Biological Control of the Weed Hemp Sesbania (Sesbania exaltata) in Rice (Oryza sativa) by the Fungus Myrothecium verrucaria
Abstract
:1. Introduction
| Common Name | Chemical Name |
|---|---|
| Acifluorfen | 5-[2-chloro-4-(trifluoromethyl)phenoxy]-2-nitrobenzoic acid |
| Bensulfuron | 2-[[[[[(4,6-dimethoxy-2-pyrimidinyl)amino] carbonyl]amino]sulfonyl]methyl]benzoic acid |
| Bentazon | 3-(1-methylethyl)-(1H)-2,1,3-benzothiadiazin-4(3H)-one 2,2-dioxide |
| Carfentrazone | α,2-dichloro-5-[4-(difluoromethyl)-4,5-dihydro-3-methyl-5-oxo-1H-1,2,4-triazol-1-yl]-4-fluorobenzenepropanoic acid |
| Chlorimuron | 2-[[[[(4-chloro-6-methoxy-2-pyrimidinyl)amino]carbonyl]amino]sulfonyl]benzoic acid |
| Fomesafen | 5-[2-chloro-4-(trifluoromethyl)phenoxy]-N-(methylsulfonyl)-2-nitrobenzamide |
| Glyphosate | N-(phosphonomethyl)glycine |
| Halosulfuron | Methyl 5-[((4,6-dimethoxy-2-pyrimidinyl)amino)carbonylamino-sulfonyl]-3-chloro-1-methyl-1H-pyrazole-4-carboxylate |
| Imazapyr | (±)-2-[4,5-dihydro-4-methyl-4-(1-methylethyl)-5-oxo-1H-imidazol-2-yl]-3-pyridinecarboxylic acid |
| Imazethapyr | 2-(4,5-dihydro-4-methyl-4-(1-methylethyl)-5-oxo-1H-imidazol-2-yl)-5-ethyl-3-pyridine-carboxylic acid |
| Lactofen | (±)-2-ethoxy-1-methyl-2-oxoethyl-5-[2-chloro-4-(trifluoro-methyl)phenoxy]-2-nitrobenzoate |
| Pendimethalin | N-(1-ethylpropyl)-3,4-dimethyl-2,6-dinitrobenzenamine |
| Triclopyr | [(3,5,6-trichloro-2-pyridinyl)oxy]acetic acid |
2. Materials and Methods
2.1. Culture, Storage, and Mass Production
2.2. Laboratory and Greenhouse Protocols
