Development of a Geo-Referenced Database for Weed Mapping and Analysis of Agronomic Factors Affecting Herbicide Resistance in Apera spica-venti L. Beauv. (Silky Windgrass)
Abstract
:1. Introduction
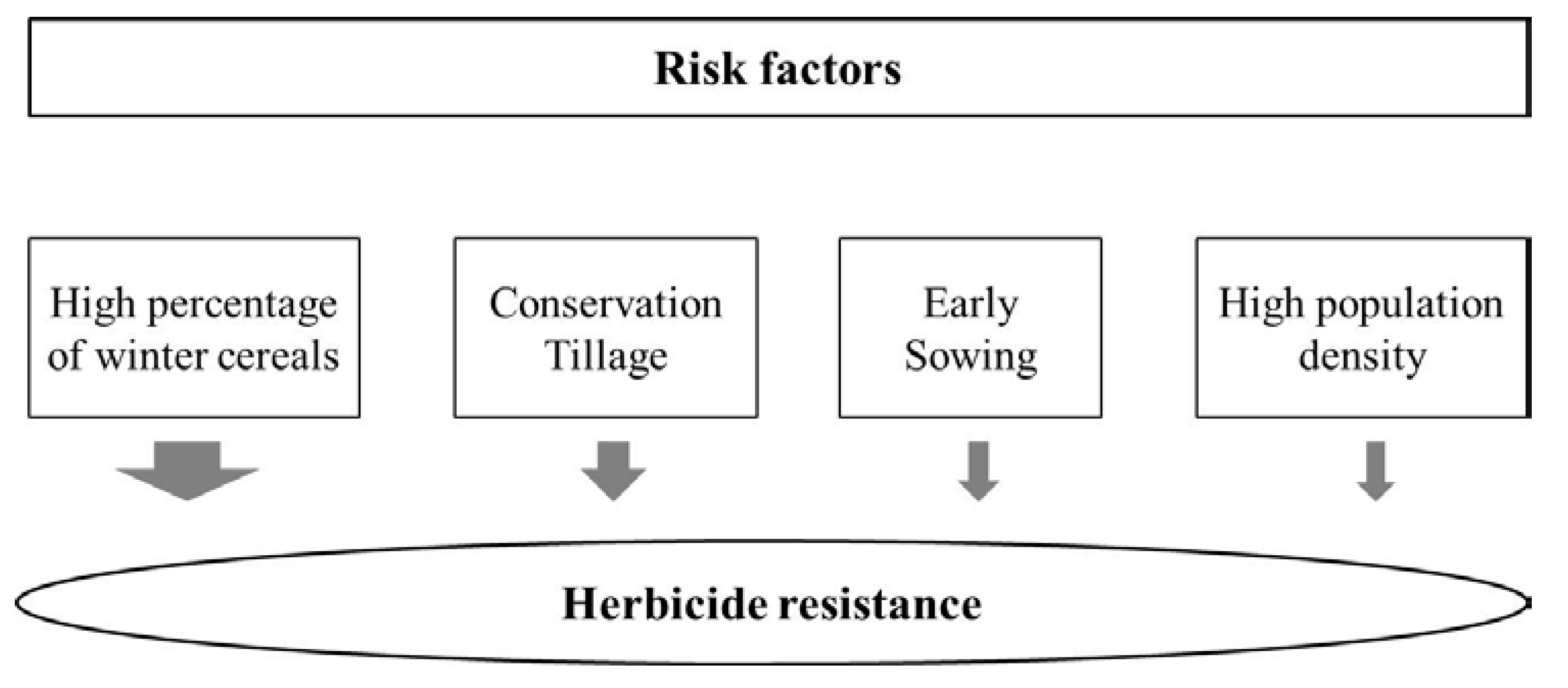
2. Results and Discussion
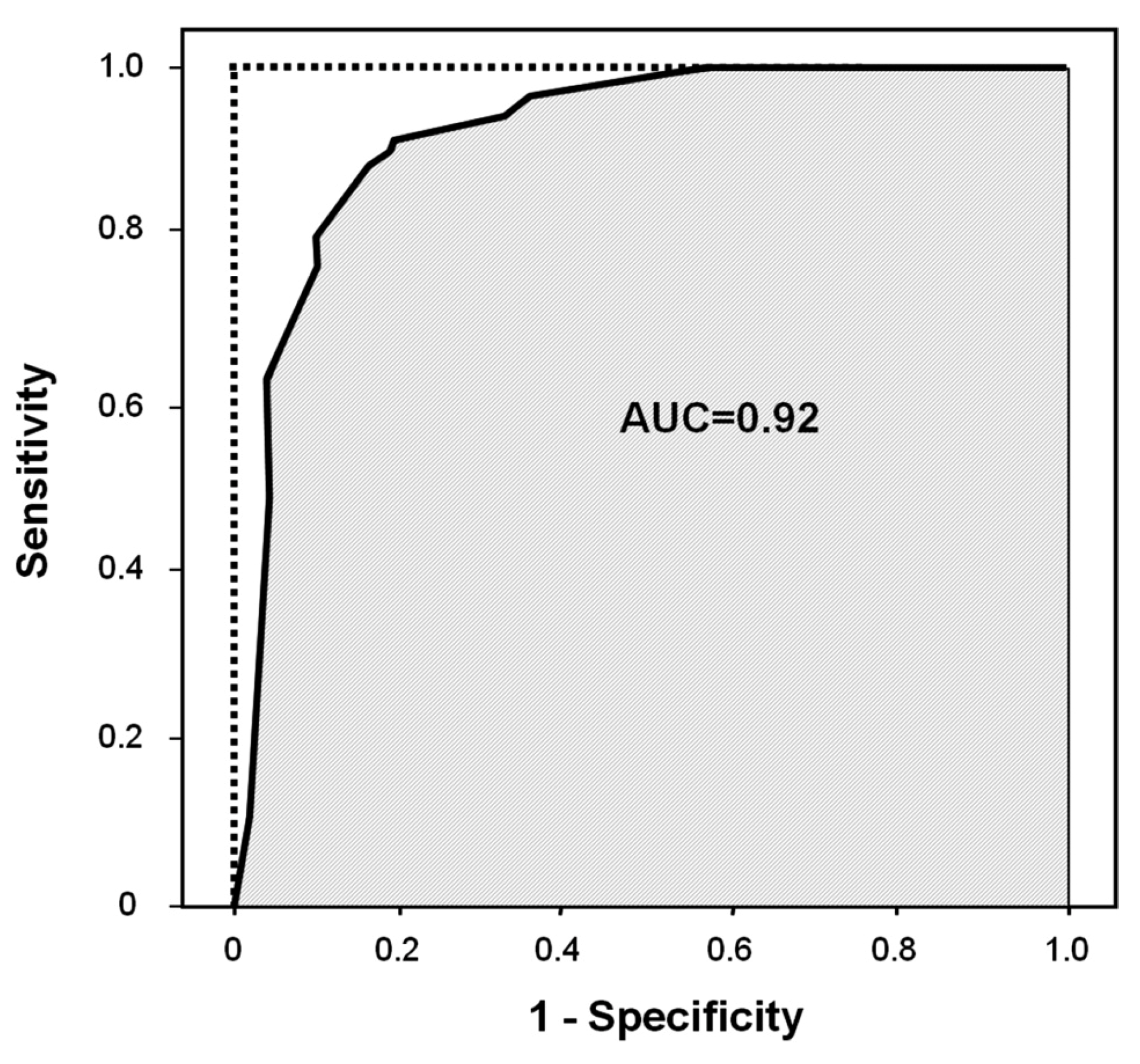
2.1. Logistic Regression Analysis
| Factors | % | Herbicide Resistance | |
|---|---|---|---|
| Yes (%) | No (%) | ||
| Resistant | |||
| Yes (verified) | 79.8 | ||
| No (not verified) | 20.2 | ||
| Total | 100 | ||
| Crop Rotation * | |||
| <50% | 11.4 | 16.7 | 83.3 |
| 50%–75% | 17.1 | 55.6 | 44.4 |
| >75%–100% | 71.5 | 95.7 | 4.3 |
| Total | 100 | ||
| Tillage | |||
| Plow | 57.0 | 74.0 | 26.0 |
| Reduced/No-Till | 43.0 | 87.6 | 12.4 |
| Total | 100 | ||
| Sowing Date ** | |||
| Middle/Late | 70.0 | 76.1 | 23.9 |
| Early | 30.0 | 88.6 | 11.4 |
| Total | 100 | ||
| Soil Texture *** | |||
| Middle/Heavy | 88.6 | 82.4 | 17.6 |
| Light | 11.4 | 60.0 | 40.0 |
