A.P.; Olson, P.D.; Colbert, D.R. Winter wheat yield reduction from interference by Italian ryegrass. Agron. J. 1976, 68, 463–466. [Google Scholar] [CrossRef]
- Reeves, T.G. Effect of annual ryegrass (Lolium rigidum Gaud.) on yield of wheat. Weed Res. 1976, 16, 57–63. [Google Scholar] [CrossRef]
- Palta, J.A.; Peltzer, S. Annual ryegrass (Lolium rigidum) reduces the uptake and utilisation of fertiliser-nitrogen by wheat. Aust. J. Agric. Res. 2001, 52, 573–581. [Google Scholar] [CrossRef]
- Gill, G.S. Why annual ryegrass is a problem in Australian agriculture. Plant Prot. Q. 1996, 11, 193–195. [Google Scholar]
- Moore, R.M. Weeds and weed control in Australia. J. Aust. Inst. Agric. Sci. 1971, 37, 181–191. [Google Scholar]
- Pannell, D.J.; Stewart, V.; Bennett, A.; Monjardino, M.; Schmidt, C.; Powles, S.B. RIM: A bioeconomic model for integrated weed management of Lolium rigidum in Western Australia. Agric. Syst. 2004, 79, 305–325. [Google Scholar] [CrossRef]
- Owen, M.J.; Walsh, M.J.; Llewellyn, R.S.; Powles, S.B. Widespread occurrence of multiple herbicide resistance in Western Australian annual ryegrass (Lolium rigidum) populations. Aust. J. Agric. Res. 2007, 58, 711–718. [Google Scholar] [CrossRef]
- Steadman, K.J.; Ellery, A.J.; Chapman, R.; Moore, A.; Turner, N.C. Maturation temperature and rainfall influence seed dormancy characteristics of annual ryegrass (Lolium rigidum). Aust. J. Agric. Res. 2004, 55, 1047–1057. [Google Scholar] [CrossRef]
- Ellery, A.J.; Gallagher, R.S.; Dudley, S.V. Dormancy and germination ecology of annual ryegrass (Lolium rigidum Gaud.). In Biology of Seeds: Recent Research Advances; Nicolas, G., Bradford, K.J., Come, D., Pritchard, H.W., Eds.; CABI Publishing: New York, NY, USA, 2003; pp. 389–396. [Google Scholar]
- Narwal, S.; Sindel, S.M.; Jessop, R.S. Dormancy and longevity of annual ryegrass (Lolium rigidum) as affected by soil type, depth, rainfall, and duration of burial. Plant Soil 2008, 310, 225–234. [Google Scholar] [CrossRef]
- Peltzer, S.; Matson, P. Understanding the weed seed bank life of important agricultural weeds. In. In Proceedings of the Crop Updates, Perth, Australia, 25-26 February 2002; Government of Western Australia: Perth, Australia, 2002. [Google Scholar]
- Jensen, P.K. Longevity of seeds of Poa pratensis and Lolium perenne as affected by simulated soil tillage practices and its implications for contamination of herbage seed crops. Grass Forage Sci. 2010, 65, 85–91. [Google Scholar] [CrossRef]
- Rampton, H.H.; Ching, T.M. Longevity and dormancy in seeds of several cool-season grasses and legumes buried in soil. Agron. J. 1966, 58, 220–222. [Google Scholar] [CrossRef]
- Rampton, H.H.; Ching, T.M. Persistence of crop seeds in soil. Agron. J. 1972, 62, 272–277. [Google Scholar] [CrossRef]
- Tan, M.-K.; Sharp, P.J.; Lu, M.-Q.; Howes, N. Genetics of grain dormancy in a white wheat. Aust. J. Agric. Res. 2006, 57, 1157–1165. [Google Scholar] [CrossRef]
- Foley, M.E.; Fennimore, S.A. Genetic basis for seed dormancy. Seed Sci. Res. 1998, 8, 173–182. [Google Scholar]
- Goggin, D.E.; Emery, R.J.N.; Powles, S.B.; Steadman, K.J. Initial characterisation of low and high seed dormancy populations of Lolium rigidum produced by repeated selection. J. Plant Physiol. 2010, 167, 1282–1288. [Google Scholar] [CrossRef]
- Wiesner, L.E.; Grabe, D.F. Effect of temperature preconditioning and cultivar on ryegrass (Lolium sp.) seed dormancy. Crop Sci. 1972, 12, 760–764. [Google Scholar] [CrossRef]
