Screening for Barley Waterlogging Tolerance in Nordic Barley Cultivars (Hordeum vulgare L.) Using Chlorophyll Fluorescence on Hydroponically-Grown Plants
Abstract
:1. Introduction
2. Experimental Section
2.1. Soil Studies
2.2. Hydroponics
2.3. Chlorophyll Fluorescence
2.4. Statistical Analysis
3. Results
3.1. Growth Reduction in Waterlogged Soil
| Cultivar | Shoot weight (mg ) | Root length (mm) | Quantum yield | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Stress | Rel(%) | Control | Stress | Rel(%) | Control | Stress | Rel(%) | |
| Alexis | 327 | 212 | 65.8 | 155 | 97 | 63 | 0.418 | 0.364 | 87.2 |
| Baronesse | 349 | 246 | 72.0 | 175 | 107 | 61 | 0.471 | 0.432 | 91.8 |
| Cecilia | 356 | 222 | 62.4 | 170 | 99 | 59 | 0.408 | 0.372 | 91.4 |
| Barke | 356 | 244 | 68.4 | 155 | 89 | 58 | 0.388 | 0.383 | 98.8 |
| Henni | 294 | 245 | 83.7 | 164 | 127 | 78 | 0.462 | 0.440 | 95.2 |
| Scarlett | 297 | 205 | 69.8 | 180 | 92 | 51 | 0.439 | 0.394 | 89.8 |