| Total | 100 | ||
| Weed Density **** | |||
| Low/Middle | 43.3 | 73.7 | 26.3 |
| High | 56.7 | 84.6 | 15.4 |
| Total | 100 | ||
| Statistical method | Value | Degrees of freedom | p-value |
|---|---|---|---|
| Goodness of fit | |||
| Log Likelihood (LL) | −64.10 | ||
| Cox-Snell R2 | 0.40 | ||
| Nagelkerke R2 | 0.64 | ||
| Calibration | |||
| Hosmer-Lemeshow Chi2 | 4.02 | 6 | 0.674 |
| DiscriminationPercentage of correct classification | 90.1 | ||
| Valid cases | 263 |
| Factors a | ORb | ORc | Wald | 95% CI | % d |
|---|---|---|---|---|---|
| Crop Rotation (ref. <50%) | 36.69 | 53.38 | |||
| 50%–75% | 6.25 | 21.13 ** | 7.21 | 2.28–195.83 | |
| >75%–100% | 112.50 | 1274.82 ** | 31.38 | 104.46–15557.23 | |
| Tillage (ref. Plow) | 2.49 | 87.67 ** | 10.05 | 5.52–1393.08 | 14.62 |
| Sowing Date (ref. Middle/Late) | 2.44 | 4.65 ** | 6.91 | 1.48–14.61 | 10.05 |
| Weed Density (ref. Low/Middle) | 1.96 | 2.11 * | 2.31 | 0.81–5.52 | 3.36 |
| Rotation * Tillage (ref. <50% & Plow) | 12.77 | 18.58 | |||
