Diurnal Leaf Starch Content: An Orphan Trait in Forage Legumes
Abstract
:1. Introduction
2. Energy Content in Forage Crops
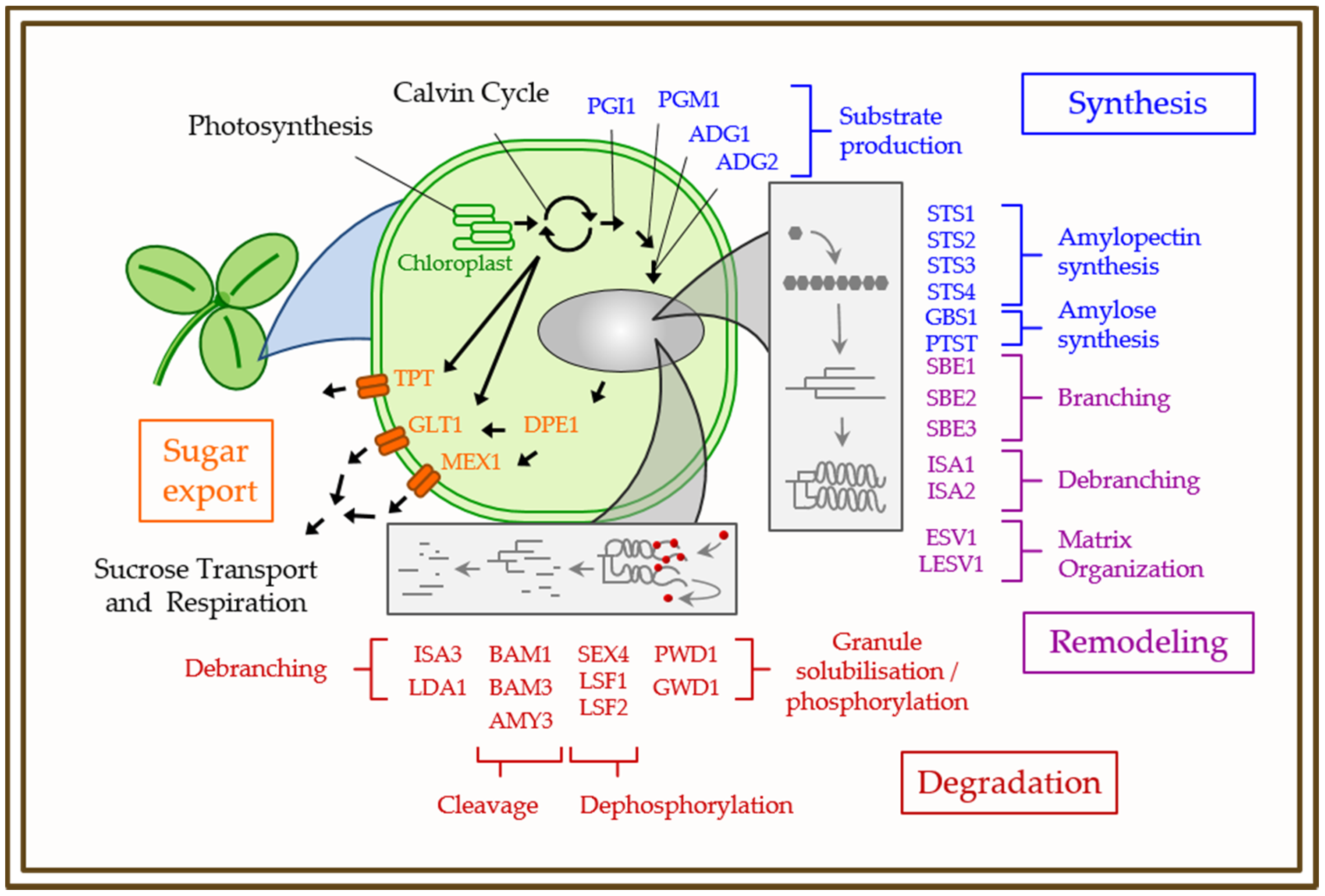
3. Physiology, Biochemistry, and Genetics of Starch Metabolism in Model Plants
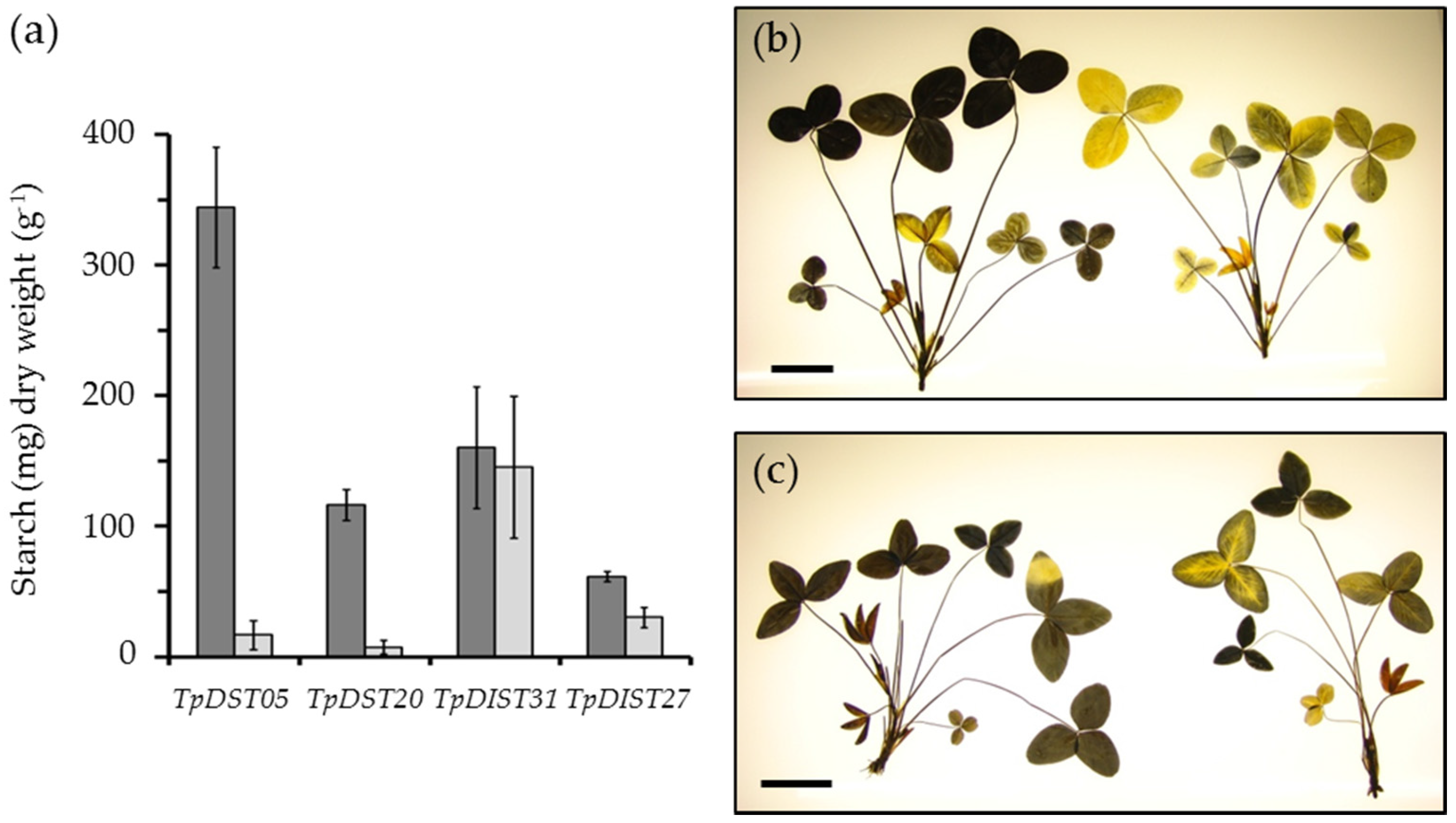
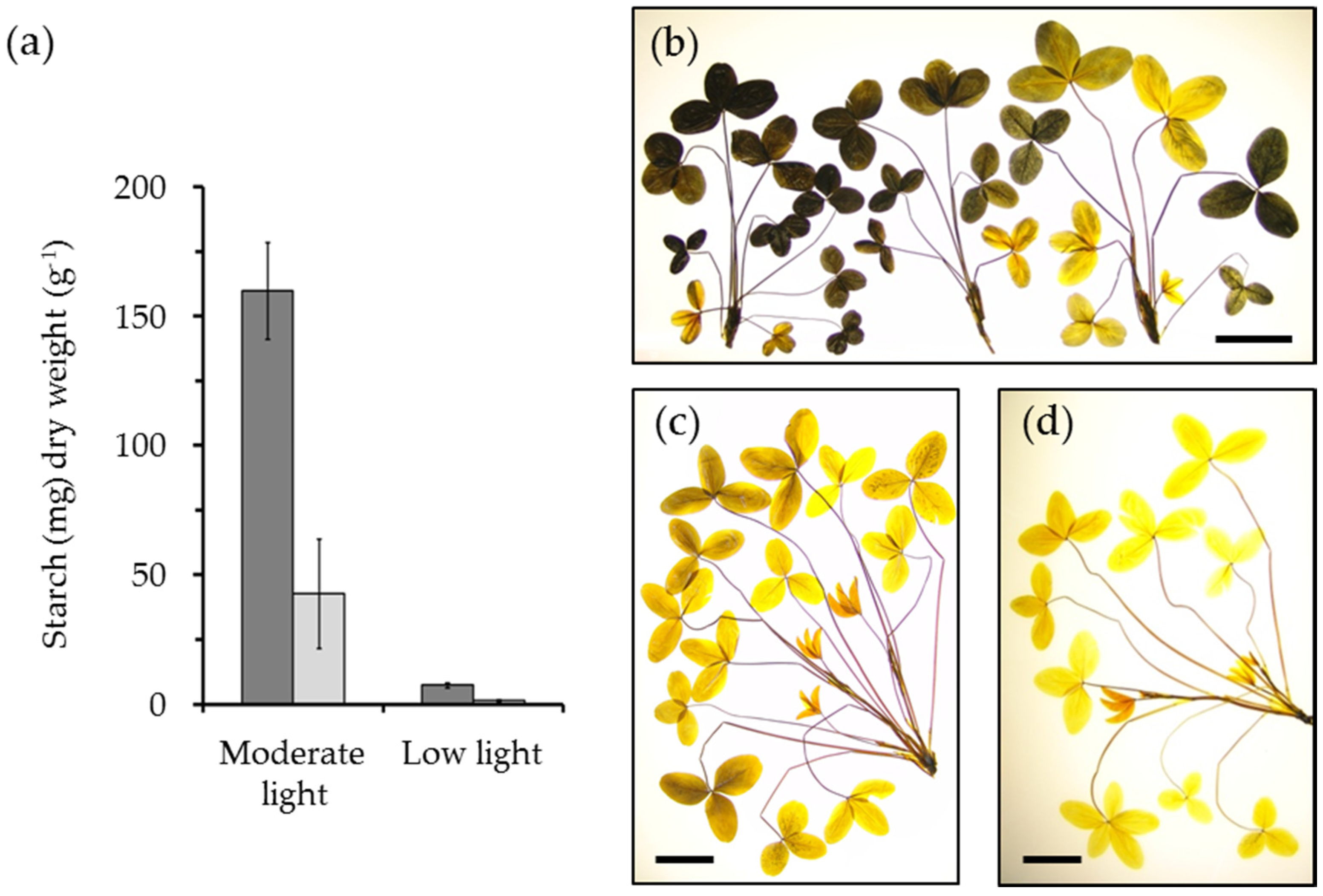
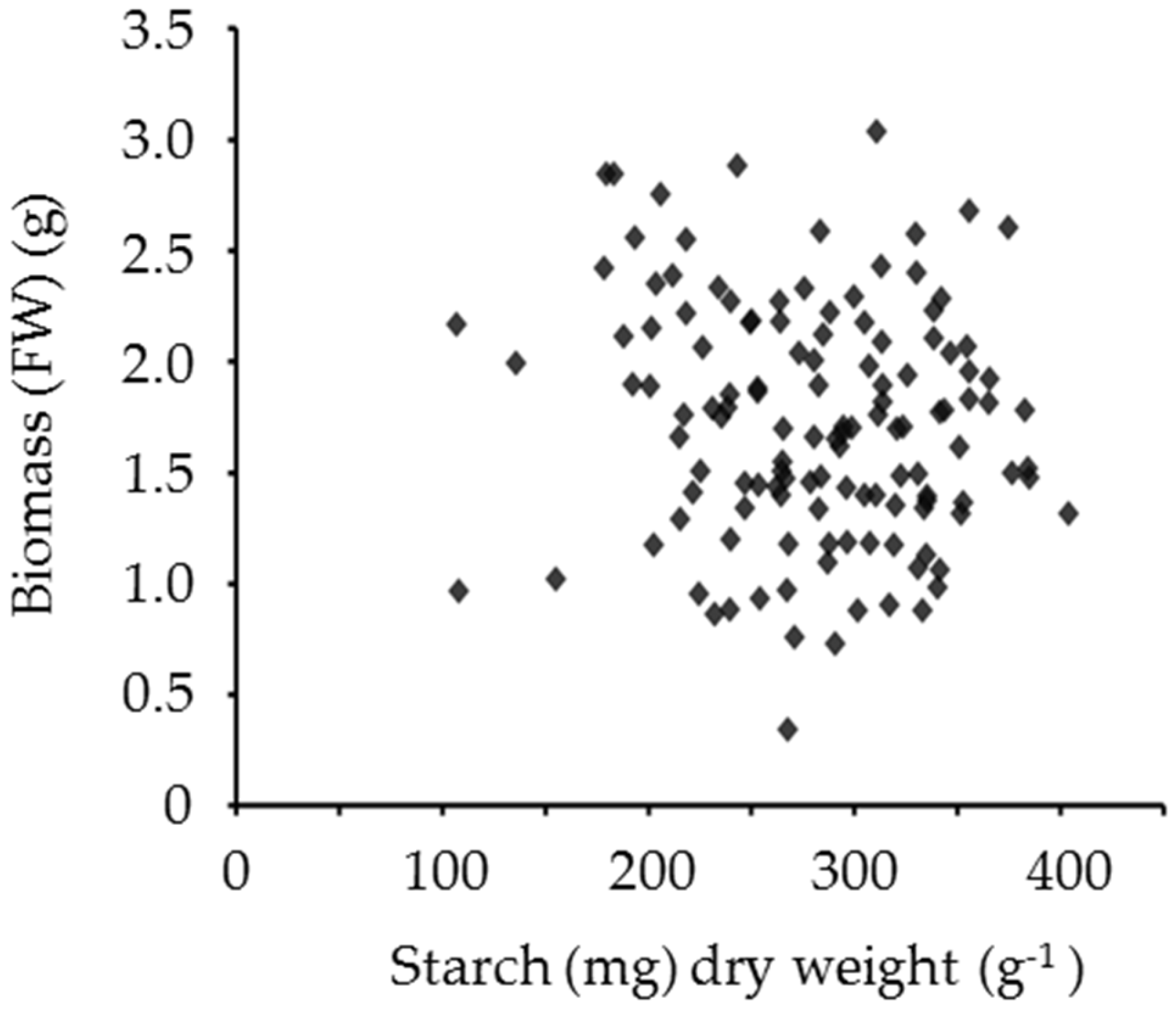
4. The Potential of Leaf Starch Content as a Trait in Forage Legumes
5. The Potential of Modern Genomic and Phenomic Tools to Develop Leaf Starch as a Trait in Forage Legumes
6. The Potential to Reduce Concentrate Feeding
7. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Steinfeld, H.; Gerber, P.; Wassenaar, T.; Castel, V.; Rosales, M.; De Haan, C. Livestock’s Long Shadow; FAO Rome: Rome, Italy, 2006. [Google Scholar]
- Robertson, G.P.; Vitousek, P.M. Nitrogen in agriculture: Balancing the cost of an essential resource. Annu. Rev. Environ. Resour. 2009, 34, 97–125. [Google Scholar] [CrossRef]
- Broderick, G.; Albrecht, K.; Owens, V.; Smith, R. Genetic variation in red clover for rumen protein degradability. Anim. Feed Sci. Technol. 2004, 113, 157–167. [Google Scholar] [CrossRef]