- Owen, M.J.; Michael, P.J.; Renton, M.; Steadman, K.J.; Powles, S.B. Towards large-scale prediction of Lolium rigidum emergence. I. Can climate be used to predict dormancy parameters? Weed Res. 2011, 51, 123–132. [Google Scholar] [CrossRef]
- Batlla, D.; Kruk, B.C.; Benech-Arnold, R.L. Very early detection of canopy presence by seeds through perception of subtle modifications in red: Far red signals. Funct. Ecol. 2000, 14, 195–202. [Google Scholar] [CrossRef]
- Mancinelli, A.L. Some thoughts about the use of predicted values of the state of phytochrome in plant photomorphogenesis research. Plant Cell Environ. 1988, 11, 429–439. [Google Scholar] [CrossRef]
- Casal, J.J.; Sánchez, R.A. Phytochromes and seed germination. Seed Sci. Res. 1998, 8, 317–329. [Google Scholar]
- Recasens, J.; Caimons, O.; Torra, J.; Taberner, A. Variation in seed germination and early growth between and within acetolactate synthase herbicide resistant and susceptible Lolium rigidum accessions. Seed Sci. Technol. 2007, 35, 32–47. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography,and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Schafer, D.E.; Chilcote, D.O. Factors influencing persistence and depletion in buried seed populations. II. The effects of soil temperature and moisture. Crop Sci. 1970, 10, 342–345. [Google Scholar] [CrossRef]
- Gramshaw, D.; Stern, W.R. Survival of annual ryegrass (Lolium rigidum Gaud.) seed in a Mediterranean type environment. II Effects of short-term burial on persistence of viable seed. Aust. J. Agric. Res. 1977, 28, 93–101. [Google Scholar] [CrossRef]
- Gramshaw, D. Germination of annual ryegrass seeds (Lolium rigidum Gaud.) as influenced by temperature, light, storage environment, and age. Aust. J. Agric. Res. 1972, 23, 779–787. [Google Scholar] [CrossRef]
- Gramshaw, D. Temperature/light interactions and the effect of seed source on germination of annual ryegrass (Lolium rigidum Gaud.) seeds. Aust. J. Agric. Res. 1976, 27, 779–786. [Google Scholar] [CrossRef]
- Shen, J.B.; Xu, L.Y.; Jin, X.Q.; Chen, J.H.; Lu, H.F. Effect of temperature regime on germination of seed of perennial ryegrass (Lolium perenne). Grass Forage Sci. 2008, 63, 249–256. [Google Scholar] [CrossRef]
- Hill, M.J.; Pearson, C.J.; Kirby, A.C. Germination and seedling growth of prairie grass, tall fescue and Italian ryegrass at different temperatures. Aust. J. Agric. Res. 1985, 36, 13–24. [Google Scholar] [CrossRef]
- Turner, N.C.; Thomson, C.J.; Rawson, H.M. Effect of temperature on germination and early growth of subterranean clover, capeweed and ryegrass. Grass Forage Sci. 2001, 56, 97–104. [Google Scholar] [CrossRef]
- Steadman, K.J.; Crawford, A.D.; Gallagher, R.S. Dormancy release in Lolium rigidum seeds is a function of thermal after-ripening time and water content. Funct. Plant Biol. 2003, 30, 345–352. [Google Scholar] [CrossRef]
- Larsen, S.U.; Andreasen, C. Light and heavy turfgrass seeds differ in germination percentage and mean germination thermal time. Crop Sci. 2004, 44, 1710–1720. [Google Scholar] [CrossRef]
- Vila-Aiub, M.M.; Neve, P.; Steadman, K.J.; Powles, S.B. Ecological fitness of a multiple herbicide-resistant Lolium rigidum population: Dynamics of seed germination and seedling emergence of resistant and susceptible phenotypes. J. Appl. Ecol. 2005, 42, 288–298. [Google Scholar] [CrossRef]
- Steadman, K.J.; Bignell, G.P.; Ellery, A.J. Field assessment of thermal after-ripening time for dormancy release prediction in Lolium rigidum seeds. Weed Res. 2003, 43, 458–465. [Google Scholar] [CrossRef]
