Implications of Environmental Stress during Seed Development on Reproductive and Seed Bank Persistence Traits in Wild Oat (Avena fatua L.)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Seed Production and Reproductive Allocation
| Per Plant | |||||
|---|---|---|---|---|---|
| Stress Environment | Maturation Group | Seed Yield | Seed Number | Biomass | RA |
| 2005 | g | g | g seed g biomass−1 | ||
| No Stress | 1 | 6.6 | 289 | 26 | 1.9 |
| 2 | 9.5 | 465 | |||
| 3 | 13.6 | 655 | |||
| 4 | 17.4 | 840 | |||
| total | 47 | 2250 | |||
| Drought | 1 | 0.7 | 38 | 9 | 0.6 |
| 2 | 1.05 | 54 | |||
| 3 | 1.55 | 81 | |||
| 4 | 1.9 | 113 | |||
| total | 5.2 | 286 | |||
| 50% Shade | Pooled | 1.2 | 65 | 9 | 0.1 |
| 70% Shade | Pooled | 0.2 | 9 | 4 | <0.1 |
| 2006 | |||||
| No stress | Early | 5.6 | 241 | 15.6 | 0.8 |
| Late | 6.6 | 325 | |||
| total | 12.2 | 566 | |||
| 50% Shade | Early | 0.7 | 40 | 5.4 | 0.2 |
| Late | 0.6 | 30 | |||
| total | 1.3 | 70 | |||
| 70% Shade | Pooled | 0.7 | 72 | 4.6 | 0.1 |
2.2. Primary Physiological Seed Dormancy

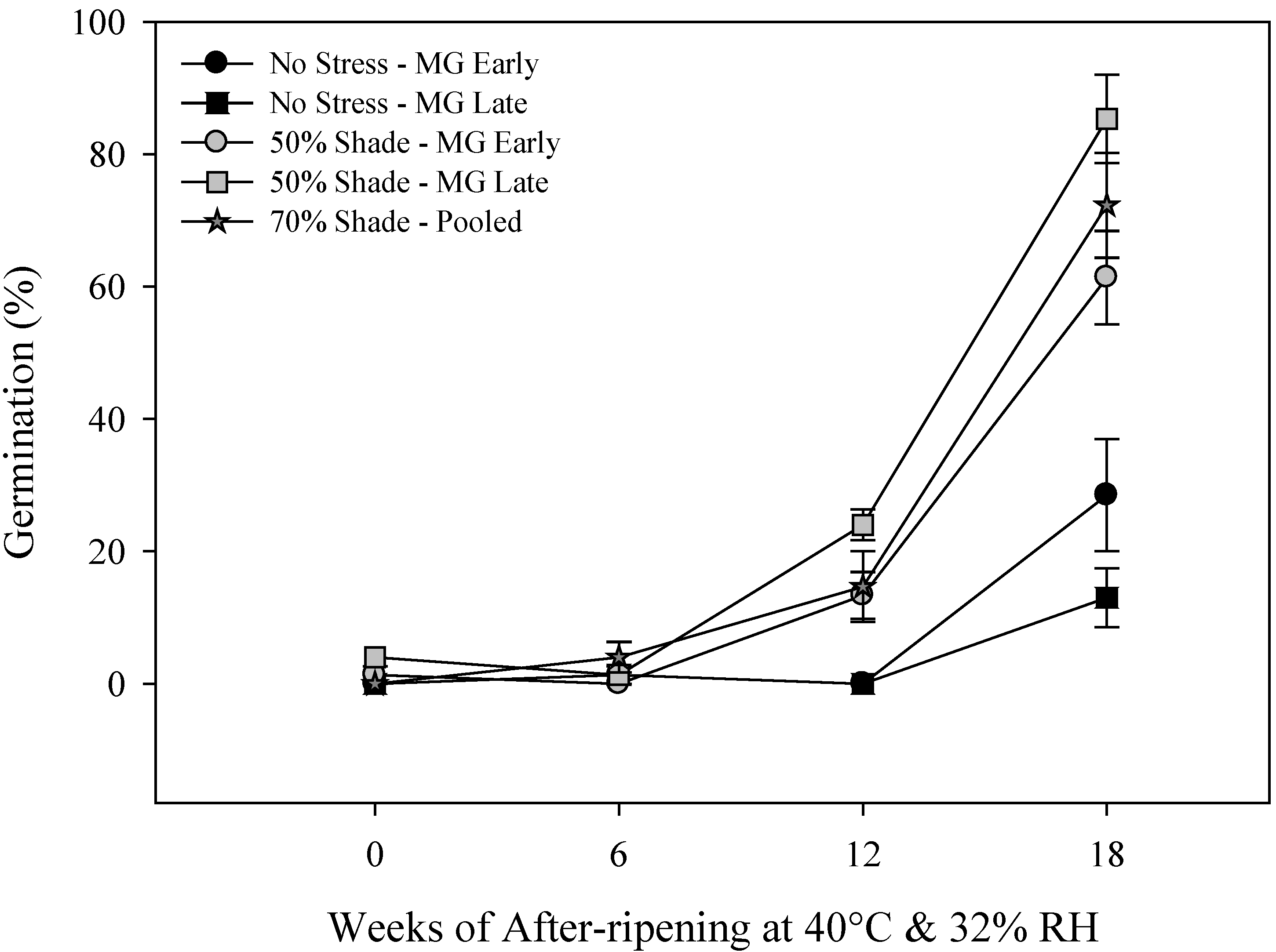
2.3. Seed and Seedling Vigor
| Weeks of Aging | Days to Emergence | Total Seedlings Emerged | Individual Seedling Biomass | Treatment (trt) Biomass |
|---|---|---|---|---|
| 2005 | d seedling−1 | seedlings 14 day−1 | mg seedling−1 | mg trt−1 |
| 0 | 5.1 a | 18.5 a | 17.0 a | 335 a |
| 4 | 4.9 a | 15.9 b | 23.6 a | 370 a |
| 8 | 8.8 b | 8.3 c | 19.9 a | 150 b |
| pvalue | <0.0001 | <0.0001 | n.s. | <0.0001 |
| 2006 | ||||
| 0 | 5.3 a | 17.8 a | 15.6 a | 291 a |
| 4 | 5.1 a | 13.7 b | 20.8 a | 284 a |
| 8 | 7.5 b | 6.6 c | 18.2 a | 121 b |
| pvalue | <0.0001 | <0.0001 | n.s. | <0.0001 |
2.4. Discussion
3. Experimental Section
3.1. Plant Materials and Stress Environments

3.2. Seed Dormancy and Viability
3.3. Seed Vigor Evaluation
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Gilliom, R.J. Pesticides in U. S. streams and groundwater. Environ. Sci. Technol. 2007, 41, 3408–3413. [Google Scholar] [CrossRef]
- Jasieniuk, M.J.; Brule-Babel, A.L.; Morrison, I.N. The evolution and genetics of herbicide resistance in weeds. Weed Sci. 1996, 44, 176–193. [Google Scholar]
- Norris, R.F. Ecological Implications of Using Thresholds for Weed Management. In Expanding the Context of Weed Management; Buhler, D.D., Ed.; Hawthorn Press: New York, NY, USA, 1999; pp. 31–58. [Google Scholar]
- Gallagher, R.S.; Fuerst, E.P. The Ecophysiology Basis of Weed Seed Longevity in the Soil. In Seed Science and Technology; Basra, A.S., Ed.; Haworth Food Products Press: Binghamton, NY, USA, 2006; pp. 521–557. [Google Scholar]