| Meltan | 288 | 211 | 75.3 | 180 | 96 | 53 | 0.456 | 0.413 | 90.6 |
| Otira | 318 | 260 | 85.2 | 150 | 124 | 83 | 0.455 | 0.439 | 96.3 |
| Pongo | 286 | 173 | 62.9 | 138 | 98 | 72 | 0.418 | 0.385 | 92.2 |
| Optic | 326 | 228 | 70.7 | 184 | 101 | 55 | 0.450 | 0.391 | 86.8 |
| Gustav | 262 | 182 | 70.6 | 173 | 101 | 58 | 0.482 | 0.416 | 86.2 |
| Quench | 326 | 215 | 69.1 | 191 | 102 | 54 | 0.459 | 0.421 | 91.8 |
| SEM | 10.8 | 13.2 | 4.5 | 3.9 | 2.0 | 2.0 | 0.006 | 0.006 | 1.56 |
| Treatment | *** | *** | *** | ||||||
| Cultivar | ns | *** | * | *** | *** | *** | |||
| T x C | ** | *** | *** | ||||||
3.2. Seedling Root Growth in Oxygen-Depleted Nutrient Solution
| WLT | SWc | SWs | RLc | RLs | RLs/RLc | |
|---|---|---|---|---|---|---|
| Shoot weight control (SWc) | −0.24 | 1.00 | ||||
| Shoot weight stress (SWs) | 0.61* | 0.61* | 1.00 | |||
| Root length control (RLc) | 0.01 | 0.03 | 0.02 | 1.00 | ||
| Root length stress (RLs) | 0.81** | −0.13 | 0.53 | −0.16 | 1.00 | |