- Lush, W.M.; Groves, R.H.; Kaye, P.E. Presowing hydration-dehydration treatments in relation to seed germination and early seedling growth of wheat and ryegrass. Aust. J. Plant Physiol. 1981, 8, 409–425. [Google Scholar] [CrossRef]
- Gallagher, R.S.; Steadman, K.J.; Crawford, A.D. Alleviation of dormancy in annual ryegrass (Lolium rigidum) seeds by hydration and after-ripening. Weed Sci. 2004, 52, 968–975. [Google Scholar] [CrossRef]
- Gill, G.S. Ecology of annual ryegrass. Plant Prot. Q. 1996, 11, 195–198. [Google Scholar]
- Chauhan, B.S.; Gill, G.; Preston, C. Influence of environmental factors on seed germination and seedling emergence of rigid ryegrass (Lolium rigidum). Weed Sci. 2006, 54, 1004–1012. [Google Scholar] [CrossRef]
- Lush, W.M.; Groves, R.H. Germination, emergence and surface establishment of wheat and ryegrass in response to natural and artificial hydration-dehydration cycles. Aust. J. Agric. Res. 1981, 32, 731–739. [Google Scholar] [CrossRef]
- Long, R.L.; Williams, K.; Griffiths, E.M.; Flematti, G.R.; Merritt, D.J.; Stevens, J.C.; Turner, S.R.; Powles, S.B.; Dixon, K.W. Prior hydration of Brassica tournefortii seeds reduces the stimulatory effect of karrikinolide on germination and increases seed sensitivity to abscisic acid. Ann. Bot. 2010, 105, 1063–1070. [Google Scholar] [CrossRef]
- Steadman, K.J.; Bignell, G.P.; Michael, P.J. Stimulating dormancy release and emergence of annual ryegrass (Lolium rigidum) seeds using short-term hydrated storage in darkness. Aust. J. Agric. Res. 2004, 55, 787–795. [Google Scholar] [CrossRef]
- Steadman, K.J. Dormancy release during hydrated storage in Lolium rigidum seeds is dependent on temperature, light quality, and hydration status. J. Exp. Bot. 2004, 55, 929–937. [Google Scholar] [CrossRef]
- Goggin, D.E.; Powles, S.B.; Toorop, P.E.; Steadman, K.J. Dark-mediated dormancy release in stratified Lolium rigidum seeds is associated with higher activities of cell wall-modifying enzymes and an apparent increase in gibberellin sensitivity. J. Plant Physiol. 2011, 168, 527–533. [Google Scholar] [CrossRef]
- Goggin, D.E.; Steadman, K.J.; Emery, R.J.N.; Farrow, S.C.; Benech-Arnold, R.L.; Powles, S.B. ABA inhibits germination but not dormancy release in mature imbibed seeds of Lolium rigidum Gaud. J. Exp. Bot. 2009, 60, 3387–3396. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Gill, G.; Preston, C. Influence of tillage systems on vertical distribution, seedling recruitment and persistence of rigid ryegrass (Lolium rigidum) seed bank. Weed Sci. 2006, 54, 669–676. [Google Scholar] [CrossRef]
- Cheam, A.; Hashem, A.; Bowran, D.; Lee, A. Autumn tickle can influence dormancy and breakdown of wild radish and annual ryegrass seeds. In Proceedings of the Crop Updates, Perth, Australia, 23-24 February 1998; Government of Western Australia: Perth, Australia, 1998. [Google Scholar]
- Chauhan, B.S.; Gill, G.S.; Preston, C. Effect of seeding systems and dinitroaniline herbicides on emergence and control of rigid ryegrass (Lolium rigidum) in wheat. Weed Technol. 2007, 21, 53–58. [Google Scholar] [CrossRef]
- Andrews, M.; Douglas, A.; Jones, A.V.; Milburn, C.E.; Porter, D.; McKenzie, B.A. Emergence of temperate pasture grasses from different sowing depths: Importance of seed weight, coleoptile plus mesocotyl length and shoot strength. Ann. Appl. Biol. 1997, 130, 549–560. [Google Scholar] [CrossRef]