| (50%–75%) & (Reduced/ No–Till) | 0.04 ** | 4.61 | 0.00–0.75 | ||
| (>75%–100%) & (Reduced/ No–Till) | 0.01 ** | 12.13 | 0.00–0.09 | ||
| Constant | 0.02 | 13.17 |
3. Experimental Section
3.1. Sampling Strategy
3.2. Screening for Herbicide Resistance
3.2.1. Greenhouse Screening: Seed Germination Tests
3.2.2. Greenhouse Screening: Whole-Plant Pot Assays
| Trade nameSurfactant (%) | Active ingredient (a.i.) | Amount of active ingredient (g a.i./Kg-L) | Applied doses (g a.i./ha) |
|---|---|---|---|
| Lexus® Trend 90® * (0.1%) | flupyrsulfuron-methyl | 500 | 15, 60 |
| Glean® Trend 90® (0.1%) | chlorsulfuron | 750 | 15, 60 |
| Monitor® MonFastTM ** (0.1%) | sulfosulfuron | 800 | 10, 40 |
| Atlantis® OD | mesosulfuron iodosulfuron | 10.44 2 | 7.4, 29.6 |
| Oust® XP Trend 90® (0.1%) | sulfometuron-methyl | 750 | 37.5, 150 |
| Ralon® Super Trend 90® (0.1%) | fenoxaprop-P-ethyl | 69 | 82.8, 331.2 |
| Select® 240 EC Parasommer® *** (0.5%) | clethodim | 241.9 | 241.9, 967.6 |