2.3. Field Experiment Protocols
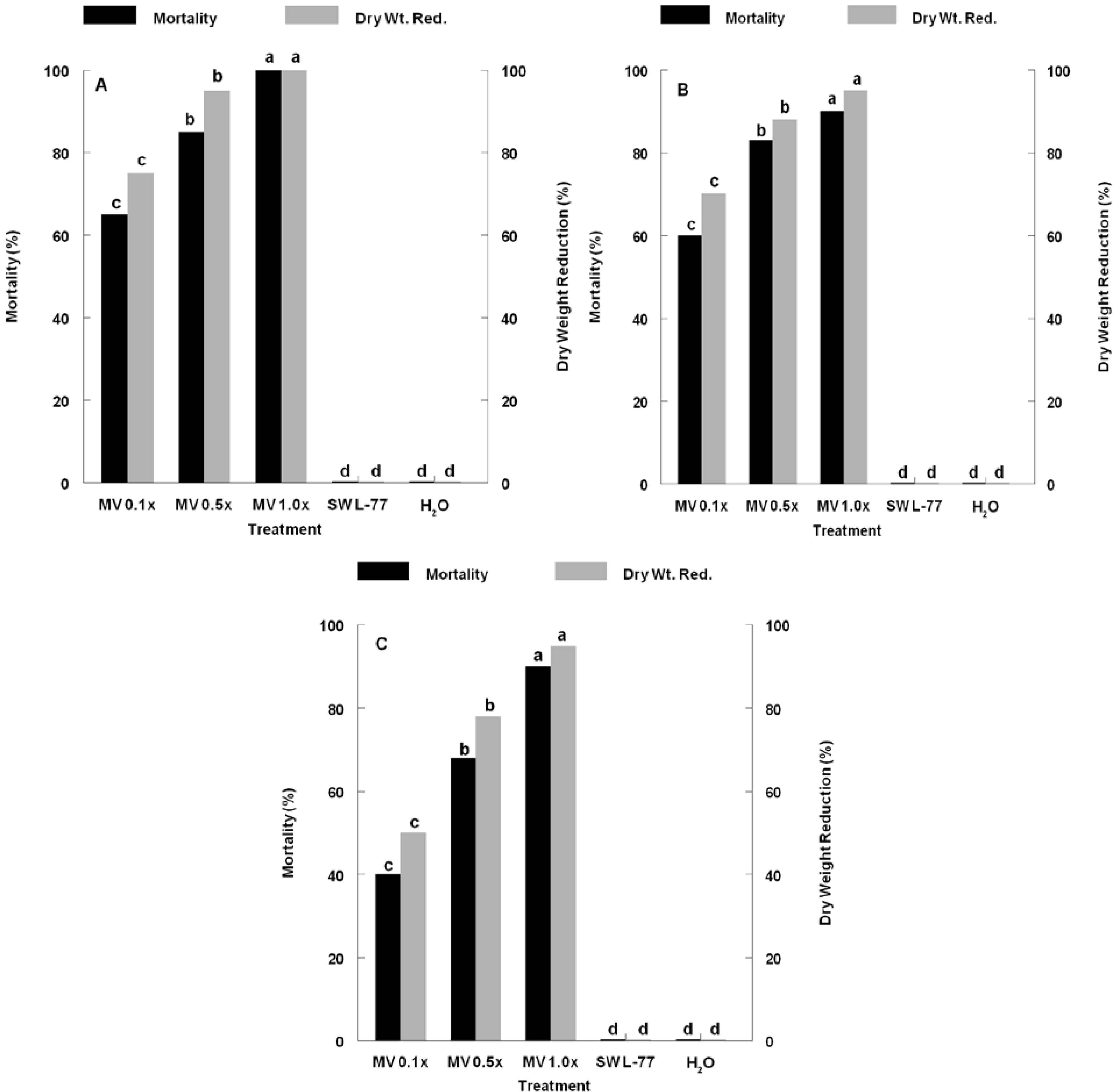
3. Results and Discussion





4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Woon, C.K. Effect of two row spacings and hemp sesbania competition on sunflower. J. Agron. Crop Sci. 1987, 159, 15–20. [Google Scholar] [CrossRef]
- Poisonous Plants of the Southern United States; Bulletin No. 818; Agricultural Extension Service, University of Tennessee: Knoxville, TN, USA, 1980; p. 12.
- Dowler, C.C. Weed survey—Southern states. Proc. South. Weed Sci. Soc. 1992, 45, 392–407. [Google Scholar]
- Lorenzi, H.J.; Jeffery, L.S. Weeds of the United States and Their Control; Van Nostrand Reinhold: New York, NY, USA, 1987. [Google Scholar]
- Lovelace, M.L.; Oliver, L.R. Effects of interference and tillage on hemp sesbania and pitted morningglory emergence and seed production. Proc. South. Weed Sci. Soc. 2000, 53, 202. [Google Scholar]
- King, C.A.; Purcell, L.C. Interference between hemp sesbania (Sesbania exaltata) and soybean (Glycine max) in response to irrigation and nitrogen. Weed Sci. 1997, 45, 91–97. [Google Scholar]
- Norsworthy, J.K.; Oliver, L.R. Hemp sesbania interference in drill-seeded glyphosate-resistant soybean. Weed Sci. 2000, 50, 34–41. [Google Scholar] [CrossRef]
- Everest, J.W.; Powe, T.A., Jr.; Freeman, J.D. Sesbania . Poisonous Plants of the Southeastern United States; Alabama Cooperative Extension System [ACES] Publications: Auburn, AL, USA, 2010. Available online: http://www.aces.edu/pubs/docs/A/ANR-0975/ (accessed on 17 August 2013).