- Sinclair, K.; Beale, P.J. Critical factors influencing no-till establishment of short-term ryegrass (Lolium multiflorum) into a kikuyu (Pennisetum clandestinum) pasture. Crop Pasture Sci. 2010, 61, 192–200. [Google Scholar] [CrossRef]
- Cirujeda, A.; Taberner, A. Cultural control of herbicide-resistant Lolium rigidum Gaud. populations in winter cereal in northeastern Spain. Span. J. Agric. Res. 2009, 7, 146–154. [Google Scholar]
- Newman, P. Mouldboard ploughing of sandplain soils-More grain, fewer weeds. In Proceedings of the Crop Updates, Perth, Australia, 21-22 February 2011; Government of Western Australia: Perth, Australia, 2011. [Google Scholar]
- Pedersen, B.P.; Neve, P.; Andreasen, C.; Powles, S.B. Ecological fitness of a glyphosate-resistant Lolium rigidum population: Growth and seed production along a competition gradient. Basic Appl. Ecol. 2007, 8, 258–268. [Google Scholar] [CrossRef]
- Gill, G.S.; Cousens, R.D.; Allan, M.R. Germination, growth, and development of herbicide resistant and susceptible populations of rigid ryegrass (Lolium rigidum). Weed Sci. 1996, 44, 252–256. [Google Scholar]
- Ghersa, C.M.; Martínez-Ghersa, M.A.; Brewer, T.G.; Roush, M.L. Selection pressures for diclofop-methyl resistance and germination time of Italian ryegrass. Agron. J. 1992, 86, 823–828. [Google Scholar]
- Gundel, P.E.; Martínez-Ghersa, M.A.; Ghersa, C.M. Dormancy, germination and ageing of Lolium multiflorum seeds following contrasting herbicide selection regimes. Eur. J. Agron. 2008, 28, 606–613. [Google Scholar] [CrossRef]
- Owen, M.J.; Michael, P.J.; Renton, M.; Steadman, K.J.; Powles, S.B. Towards large-scale prediction of Lolium rigidum (annual ryegrass) emergence. II. Correlation between dormancy and herbicide resistance levels suggests an impact of cropping systems. Weed Res. 2011, 51, 133–141. [Google Scholar] [CrossRef]
- Neve, P.; Powles, S. Recurrent selection with reduced herbicide rates results in the rapid evolution of herbicide resistance in Lolium rigidum. Theor. Appl. Genet. 2005, 110, 1154–1166. [Google Scholar] [CrossRef]
- Batlla, D.; Benech-Arnold, R.L. Predicting changes in dormancy level in weed seed soil banks: Implications for weed management. Crop Prot. 2007, 26, 189–197. [Google Scholar] [CrossRef]
- Ashworth, M.; Desbiolles, J.; Tola, E. Disc Seeding in Zero-Till Farming Systems: A Review of Technology and Paddock Issues; Western Australian No-Tillage Farmer’s Association: Northam, Australia, 2010. [Google Scholar]
- Mutikainen, P.; Walls, M.; Ojala, A. Effects of simulated herbivory on tillering and reproduction in an annual ryegrass, Lolium remotum. Oecologia 1993, 95, 54–60. [Google Scholar]
- Warringa, J.W.; Marinissen, M.J. The effect of light intensity after anthesis on dry matter distribution and seed yield of Lolium perenne L. Grass Forage Sci. 1996, 51, 103–110. [Google Scholar] [CrossRef]
- Hashem, A.; Radosevich, S.R.; Roush, M.L. Effect of proximity factors on competition between winter wheat (Triticum aestivum) and Italian ryegrass (Lolium multiflorum). Weed Sci. 1998, 46, 181–190. [Google Scholar]
- Gonzalez-Andujar, J.L.; Fernandez-Quintanilla, C. Modelling the population dynamics of ryegrass (Lolium rigidum) under various weed management systems. Crop Prot. 2004, 23, 723–729. [Google Scholar] [CrossRef]
- Dear, B.S.; Hodge, A.; Lemerle, D.; Pratley, J.E.; Orchard, B.A.; Kaiser, A.G. Influence of forage legume species, seeding rate and seed size on competitiveness with annual ryegrass (Lolium rigidum) seedlings. Aust. J. Exp. Agric. 2006, 46, 627–636. [Google Scholar] [CrossRef]