- Frame, J.; Charlton, J.; Laidlaw, A.S. Temperate Forage Legumes; Cab International: Wallingford, UK, 1998. [Google Scholar]
- Nkrumah, J.; Okine, E.; Mathison, G.; Schmid, K.; Li, C.; Basarab, J.; Price, M.; Wang, Z.; Moore, S. Relationships of feedlot feed efficiency, performance, and feeding behavior with metabolic rate, methane production, and energy partitioning in beef cattle. J. Anim. Sci. 2006, 84, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Nocek, J.E.; Tamminga, S. Site of digestion of starch in the gastrointestinal tract of dairy cows and its effect on milk yield and composition. J. Dairy Sci. 1991, 74, 3598–3629. [Google Scholar] [CrossRef]
- Reynolds, C. Production and metabolic effects of site of starch digestion in dairy cattle. Anim. Feed Sci. Technol. 2006, 130, 78–94. [Google Scholar] [CrossRef]
- Taylor, N.; Quesenberry, K. Red Clover Science. Current Plant Sciences and Biology in Agriculture; Kluwer Academic Publisher: Dordrecht, The Netherlands, 1996; Volume 28, pp. 141–160. [Google Scholar]
- Graham, P.H.; Vance, C.P. Legumes: Importance and constraints to greater use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Gibon, A. Managing grassland for production, the environment and the