| Arelon® flüssig | isoproturon | 500 | 1500, 6000 |
3.3. Field History
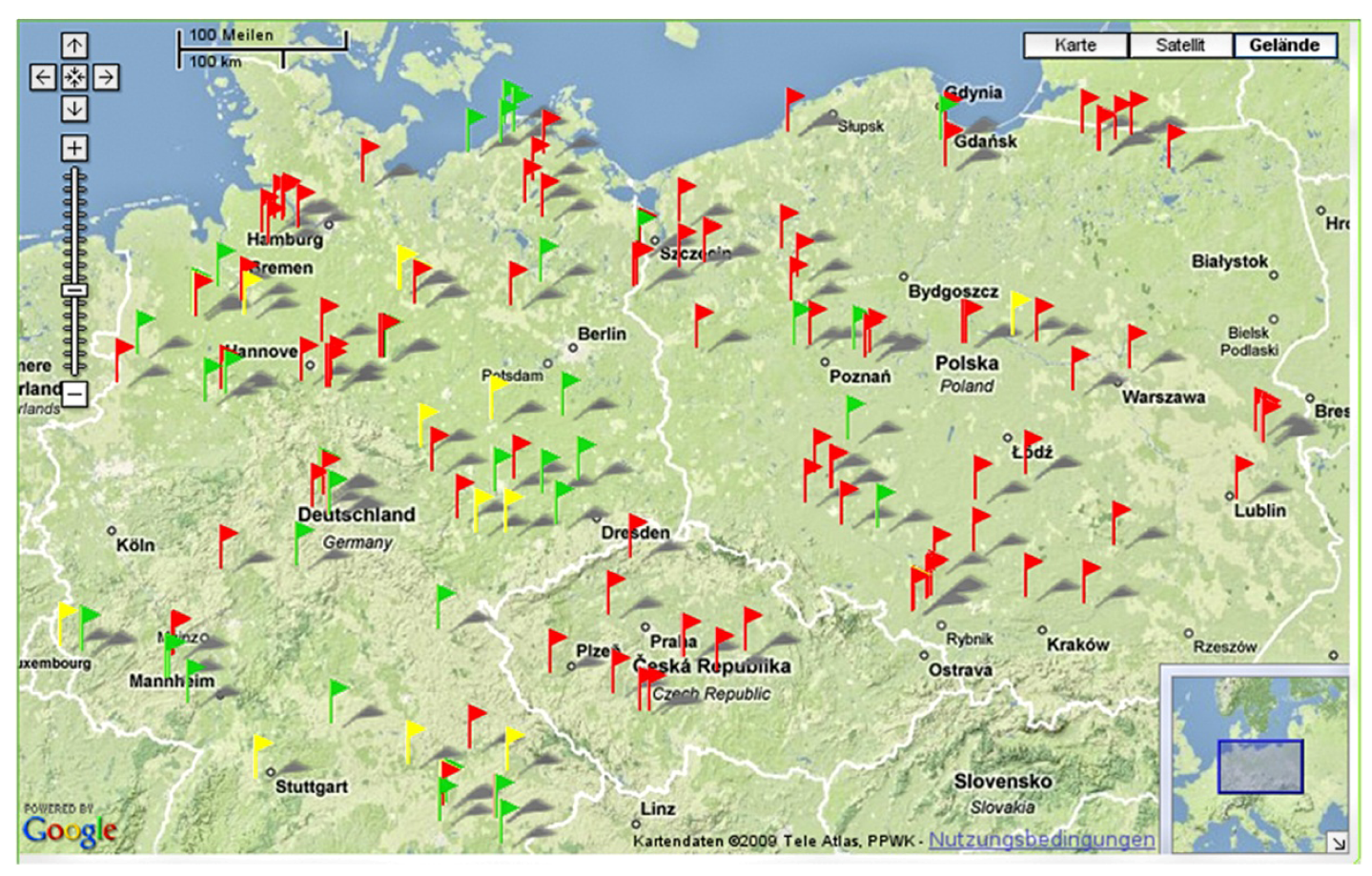
3.4. Technical Features of the GIS Database “Weedscout”
3.5. Presentation of the Model and Statistical Analyses of the Data

4. Conclusions
Acknowledgments
References
- Melander, B. Impact of drilling date on Apera spica-venti L. and Alopecurus myosuroides Huds. in winter cereals. Weed Res. 1995, 35, 157–166. [Google Scholar] [CrossRef]
- Zwerger, P.; Ammon, H.U. Unkraut: Ökologie und Bekämpfung; Eugen Ulmer: Stuttgart, Germany, 2002. [Google Scholar]
- Owen, M.J.; Walsh, M.J.; Llewellyn, R.S.; Powles, S.B. Widespread occurrence of multiple herbicide resistance in Western Australian annual ryegrass (Lolium rigidum) populations. Aust. J. Exp. Agric. 2007, 58, 711–718. [Google Scholar]
- Heap, I.M. International Survey of Herbicide Resistant Weeds. Available online: http://www.weedscience.org (accessed on 5 December 2012).