- Bar, A.R.; Baggie, I.; Sanginga, N. The use of Sesbania (Sesbania rostrata) and urea in lowland rice production in Sierra Leone. Agrofor. Syst. 2000, 48, 111–118. [Google Scholar] [CrossRef]
- Herrera, W.T.; Garrity, D.P.; Vejpas, C. Management of Sesbania rostrata green manure crops grown prior to rain-fed lowland rice on sandy soils. Field Crops Res. 1997, 49, 259–268. [Google Scholar] [CrossRef]
- Shaw, D.R.; Arnold, J.C. Weed control from herbicide combinations with glyphosate. Weed Technol. 2002, 16, 1–6. [Google Scholar] [CrossRef]
- Vidrine, P.R.; Reynolds, D.B.; Griffin, J.L. Postemergence hemp sesbania (Sesbania exaltata) control in soybean (Glycine max). Weed Technol. 1992, 6, 374–377. [Google Scholar]
- Norris, J.L.; Shaw, D.R.; Snipes, C.E. Weed control from herbicide combinations with three formulations of glyphosate. Weed Technol. 2001, 15, 552–558. [Google Scholar] [CrossRef]
- Pellerin, K.J.; Webster, E.P.; Zhang, W.; Blouin, D.C. Herbicide mixtures in water-seeded imidazolinone-resistant rice (Oryza sativa). Weed Technol. 2003, 17, 836–841. [Google Scholar] [CrossRef]
- Pellerin, K.J.; Webster, E.P.; Zhang, W.; Blouin, D.C. Potential use of imazethapyr mixtures in drill-seeded imidazolinone-resistant rice. Weed Technol. 2004, 18, 1037–1042. [Google Scholar] [CrossRef]
- Jordan, D.L.; York, A.C.; Griffin, J.L.; Clay, P.A.; Vidrine, P.R.; Reynolds, D.B. Influence of application variables of efficacy on glyphosate. Weed Technol. 1997, 11, 354–362. [Google Scholar]
- Lich, J.M.; Renner, K.A.; Penner, D. Interaction of glyphosate with postemergence soybean (Glycine max) herbicides. Weed Sci. 1997, 45, 12–21. [Google Scholar]
- Oliver, L.R.; Taylor, S.E.; Gander, J.R. Influence of application timing and rate of glyphosate on weed control in soybean. Proc. South. Weed Sci. Soc. 1996, 49, 57. [Google Scholar]
- LaMastus, F.E.; Shaw, D.R.; Smith, M.C. Influence of application timing and rate on weed control in Roundup-Ready soybean. Proc. South. Weed Sci. Soc. 1998, 51, 8. [Google Scholar]
- Miller, D.K.; Milligan, J.L.; Wilson, C.F. Evaluation of reduced rate preemergence herbicides in Roundup-Ready soybean. Proc. South. Weed Sci. Soc. 1998, 51, 271–272. [Google Scholar]
- Vidrine, P.R.; Griffin, J.L.; Jordan, D.L.; Miller, D.K. Postemergence weed control in soybeans utilizing glyphosate and chlorimuron. Proc. South. Weed Sci. Soc. 1997, 50, 175. [Google Scholar]
- Ellis, J.M.; Griffin, J.L. Benefits of soil-applied herbicides in glyphosate-resistant soybean (Glycine max). Weed Technol. 2002, 16, 541–547. [Google Scholar] [CrossRef]
- Common and chemical names of herbicides approved by the Weed Science Society of America. Weed Sci. 2010, 58, 511–518. [CrossRef]
- Hallett, S.G. Where are the bioherbicides? Weed Sci. 2005, 53, 404–415. [Google Scholar] [CrossRef]
- Rosskopf, E.N.; Charudattan, R.; Kadir, J.B. Use of Plant Pathogens in Weed Control. In Handbook of Biological Control; Bellows, T.S., Fisher, T.W., Eds.; Academic Press: New York, NY, USA, 1999; pp. 891–918. [Google Scholar]
- Charudattan, R. Biological control of weeds by means of plant pathogens: Significance for integrated weed management in modern agro-ecology. BioControl 2001, 46, 229–260. [Google Scholar] [CrossRef]