landscape. Challenges at the farm and the landscape level. Livest. Prod. Sci. 2005, 96, 11–31. [Google Scholar] [CrossRef]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Edmeades, G.O. Breeding and cereal yield progress. Crop Sci. 2010, 50, S-85–S-98. [Google Scholar] [CrossRef]
- Kingston-Smith, A.; Marshall, A.; Moorby, J. Breeding for genetic improvement of forage plants in relation to increasing animal production with reduced environmental footprint. Animal 2013, 7, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, A.; Dhont, C.; Bipfubusa, M.; Chalifour, F.-P.; Drouin, P.; Beauchamp, C.J. Improving salt stress responses of the symbiosis in alfalfa using salt-tolerant cultivar and rhizobial strain. Appl. Soil Ecol. 2015, 87, 108–117. [Google Scholar] [CrossRef]
- Brito, A.; Tremblay, G.; Bertrand, A.; Castonguay, Y.; Bélanger, G.; Michaud, R.; Lafrenière, C.; Martineau, R.; Berthiaume, R. Alfalfa baleage with increased concentration of nonstructural carbohydrates supplemented with a corn-based concentrate did not improve production and nitrogen utilization in early lactation dairy cows. J. Dairy Sci. 2014, 97, 6970–6990. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.; Pollock, C. Changes in stolon carbohydrates during the winter in four varieties of white clover (trifolium repensl.) with contrasting hardiness. Ann. Bot. 1998, 81, 97–107. [Google Scholar] [CrossRef]
- Humphreys, M. Water-soluble carbohydrates in perennial ryegrass breeding. Grass Forage Sci. 1989, 44, 237–244. [Google Scholar] [CrossRef]
- Turner, L.; Cairns, A.; Armstead, I.; Ashton, J.; Skøt, K.; Whittaker, D.; Humphreys, M. Dissecting the regulation of fructan metabolism in perennial ryegrass (Lolium perenne) with quantitative trait locus mapping. New Phytol. 2006, 169, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.B.; Humphreys, M.O.; Cairns, A.J.; Pollock, C.J. Comparison of growth and carbohydrate accumulation in seedlings of two varieties of Lolium perenne. J. Plant Physiol. 2001, 158, 891–897. [Google Scholar] [CrossRef]
- Moorby, J.; Evans, R.; Scollan, N.; MacRae, J.; Theodorou, M. Increased concentration of water-soluble carbohydrate in perennial ryegrass (Lolium perenne L.). Evaluation in dairy cows in early lactation. Grass Forage Sci. 2006, 61, 52–59. [Google Scholar] [CrossRef]