- Hatzios, K.K.; Penner, D. Interaction of herbicides with other agricultural chemicals in higher plants. Weed Sci. 1985, 1, 1–64. [Google Scholar]
- Beckie, H.J.; Hall, L.M.; Meers, S.; Laslo, J.J.; Stevenson, F.C. Management Practices Influencing Herbicide Resistance in Wild Oat. Weed Technol. 2004, 18, 853–859. [Google Scholar] [CrossRef]
- Melander, B.; Holst, N.; Jensen, P.K.; Hansen, E.M.; Olesen, J.E. Apera spica-venti population dynamics and impact on crop yield as affected by tillage, crop station, location and herbicide programmes. Weed Res. 2008, 48, 48–57. [Google Scholar] [CrossRef]
- Murphy, C.; Lemerle, D. Continuous cropping systems and weed selection. Euphytica 2006, 148, 61–73. [Google Scholar] [CrossRef]
- Mohler, C.L. Ecological bases for the cultural control of annual weeds. J. Prod. Agric. 1996, 9, 468–474. [Google Scholar]
- Hurle, K. Integrated management of grass weeds in arable crops. In Proceedings of the Brighton Crop Protection Conference—Weeds, Brighton, UK, 22–25 November 1993; pp. 81–88.
- Koch, W.; Hurle, K. Grundlagen der Unkrautbekämpfung; UTB Verlag: Stuttgart, Germany, 1978. [Google Scholar]
- Wallgren, B.; Avholm, K. Dormancy and Germination of Apera spica-venti L. and Alopecurus myosuroides Huds Seeds. Swed. J. Agric. Res. 1978, 8, 11–15. [Google Scholar]
- Rola, J. Dynamik von Unkrautpopulationen auf leichten Böden in Polen. J. Plant Dis. Prot. 1990, Special Issue XII, 97–100. [Google Scholar]
- Northam, F.E.; Callihan, R.H. The windgrasses (Apera Adans., Poaceae) in North America. Weed Technol. 1992, 6, 445–450. [Google Scholar]
- Andreasen, C. The Occurrence of Weed Species in Danish Arable Fields.
- Gehring, K.; Thyssen, S. Unkraut-Steckbrief, Windhalm. Bayerische Landesanstalt für Landwirtschaft, Institut für Pflanzenschutz. Available online: http://www.lfl.bayern.de (accessed on 4 September 2012).
- Kötter, U. Entwicklung und Konkurrenzverhalten von Windhalm (Apera spica-venti) in Winterweizen und Winterroggen. Gesunde Pflanzen 1991, 4, 184–189. [Google Scholar]
- Mayor, J.P.; Maillard, A. A wind bent grass biotype resistant to the herbicide isoproturon found in Changins. Suisse Agric. 1997, 29, 39–44. [Google Scholar]
- Niemann, P. Resistance of silky bentgrass (Apera spica-venti) against Isoproturon. Mitteilungen der Biologischen Bundesanstalt für Land- und Forstwirtschaft 2000, 376, 147–148. [Google Scholar]
- Marczewska, K.; Rola, H. Biotypes of Apera spica-venti and Centaurea cyanus resistant to chlorsulfuron in Poland. In Proceedings of the 13th EWRS Symposium, Bari, Italy, 19–23 June 2005.
- Novakova, K.; Soukup, J.; Wagner, J.; Hamouz, P.; Namestek, J. Chlorsulfuron resistance in silky bent-grass (Apera spica-venti L. Beauv.) in the Czech Republic. J. Plant Dis. Prot. 2006, Special issue XX, 139–146. [Google Scholar]
- Balgheim, N.; Wagner, J.; Gerhards, R. ALS-inhibitor resistant Apera spica-venti (L.) Beauv. due to target-site mutation. In Proceedings of the 14th European Weed Research Society Symposium, Hamar, Norway, 17–21 June 2007.