- Charudattan, R. Ecological, practical, and political inputs into selection of weed targets: What makes a good biological control target? Bio. Control. 2005, 35, 183–196. [Google Scholar] [CrossRef]
- Weaver, M.A.; Lyn, M.E.; Boyette, C.D.; Hoagland, R.E. Bioherbicides for Weed Control. In Non-Chemical Weed Management; Updhyaya, M.K., Blackshaw, R.E, Eds.; CABI, International: Cambridge, MA, USA, 2007; pp. 93–110. [Google Scholar]
- Hoagland, R.E. Microbes and Microbial Products as Herbicides; American Chemical Society: Washington, DC, USA, 1990. [Google Scholar]
- Boyette, C.D.; Abbas, H.K. Host range alteration of the bioherbicidal fungus Alternaria crassa with fruit pectin and plant filtrates. Weed Sci. 1994, 42, 487–491. [Google Scholar]
- Boyette, C.D. Host range and virulence of Colletotrichum truncatum, a potential mycoherbicide for hemp sesbania (Sesbania exaltata). Plant Dis. 1991, 75, 62–64. [Google Scholar] [CrossRef]
- Boyette, C.D.; Quimby, P.C., Jr.; Bryson, C.T.; Egley, G.H.; Fulgham, F.E. Biological control of hemp sesbania (Sesbania exaltata) under field conditions with Colletotrichum truncatum formulated in an invert emulsion. Weed Sci. 1993, 41, 497–500. [Google Scholar]
- Abbas, H.K.; Boyette, C.D. Solid substrate formulation of the mycoherbicide Colletotrichum truncatum for hemp sesbania (Sesbania exaltata) control. Biocontrol Sci. Technol. 2000, 10, 297–304. [Google Scholar]
- Boyette, C.D.; Hoagland, R.E.; Weaver, M.A. Biocontrol efficacy of Colletotrichum truncatum for hemp sesbania (Sesbania exaltata) control is enhanced with unrefined corn oil and surfactants. Weed Biol. Manag. 2007, 7, 70–76. [Google Scholar] [CrossRef]
- Cartwright, K.; Boyette, D.; Roberts, M. Lockdown: Collego bioherbicide gets a second act. Phytopathology 2010, 100, S162. [Google Scholar]
- Templeton, G.E.; Smith, R.J., Jr.; TeBeest, D.O. Perspectives on mycoherbicides two decades after discovery of the Collego pathogen. In Proceedings of the VII International Symposium on the Biological Control of Weeds, Rome, Italy, 6–11 March 1988; pp. 553–558.
- Boyette, C.D.; Bowling, A.J.; Vaughn, K.C.; Hoagland, R.E.; Stetina, K.C. Induction of infection of Sesbania exaltata by Colletotrichum gloeosporioides f. sp. aeschynomene formulated in an invert emulsion. World J. Microbiol. Biotechnol. 2010, 26, 951–956. [Google Scholar] [CrossRef]
- Boyette, C.D.; Gealy, D.; Hoagland, R.E.; Vaughn, K.C.; Bowling, A.J. Hemp sesbania (Sesbania exaltata) control in rice (Oryza sativa) with the bioherbicidal fungus Colletotrichum gloeosporioides f. sp. aeschynomene formulated in an invert emulsion. Biocontrol Sci. Technol. 2011, 21, 1399–1407. [Google Scholar] [CrossRef]
- Boyette, C.D.; Walker, H.L.; Abbas, H.K. Biological control of kudzu (Pueraria lobata) with an isolate of Myrothecium verrucaria. Biocontrol Sci. Technol. 2002, 12, 75–82. [Google Scholar] [CrossRef]
- Hoagland, R.E.; McCallister, T.S.; Boyette, C.D.; Weaver, M.A.; Beecham, R.V. Effects of Myrothecium verrucaria on morning-glory (Ipomoea) species. Allelopath. J. 2011, 27, 151–162. [Google Scholar]
- Walker, H.L.; Tilley, A.M. Evaluation of an isolate of Myrothecium verrucaria from sicklepod (Senna obtusifolia) as a potential mycoherbicide agent. Biol. Control 1997, 10, 104–112. [Google Scholar] [CrossRef]