- Holman, J.D.; Bussan, A.J.; Maxwell, B.D.; Miller, P.R.; Mickelson, J.A. Persian darnel (Lolium persicum) fecundity response to spring wheat, canola and sunflower interference. Weed Technol. 2006, 20, 430–437. [Google Scholar] [CrossRef]
- Sim, L.C.; Froud-Williams, R.J.; Gooding, M.J. The influence of winter oilseed rape (Brassica napus ssp. oleifera var. biennis) canopy size on grass weed growth and grass weed seed return. J. Agric. Sci. 2007, 145, 313–327. [Google Scholar]
- Walsh, M.J.; Devlin, R.D.; Powles, S.B. Potential for preseason herbicide application to prevent weed emergence in the subsequent growing season. 1. Identification and evaluation of possible herbicides. Weed Technol. 2004, 18, 228–235. [Google Scholar] [CrossRef]
- Steadman, K.J.; Eaton, D.M.; Plummer, J.A.; Ferris, D.G.; Powles, S.B. Late-season non-selective herbicide application reduces Lolium rigidum seed numbers, seed viability, and seedling fitness. Aust. J. Agric. Res. 2006, 57, 133–141. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Gill, G.S.; Preston, C. Timing and dose of metolachlor affect rigid ryegrass (Lolium rigidum) control in wheat. Weed Technol. 2007, 21, 225–229. [Google Scholar] [CrossRef]
- Cobley, B.; Nutt, B. Controlling annual ryegrass seed-set during a pasture legume phase. In Proceedings of the Crop Updates, Perth, Australia, 22-23 February 1999; Government of Western Australia: Perth, Australia, 1999. [Google Scholar]
- Stanton, R.; Piltz, J.; Pratley, J.; Kaiser, A.; Hudson, D.; Dill, G. Annual ryegrass (Lolium rigidum) seed survival and digestibility in cattle and sheep. Aust. J. Exp. Agric. 2002, 42, 111–115. [Google Scholar] [CrossRef]
- Gramshaw, D.; Stern, W.R. Survival of annual ryegrass (Lolium rigidum Gaud.) seed in a Mediterranean type environment. I. Effect of summer grazing by sheep on seed numbers and seed germination in autumn. Aust. J. Agric. Res. 1977, 28, 81–91. [Google Scholar] [CrossRef]
- Harris, G.S. The periodicity of germination in some grass species. N. Z. J. Agric. Res. 1961, 4, 253–260. [Google Scholar] [CrossRef]
- Falloon, R.E. Fungi pathogenic to ryegrass seedlings. Plant Soil 1985, 86, 79–86. [Google Scholar] [CrossRef]
- Brodie, G.; Harris, G.; Pasma, L.; Travers, A.; Leyson, D.; Lancaster, C.; Woodworth, J. Microwave soil heating for controlling ryegrass seed germination. Trans. ASABE 2009, 52, 295–302. [Google Scholar]
- Walsh, M.J.; Powles, S.B. Management strategies for herbicide-resistant weed populations in Australian dryland crop production systems. Weed Technol. 2007, 21, 332–338. [Google Scholar] [CrossRef]
- Walsh, M.J.; Harrington, R.B.; Powles, S.B. Harrington seed destructor: A new nonchemical weed control tool for global grain crops. Crop Sci. 2012, 52, 1343–1347. [Google Scholar] [CrossRef]
- Jacob, H.S.; Minkey, D.M.; Gallagher, R.S.; Borger, C.P. Variation in postdispersal weed seed predation in a crop field. Weed Sci. 2006, 54, 148–155. [Google Scholar] [CrossRef]
- Appleby, A.P.; Brenchley, R.G. Influence of paraquat on seed germination. Weed Sci. 1968, 16, 484–485. [Google Scholar]
- Watkin, E.M.; Sagar, G.R. Effect of paraquat on seed germination. Weed Res. 1972, 12, 195–198. [Google Scholar] [CrossRef]
- Goggin, D.E.; Powles, S.B.; Steadman, K.J. Selection for low or high primary dormancy in Lolium rigidum Gaud seeds results in constitutive differences in stress protein expression and peroxidase activity. J. Exp. Bot. 2011, 62, 1037–1047. [Google Scholar] [CrossRef]