- Delabays, N.; Mermillod, C.; Bohren, C. First case of resistance to sulfonylurea herbicides reported in Switzerland: A biotype of loose silky-bent (Apera spica-venti (L.) Beauv.). J. Plant Dis. Prot. 2006, Special Issue XX, 139–146. [Google Scholar]
- Bárberi, P. Weed Management for Developing Countries, 120 Addendum, 1st; Labrada, R., Ed.; FAO (Food Agricultural Organization of the United Nations): Roma, Italy, 2003. [Google Scholar]
- Cardina, J.; Webster, T.M.; Herms, C.P. Long-term tillage and rotation effects on soil seedbank characteristics. Annals of Applied Biology. 1998, 5, 213–220. [Google Scholar]
- Froud-Williams, R.J.; Chancellor, R.J.; Drennan, D.S.H. Potential changes in weed floras associated with reduced-cultivation systems for cereal production in temperate regions. Weed Res. 1981, 21, 99. [Google Scholar] [CrossRef]
- Gill, G.S.; Poole, M.L.; Holmes, J.E. Competition between wheat and brome grass in Western Australia. Aust. J. Exp. Agric. 1987, 27, 291–294. [Google Scholar] [CrossRef]
- Hartzler, B. Weed population dynamics. In Proceedings of the 2000 Integrated Crop Management Conference, Ames, IA, USA, 20–29 November 2000.
- OEPP/EPPO. General introduction to the EPPO guidelines for efficacy evaluation of plant protection products. OEPP/EPPO Bull. 1989, 19, 183–195.
- Proplanta GmbH & Co. KG. Available online: http://www.proplanta.de (accessed on 19 December 2012).
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]
- Long, J.S. Regression models for categorical and limited dependent variables. Sage Publ. 1997, 7, 11–33. [Google Scholar]
- Pampel, F.C. Logistic regression: A primer. Sage University Papers Series on Quantitative Applications in the Social Sciences. Sage Publ. 2000, 7–132. [Google Scholar]
- Escobar, G.J.; Greene, J.D.; Scheirer, P.; Gardner, M.N.; Draper, D.; Kipnis, P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient and laboratory databases. Medical care 2008, 46, 232–239. [Google Scholar] [CrossRef]
- Davis, A.S.; Renner, K.A. Influence of seed depth and pathogens on fatal germination on velvetleaf (Abutilon theophrasti) and giant foxtail (Setaria faberi). Weed Sci. 2007, 55, 30–35. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Massa, D.; Kaiser, Y.I.; Andújar-Sánchez, D.; Carmona-Alférez, R.; Mehrtens, J.; Gerhards, R. Development of a Geo-Referenced Database for Weed Mapping and Analysis of Agronomic Factors Affecting Herbicide Resistance in Apera spica-venti L. Beauv. (Silky Windgrass). Agronomy 2013, 3, 13-27. https://doi.org/10.3390/agronomy3010013
Massa D, Kaiser YI, Andújar-Sánchez D, Carmona-Alférez R, Mehrtens J, Gerhards R. Development of a Geo-Referenced Database for Weed Mapping and Analysis of Agronomic Factors Affecting Herbicide Resistance in Apera spica-venti L. Beauv. (Silky Windgrass). Agronomy. 2013; 3(1):13-27. https://doi.org/10.3390/agronomy3010013
Chicago/Turabian StyleMassa, Dario, Yasmin I. Kaiser, Dionisio Andújar-Sánchez, Rocío Carmona-Alférez, Jörg Mehrtens, and Roland Gerhards. 2013. "Development of a Geo-Referenced Database for Weed Mapping and Analysis of Agronomic Factors Affecting Herbicide Resistance in Apera spica-venti L. Beauv. (Silky Windgrass)" Agronomy 3, no. 1: 13-27. https://doi.org/10.3390/agronomy3010013