- Anderson, K.I.; Hallett, S.G. Bioherbicidal spectrum and activity of Myrothecium verrucaria. Weed Sci. 2004, 22, 623–627. [Google Scholar] [CrossRef]
- Abbas, H.K.; Johnson, B.J.; Shier, W.T.; Tak, H.; Jarvis, B.B.; Boyette, C.D. Phytotoxicity and mammalian cytotoxicity of macrocyclic trichothecenes from Myrothecium verrucaria. Phytochemistry 2002, 59, 309–313. [Google Scholar] [CrossRef]
- Conway, K.E. Evaluation of Cercospora rodmanii as a biological control of water hyacinth. Phytopathology 1976, 66, 914–917. [Google Scholar] [CrossRef]
- Boyette, C.D.; Weidemann, G.J.; TeBeest, D.O.; Quimby, P.C., Jr. Biological control of jimsonweed (Datura stramonium) with Alternaria crassa. Weed Sci. 1991, 39, 673–667. [Google Scholar]
- Ghorboni, R.; Seel, W.; Litterick, A.; Leifert, C. Evaluation of Alternaria alternata for biological control of Amaranthus retroflexus. Weed Sci. 2000, 48, 474–480. [Google Scholar] [CrossRef]
- Elzein, A.; Kroschel, J. Fusarium oxysporum Foxy 2 shows potential to control both Striga heronthica and S. asiatica. Weed Res. 2004, 44, 433–438. [Google Scholar] [CrossRef]
- Boyette, C.D.; Weaver, M.A.; Hoagland, R.E.; Stetina, K.C. Submerged culture of a mycelial formulation of a bioherbicidal strain of Myrothecium verrucaria with mitigated mycotoxin production. World J. Microbiol. Biotechnol. 2008, 24, 2721–2726. [Google Scholar] [CrossRef]
- Tuite, J. Plant Pathological Methods: Fungi and Bacteria; Burgess Publication Co.: Minneapolis, MN, USA, 1969. [Google Scholar]
- Horsfall, J.G.; Barratt, R.W. An improved grading system for measuring diseases. Phytopathology 1945, 35, 655. [Google Scholar]
- Steele, R.G.D.; Torrey, J.H.; Dickeys, D.A. Multiple Comparisons. In Principles and Procedures of Statistics—A Biometrical Approach; McGraw Hill: New York, NY, USA, 1997; p. 365. [Google Scholar]
- Boyette, C.D.; Hoagland, R.E. Biological Control of Hemp sesbania (Sesbania exaltata) and sicklepod (Senna obtusifolia) in soybean with anthracnose pathogen mixtures. Weed Technol. 2010, 24, 551–556. [Google Scholar] [CrossRef]
- Müller-Schärer, H. Finding solutions for biological control of weeds in European crop systems. BioControl 2001, 46, 125–126. [Google Scholar] [CrossRef]
- Amsellem, Z.; Barghouthi, S.; Cohen, B.; Goldwasser, Y.; Gressel, J.; Hornok, L.; Kerenyi, A.; Kleifeld, Y.; Klein, O.; Kroschel, J.; et al. Recent advances in the biocontrol of Orobanche (broomrape) species. BioControl 2001, 46, 211–228. [Google Scholar] [CrossRef]
- Bürki, H.M.; Lawrie, J.; Greaves, M.P.; Down, V.M.; Jüttersonke, B.; Cagán, L.; Vráblová, V.; Ghorbani, R.; Hassan, E.A.; Schroeder, D. Biocontrol of Amaranthus spp. in Europe: State of the art. BioControl 2001, 46, 197–210. [Google Scholar] [CrossRef]
- Défago, G.; Ammon, H.U.L.; Cagán, B.; Draeger, B.; Greaves, M.P.; Guntli, D.; Hoeke, D.; Klimes, L.; Lawrie, J.; Moënne-Loccoz, Y.; et al. Towards the biocontrol of bindweeds with a mycoherbicide. BioControl 2001, 46, 157–173. [Google Scholar] [CrossRef]
- Frantzen, J.; Paul, N.D.; Müller-Schärer, H. The system management approach of biological weed control: Some theoretical considerations and aspects of application. BioControl 2001, 46, 139–155. [Google Scholar] [CrossRef]