| RLs/RLc | 0.55 | −0.11 | 0.34 | −0.72** | 0.80** | 1.00 |
| QY control (QYc) | 0.56 | −0.51 | 0.01 | 0.49 | 0.51 | 0.05 |
| QYstress (QYs) | 0.82*** | −0.28 | 0.41 | 0.21 | 0.75** | 0.40 |
| QYs/QYc | 0.34 | 0.36 | 0.60* | −0.43 | 0.32 | 0.49 |
3.3. Quantum Yield of Seedlings Grown in Oxygen-Depleted Solution

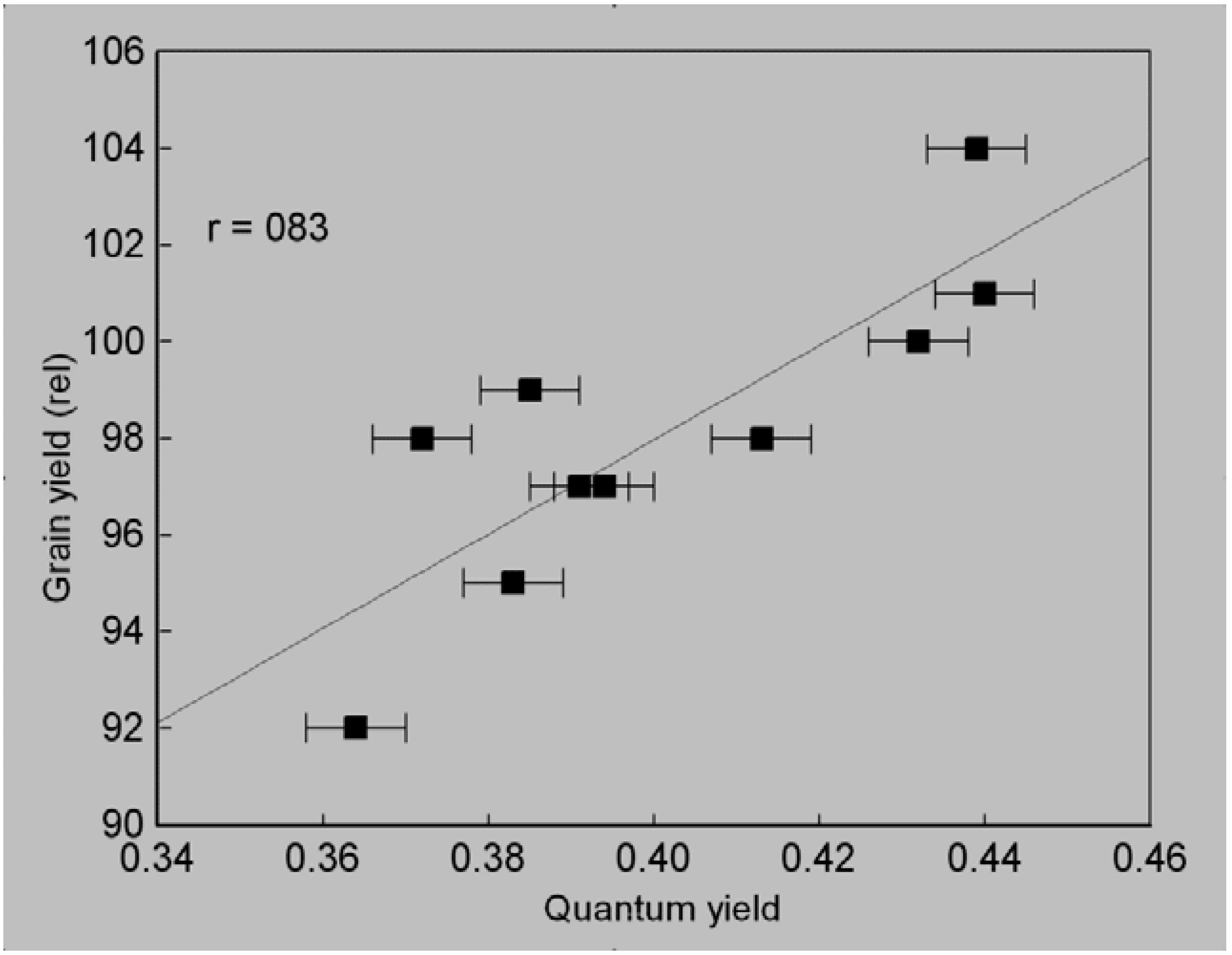
3.4. Selection Efficiency of Quantum Yield
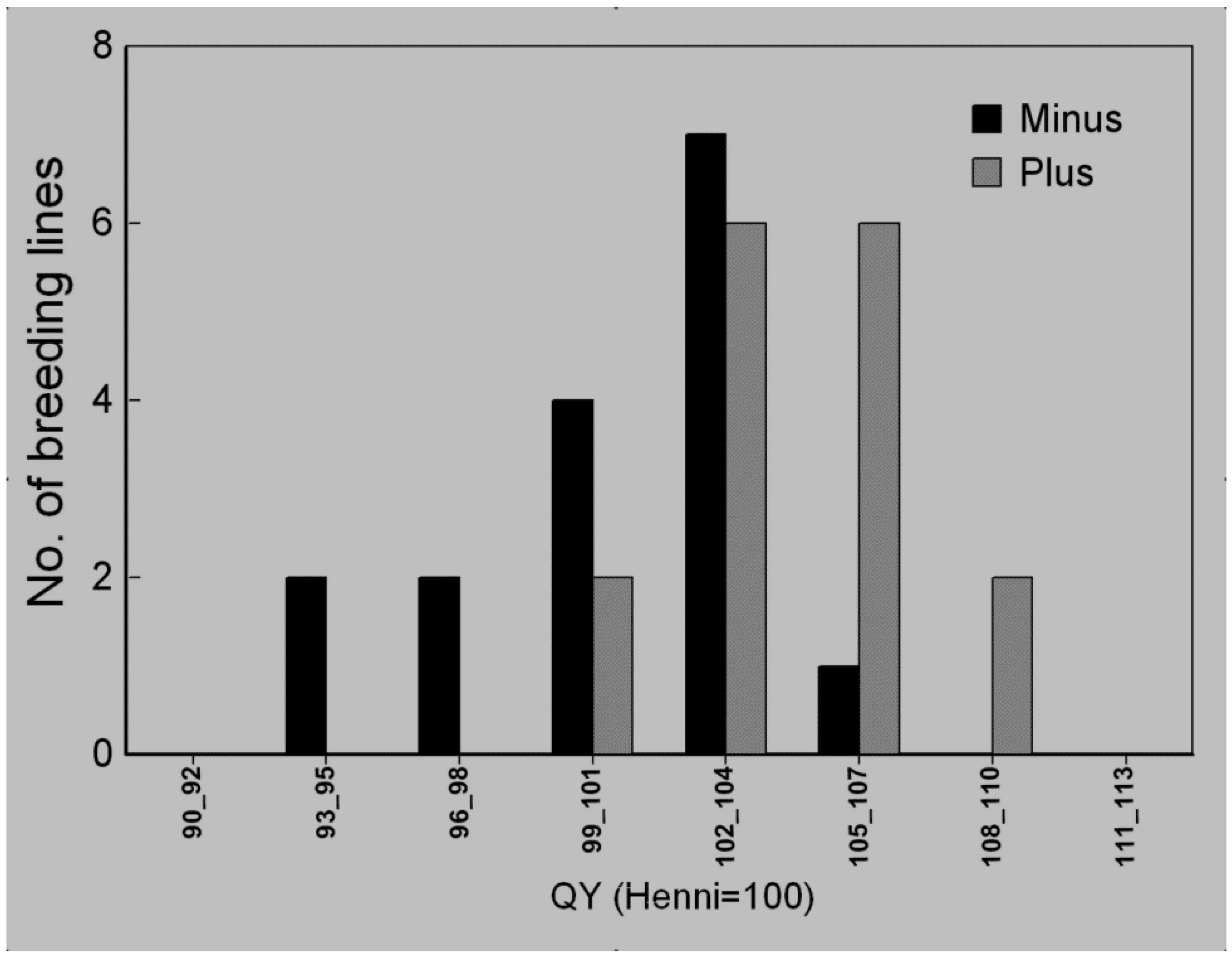
3.5. Waterlogging Tolerance of Nordic Barley Cultivars
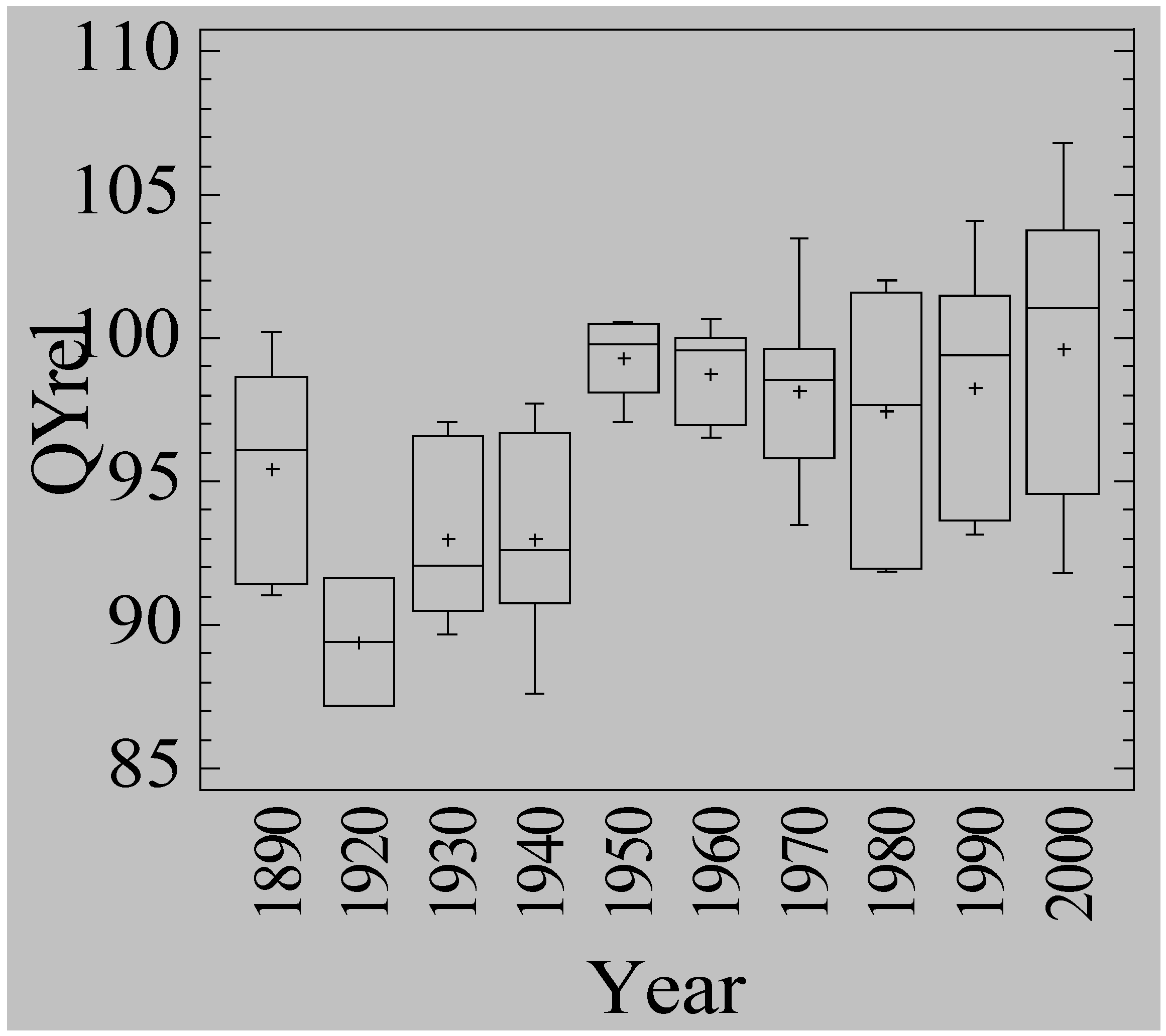
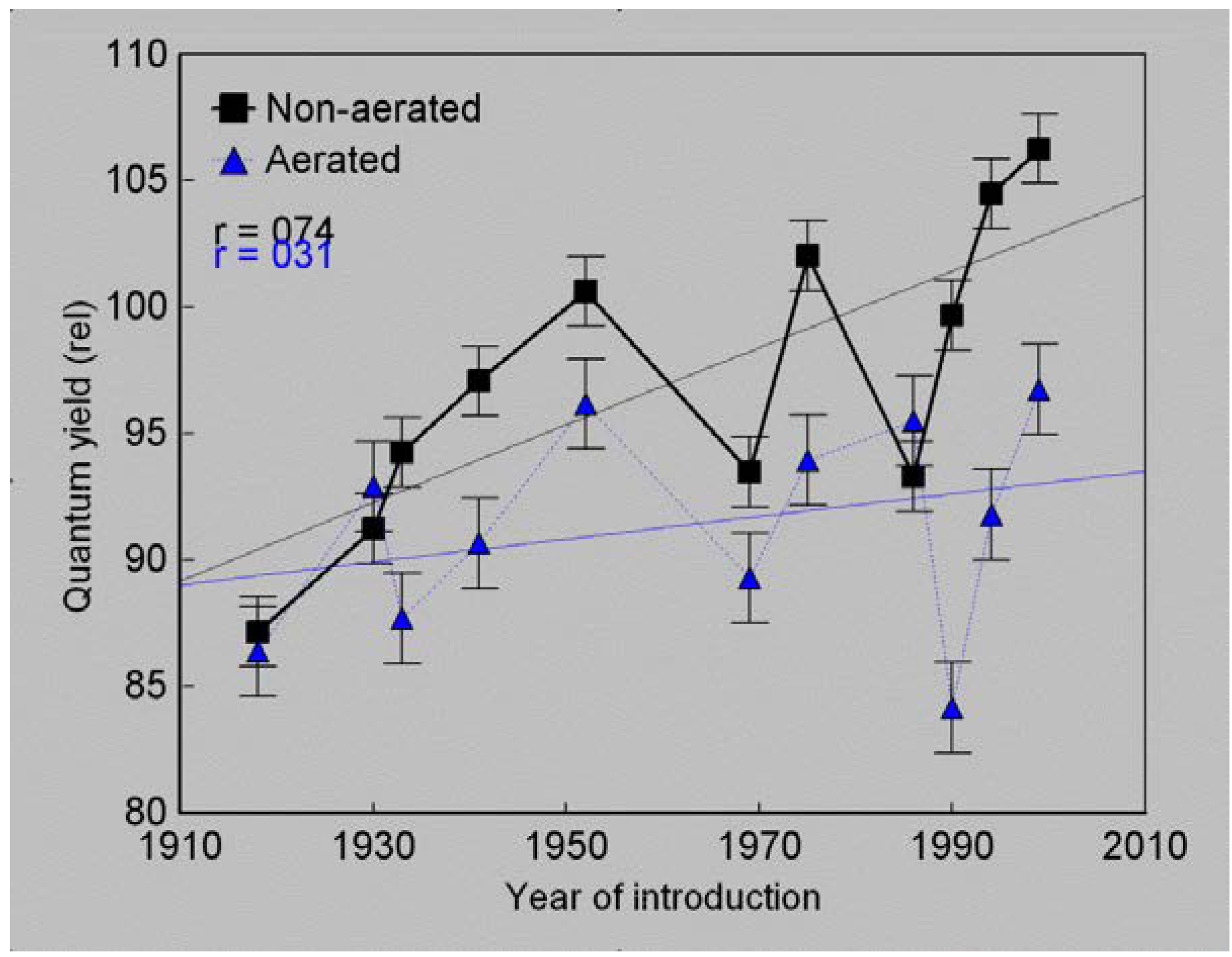
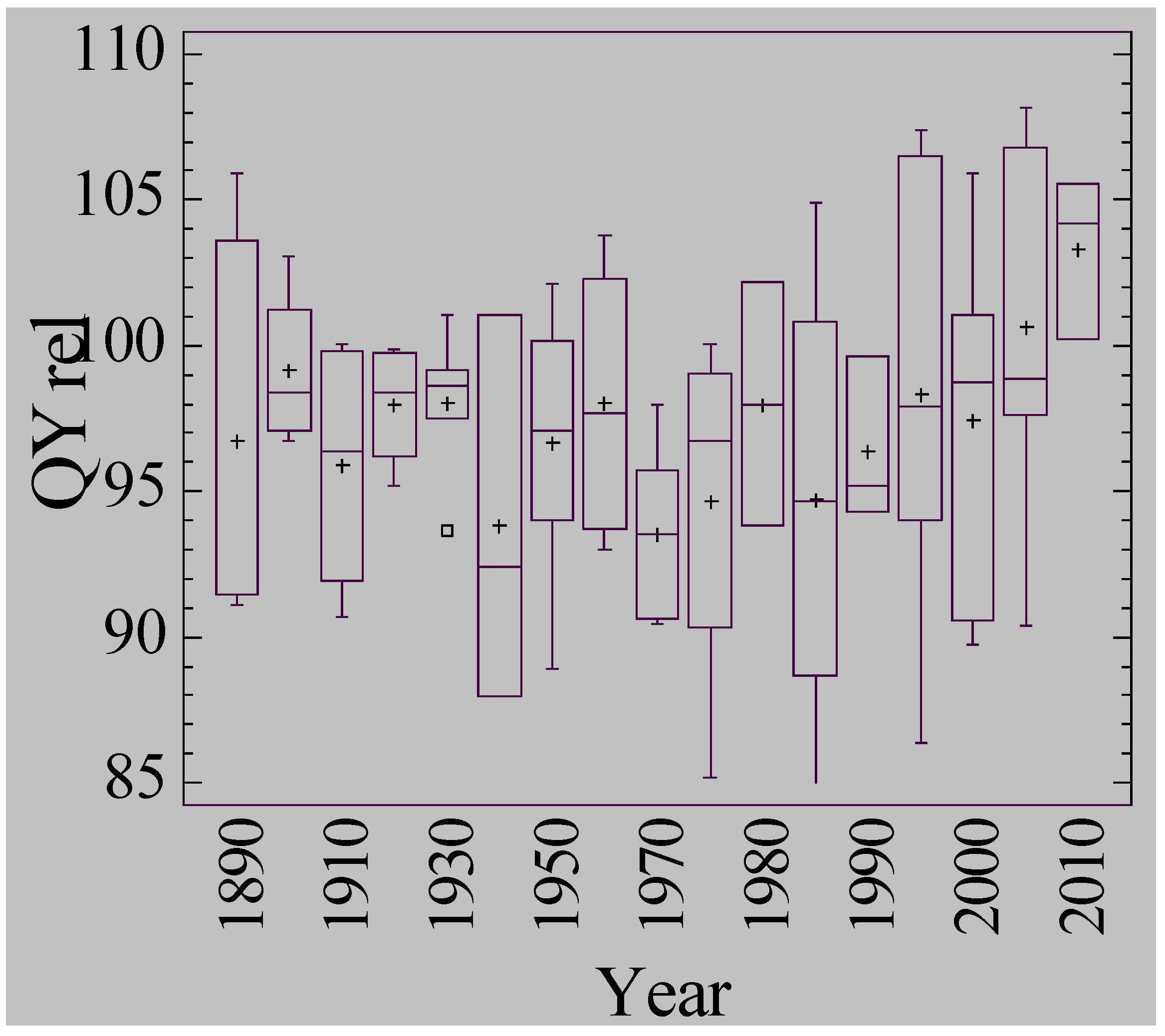
4. Discussion
5. Conclusions
Acknowledgments
References and Notes
- Setter, T.L.; Waters, I. Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant Soil 2003, 253, 1–34. [Google Scholar] [CrossRef]
- Zhou, M.X.; Li, H.B.; Mendham, N.J. Combining ability of waterlogging tolerance in barley. Crop Sci. 2007, 47, 278–284. [Google Scholar] [CrossRef]