- Sawhney, R.; Naylor, J.M. Dormancy studies in seed of Avena fatua 13: Influence of drought stress during seed development on the duration of seed dormancy. Can. J. Bot. 1982, 60, 1016–1020. [Google Scholar] [CrossRef]
- Peters, N.C.B. Production and dormancy of wild oat (Avena fatua) seed fromplants grown under water stress. Ann. Appl. Biol. 1982, 100, 189–196. [Google Scholar] [CrossRef]
- Gallagher, R.S.; Granger, K.L.; Keser, L.H.; Rossi, J.; Pittmann, D.; Rowland, S.; Burnham, M.; Fuerst, E.P. Shade and drought stress-induced changes in phenolic content of wild oat (Avena fatua L.) seed. J. Stress Physiol. 2010, 6, 90–107. [Google Scholar]
- Leishman, M.R.; Wright, I.J.; Moles, A.T.; Westoby, M. The Evolutionary Ecology of Seed Size. In Seed: The Ecology of Regeneration in Plant Communities, 2nd ed.; CABI Publishing: Wallingford, UK, 2000; pp. 31–58, 410. [Google Scholar]
- Simpson, G.M. Seed Dormancy in Grasses; Cambridge University Press: Cambridge, UK, 1990; p. 308. [Google Scholar]
- De Luna, L.Z.; Kennedy, A.C.; Hansen, J.C.; Paulitz, T.C.; Gallagher, R.S.; Fuerst, E.P. Mycobiota on wild oat (Avena fatua L.) seed and their caryopsis decay potential. Plant Health Progr. 2011. [Google Scholar] [CrossRef]
- Stoller, E.W.; Myers, R.A. Effects of shading and soybean Glycine max (L.) interference on Solanum ptycanthum (Dun.) (eastern black nightshade) growth and development. Weed Res. 1989, 29, 307–316. [Google Scholar] [CrossRef]
- Van Hinsberg, A. Maternal and ambient environmental effects of light on germination in Plantaga lanceolata: Correlated responses to selection on leaf length. Funct. Ecol. 1998, 12, 825–833. [Google Scholar] [CrossRef]
- Bello, I.A.; Owen, M.; Hatterman-Valenti, H.M. Effect of shade on velvetleaf (Abutilon theophrasti) growth, seed production, and dormancy. Weed Technol. 1995, 9, 452–455. [Google Scholar]
- Benvenuti, S.; Macchia, M.; Stefani, A. Effects of shade on reproduction and some morphological characteristics of Abutilon theophrasti Medicus, Datura stramonium L. and Sorghum halepense L. Weed Res. 1994, 34, 283–288. [Google Scholar] [CrossRef]
- Turnbull, C.G.N. Shoot architecture II: Control of Branching. In Plant Architechture and Its Manipulation: Annual Plant Reviews 17; CRC Press: Boca Raton, FL, USA, 2005; pp. 92–120. [Google Scholar]
- Foley, M.E.; Fennimore, S.A. Genetic basis for seed dormancy. Seed Sci. Res. 1998, 8, 173–182. [Google Scholar]
- Foley, M.E. Temperature and water status affecting afterripening in wild oat (Avena fatua). Weed Sci. 1994, 42, 200–204. [Google Scholar]