- Netland, J.; Dutton, L.C.; Greaves, M.P.; Baldwin, M.; Vurro, M.; Evidente, J.; Einhorn, G.; Scheepens, P.C.; French, L.W. Biological control of Chenopodium album L. in Europe. BioControl 2001, 46, 175–196. [Google Scholar] [CrossRef]
- Scheepens, P.C.; Müller-Schärer, H.; Kempenaar, C. Opportunities for biological weed control in Europe. BioControl 2001, 46, 127–138. [Google Scholar] [CrossRef]
- Abbas, H.K.; Tak, H.; Boyette, C.D.; Shier, W.T.; Jarvis, B.B. Macrocyclic trichothecenes are undetectable in kudzu (Pueraria montana) plants treated with a high-producing isolate of Myrothecium verrucaria. Phytochemistry 2001, 58, 269–276. [Google Scholar] [CrossRef]
- Milhollon, R.W.; Berner, D.K.; Paxson, L.K.; Jarvis, B.B.; Bean, G.W. Myrothecium verrucaria for control of annual morningglories in sugarcane. Weed Technol. 2003, 17, 276–283. [Google Scholar] [CrossRef]
- Weaver, M.A.; Boyette, C.D.; Hoagland, R.E. Bioherbicidal activity from washed spores of Myrothecium verrucaria. World J. Microbiol. Biotechnol. 2012, 28, 1941–1946. [Google Scholar] [CrossRef]
- Domsch, K.H.; Gams, W.; Anderson, T.H. Myrothecium . In Compendium of Soil Fungi; Academic Press: New York, NY, USA, 1980; pp. 481–487. [Google Scholar]
- Nguyen, T.H.; Mathur, S.B.; Neergaard, P. Seed-borne species of Myrothecium and their pathogenic potential. Trans. Br. Mycol. Soc. 1973, 61, 347–354. [Google Scholar] [CrossRef]
- Yang, S.; Jong, S. C. Host range determination of Myrothecium verrucaria isolated from leafy spurge. Plant Dis. 1995, 79, 994–997. [Google Scholar] [CrossRef]
- Weaver, M.A.; Jin, X.; Hoagland, R.E.; Boyette, C.D. Improved bioherbicidal efficacy by Myrothecium verrucaria via spray adjuvants or herbicide mixtures. Biol. Control 2009, 50, 150–156. [Google Scholar] [CrossRef]
- Shivrain, V.K.; Burgos, N.R.; Moldenhauer, K.A.K.; McNew, R.W.; Baldwin, T.L. Characterization of spontaneous crosses between Clearfield rice (Oryza sativa) and red rice (Oryza sativa). Weed Technol. 2006, 20, 576–584. [Google Scholar] [CrossRef]
- Scott, R.C.; Meins, K.B.; Smith, K.L. Tank-mix partners with Newpath herbicide for hemp sesbania control in a Clearfield rice-production system. Ark. Agric. Res. Ser. 2005, 54, 225–229. [Google Scholar]
- Powles, S.B. Evolved glyphosate-resistant weeds around the world: Lessons to be learnt. Pest Manag. Sci. 2008, 64, 360–365. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Boyette, C.D.; Hoagland, R.E.; Stetina, K.C. Biological Control of the Weed Hemp Sesbania (Sesbania exaltata) in Rice (Oryza sativa) by the Fungus Myrothecium verrucaria. Agronomy 2014, 4, 74-89. https://doi.org/10.3390/agronomy4010074
Boyette CD, Hoagland RE, Stetina KC. Biological Control of the Weed Hemp Sesbania (Sesbania exaltata) in Rice (Oryza sativa) by the Fungus Myrothecium verrucaria. Agronomy. 2014; 4(1):74-89. https://doi.org/10.3390/agronomy4010074
Chicago/Turabian StyleBoyette, Clyde D., Robert E. Hoagland, and Kenneth C. Stetina. 2014. "Biological Control of the Weed Hemp Sesbania (Sesbania exaltata) in Rice (Oryza sativa) by the Fungus Myrothecium verrucaria" Agronomy 4, no. 1: 74-89. https://doi.org/10.3390/agronomy4010074