- Kjellstrom, E.; Nikulin, G.; Hansson, U.; Strandberg, G.; Ullerstig, A. 21st century changes in the European climate: Uncertainties derived from an ensemble of regional climate model simulations. Tellus Ser. A Dyn. Meteorol. Oceanogr. 2011, 63, 24–40. [Google Scholar] [CrossRef]
- Colmer, T.D.; Voesenek, L.A.C.J. Flooding tolerance: Suites of plant traits in variable environments. Funct. Plant Biol. 2009, 36, 665–681. [Google Scholar] [CrossRef]
- Ahmed, F.; Rafii, M.Y.; Ismail, M.R.; Juraimi, A.S.; Rahim, H.A.; Asfaliza, R.A.; Latif, M.A. Waterlogging tolerance of crops: Breeding, mechanism of tolerance, molecular approaches, and future prospects. BioMed. Res. Int. 2013. [Google Scholar] [CrossRef]
- Garthwaite, A.J.; von Bothmer, R.; Colmer, T.D. Diversity in root aeration traits associated with waterlogging tolerance in the genus Hordeum. Funct. Plant Biol. 2003, 30, 875–889. [Google Scholar] [CrossRef]
- Pang, J.Y.; Zhou, M.X.; Mendham, N.; Shabala, S. Growth and physiological responses of six barley genotypes to waterlogging and subsequent recovery. Aust. J. Agric. Res. 2004, 55, 895–906. [Google Scholar] [CrossRef]
- Malik, A.I.; Islam, A.K.M. R.; Colmer, T.D. Transfer of the barrier to radial oxygen loss in roots of Hordeum marinum to wheat (Triticum aestivum): Evaluation of four H-marinum-wheat amphiploids. New Phytol. 2011, 190, 499–508. [Google Scholar] [CrossRef]
- Malik, A.I.; Colmer, T.D.; Lambers, T.L.; Setter, H.T.L.; Schortemeyer, M. Short-term waterlogging has long-term effects on the growth and physiology of wheat. New Phytol. 2002, 153, 225–236. [Google Scholar] [CrossRef]
- Takeda, K.; Fukuyama, T. Tolerance to pre-germination flooding in the world collection of barley varieties. Barley Genet. 1987, V, 735–740. [Google Scholar]
- Hamachi, Y.; Yoshino, M.; Furusho, M.; Yoshida, T. Index of screening for wet endurance in malting barley. Jpn J. Breed. 1990, 40, 361–366. [Google Scholar]
- Li, H.; Vaillancourt, R.; Mendham, N.; Zhou, M. Comparative mapping of quantitative trait loci associated with waterlogging tolerance in barley (Hordeum vulgare L.). BMC Genomics 2008, 9, 401. [Google Scholar]
- Striker, G. Visiting the methodological aspects of flooding experiments: Quantitative evidence from agricultural and ecophysiological studies. J. Agron. Crop Sci. 2008, 194, 249–255. [Google Scholar] [CrossRef]
- Qiu, F.Z.; Zheng, Z.L.; Xu, S.Z. Mapping of QTL associated with waterlogging tolerance during the seedling stage in maize. Ann. Bot. 2007, 99, 1067–108. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Else, M.A.; JJanowiak, F.; Atkinson, C.J.; Jackson, M.B. Root signals and stomatal closure in relation to photosynthesis, chlorophyll a fluorescence and adventitious rooting of flooded tomato plants. Ann. Bot. 2009, 103, 313–323. [Google Scholar]
- Bertholdsson, N.-O. Pre-studies of water- and drought tolerance in Barley. unpublished.