- Steadman, K.J.; Crawford, A.D.; Gallagher, R.S. Dormancy release in Lolium rigidum seed is a function of thermal after-ripening time and seed water content. Funct. Plant Biol. 2003, 30, 345–352. [Google Scholar] [CrossRef]
- Gallagher, R.S.; Crawford, A.D.; Steadman, K.J. Alleviation of dormancy in annual ryegrass (Lolium rigidum) seed by hydration and after-ripening. Weed Sci. 2004, 52, 968–975. [Google Scholar] [CrossRef]
- Adkins, S.W.; Loewen, M.; Symons, S. Variation within pure lines of wild oats (Avena fatua) in relation to degree of primary dormancy. Weed Sci. 1986, 34, 859–864. [Google Scholar]
- Nurse, R.E.; DiTommaso, A. Corn competition alters the germinability of velvetleaf (Abutilon theophrasti) seed. Weed Sci. 2005, 53, 479–488. [Google Scholar] [CrossRef]
- Schutte, B.J.; Davis, A.S.; Renner, K.A.; Cardina, J. Maternal and burial environment effects on seed mortality of velvetleaf (Abutilon theophrasti) and Giant Foxtail (Setaria faberi). Weed Sci. 2008, 56, 834–840. [Google Scholar] [CrossRef]
- Imam, A.G.; Allard, R.W. Population studies in predominantly self-pollinated species IV: Genetic variability between and within natural populations of wild oats from different habitats in California. Genetics 1965, 51, 49–62. [Google Scholar]
- Dhingra, O.D.; Sinclair, J.B. Basic Plant Pathology Methods, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1995; p. 434. [Google Scholar]
- AOSA (Association of Official Seed Analysis), Tetrazolium Testing Handbook: Contribution No. 29 to the Handbook on Seed Testing; Peters, J. (Ed.) The Association of Official Seed Analysts: Lincoln, NE, USA, 2000.
- Coble, H.D.; Mortensen, D.A. The threshold concept and its application to weed science. Weed Technol. 1992, 6, 191–195. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gallagher, R.S.; Granger, K.L.; Snyder, A.M.; Pittmann, D.; Fuerst, E.P. Implications of Environmental Stress during Seed Development on Reproductive and Seed Bank Persistence Traits in Wild Oat (Avena fatua L.). Agronomy 2013, 3, 537-549. https://doi.org/10.3390/agronomy3030537
Gallagher RS, Granger KL, Snyder AM, Pittmann D, Fuerst EP. Implications of Environmental Stress during Seed Development on Reproductive and Seed Bank Persistence Traits in Wild Oat (Avena fatua L.). Agronomy. 2013; 3(3):537-549. https://doi.org/10.3390/agronomy3030537
Chicago/Turabian StyleGallagher, Robert S., Kristen L. Granger, Amanda M. Snyder, Dennis Pittmann, and E. Patrick Fuerst. 2013. "Implications of Environmental Stress during Seed Development on Reproductive and Seed Bank Persistence Traits in Wild Oat (Avena fatua L.)" Agronomy 3, no. 3: 537-549. https://doi.org/10.3390/agronomy3030537