- Huang, J.H.; Fu, R.; Liang, C.X.; Dong, D.F.; Luo, X.L. Allelopathic effects of cassava (Manihot esculenta Crantz) on radish (Raphanus sativus L.) and (Lolium perenne L.). Allelopath. J. 2010, 25, 155–162. [Google Scholar]
- Ammann, N.; Pieterse, P.J. Effects of Artemisia afra leaf extracts on seed germination of selected crop and weed species. S. Afr. J. Plant Soil 2005, 22, 263–265. [Google Scholar]
- Petroski, R.J.; Dornbos, D.L.; Powell, R.G. Germination and growth inhibition of annual ryegrass (Lolium multiflorum L.) and alfalfa (Medicago sativa L.) by loline alkaloids and synthetic N-acylloline derivatives. J. Agric. Food Chem. 1990, 38, 1716–1718. [Google Scholar] [CrossRef]
- Lill, R.E.; McWha, J.A.; Cole, A.L.J. The influence of volatile substances from incubated litter of Pinus radiata on seed germination. Ann. Bot. 1979, 43, 81–85. [Google Scholar]
- Pepperman, A.B.; Bradow, J.M. Strigol analogs as germination regulators in weed and crop seeds. Weed Sci. 1988, 36, 719–725. [Google Scholar]
- Kato-Noguchi, H.; Macías, F.A. Inhibition of germination and α-amylase induction by 6-methoxy-2-benzoxazolinone in twelve plant species. Biol. Plant. 2008, 52, 351–354. [Google Scholar] [CrossRef]
- Cavalieri, A.; Caporali, F. Effects of essential oils of cinnamon, lavender and peppermint on germination of Mediterranean weeds. Allelopath. J. 2010, 25, 441–451. [Google Scholar]
- Wu, H.; Pratley, J.; Haig, T. Phytotoxic effects of wheat extracts on a herbicide-resistant biotype of annual ryegrass (Lolium rigidum). J. Agric. Food Chem. 2003, 51, 4610–4616. [Google Scholar] [CrossRef]
- Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Trengove, R.D. A compound from smoke that promotes seed germination. Science 2004, 305, 977. [Google Scholar] [CrossRef]
- Stevens, J.C.; Merritt, D.J.; Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W. Seed germination of agricultural weeds is promoted by the butenolide 3-methyl-2H-furo[2,3-c]pyran-2-one under laboratory and field conditions. Plant Soil 2007, 298, 113–124. [Google Scholar] [CrossRef]
- Forcella, F. Real-time assessment of seed dormancy and seedling growth for weed management. Seed Sci. Res. 1998, 8, 201–209. [Google Scholar]
- Schutte, B.J.; Regnier, E.E.; Harrison, S.K.; Schmoll, J.T.; Spokas, K.; Forcella, F. A hydrothermal seedling emergence model for giant ragweed (Ambrosia trifida). Weed Sci. 2008, 56, 555–560. [Google Scholar] [CrossRef]
- Renton, M.; Peltzer, S.; Diggle, A. Winning the weed war with the Weed Seed Wizard! In Proceedings of the Crop Updates, Perth, Australia, 25-26 February 2007; Government of Western Australia: Perth, Australia, 2007. [Google Scholar]
- Anderson, R.L. Managing weeds with a dualistic approach of prevention and control: A review. Agron. Sustain. Dev. 2007, 27, 13–18. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Goggin, D.E.; Powles, S.B.; Steadman, K.J. Understanding Lolium rigidum Seeds: The Key to Managing a Problem Weed? Agronomy 2012, 2, 222-239. https://doi.org/10.3390/agronomy2030222
Goggin DE, Powles SB, Steadman KJ. Understanding Lolium rigidum Seeds: The Key to Managing a Problem Weed? Agronomy. 2012; 2(3):222-239. https://doi.org/10.3390/agronomy2030222
Chicago/Turabian StyleGoggin, Danica E., Stephen B. Powles, and Kathryn J. Steadman. 2012. "Understanding Lolium rigidum Seeds: The Key to Managing a Problem Weed?" Agronomy 2, no. 3: 222-239. https://doi.org/10.3390/agronomy2030222