- Bertholdsson, N.-O.; Kolodinska Brantestam, A. Breeding for improved yield in Nordic barley germplasms and its effects on early vigour, straw length and harvest index. Eur. J. Agron. 2009, 30, 266–274. [Google Scholar] [CrossRef]
- Larsson, S. A simple, rapid and non-destructive method useful for drought resistance breeding in oats (Avena sativa L.). Zeitschrift für Pflanzenzüchtung 1982, 89, 206–221. [Google Scholar]
- Walz, H. Photosynthesis yield analyzer MINI-PAM. In Handbook of Operation, 2nd ed; Heinz Walz GmbH: Effeltrich, Germany, 1999. [Google Scholar]
- Arbona, V.; López-Climent, M.F.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Maintenance of a high photosynthetic performance is linked to flooding tolerance in citrus. Environ. Exp. Bot. 2009, 66, 135–142. [Google Scholar] [CrossRef]
- Smethurst, C.F.; Shabala, S. Screening methods for waterlogging tolerance in lucerne: Comparative analysis of waterlogging effects on chlorophyll fluorescence, photosynthesis, biomass and chlorophyll content. Funct. Plant Biol. 2003, 30, 335–343. [Google Scholar] [CrossRef]
- Hague, M.E.; Oyanagi, A.; Kawaguchi, K. Aerenchyma formation in the seminal roots of Japanese wheat cultivars in relatin to growth under waterlogged conditions. Plant Product. Sci. 2012, 15, 164–173. [Google Scholar] [CrossRef]
- Vartapetian, B. Plant anaerobic stress as a novel trend in ecological physiology, biochemistry, and molecular biology: 2. Further development of the problem. Russian J. Plant Physiol. 2006, 53, 711–738. [Google Scholar] [CrossRef]
- Lynch, J.P. Roots of the second green revolution. Aust. J. Bot. 2007, 55, 493–512. [Google Scholar] [CrossRef]
- Xue, D.; Zhou, M.; Zhang, X.; Chen, S.; Wie, K.; Chen, S.; Wie, K.; Zeng, F.; Mao, Y.; Wu, F.; Zhang, G. Identification of QTLs for yield and yield components of barley under different growth conditions. J. Zhejiang Univ. Sci. B. 2011, 11, 169–176. [Google Scholar]
- Zhou, M. Accurate phenotyping reveals better QTL for waterlogging tolerance in barley. Plant Breed. 2011, 130, 203–208. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bertholdsson, N.-O. Screening for Barley Waterlogging Tolerance in Nordic Barley Cultivars (Hordeum vulgare L.) Using Chlorophyll Fluorescence on Hydroponically-Grown Plants. Agronomy 2013, 3, 376-390. https://doi.org/10.3390/agronomy3020376
Bertholdsson N-O. Screening for Barley Waterlogging Tolerance in Nordic Barley Cultivars (Hordeum vulgare L.) Using Chlorophyll Fluorescence on Hydroponically-Grown Plants. Agronomy. 2013; 3(2):376-390. https://doi.org/10.3390/agronomy3020376
Chicago/Turabian StyleBertholdsson, Nils-Ove. 2013. "Screening for Barley Waterlogging Tolerance in Nordic Barley Cultivars (Hordeum vulgare L.) Using Chlorophyll Fluorescence on Hydroponically-Grown Plants" Agronomy 3, no. 2: 376-390. https://doi.org/10.3390/agronomy3020376