- Proctor, L.; Craig, H.; Mclean, N.; Fennessy, P.; Kerslake, J.; Behrent, M.; Chuah, J.; Campbell, A. The effect of grazing high-sugar ryegrass on lamb performance. Proc. N. Z. Soc. Anim. Prod. 2015, 75, 235–238. [Google Scholar]
- Staerfl, S.; Amelchanka, S.; Kälber, T.; Soliva, C.; Kreuzer, M.; Zeitz, J. Effect of feeding dried high-sugar ryegrass (‘abermagic’) on methane and urinary nitrogen emissions of primiparous cows. Livest. Sci. 2012, 150, 293–301. [Google Scholar] [CrossRef]
- Åman, P.; Nordkvist, E. Chemical composition and in-vitro degradability of major chemical constituents of red clover harvested at different stages of maturity. J. Sci. Food Agric. 1983, 34, 1185–1189. [Google Scholar] [CrossRef]
- Mackenzie, D.; Wylam, C.B. Analytical studies on the carbohydrates of grasses and clovers. Viii.—changes in carbohydrate composition during the growth of perennial rye-grass. J. Sci. Food Agric. 1957, 8, 38–45. [Google Scholar] [CrossRef]
- Owens, V.; Albrecht, K.; Muck, R. Protein degradation and fermentation characteristics of unwilted red clover and alfalfa silage harvested at various times during the day. Grass Forage Sci. 2002, 57, 329–341. [Google Scholar] [CrossRef]
- Chouinard-Michaud, C.; Michaud, R.; Castonguay, Y.; Bertrand, A.; Bélanger, G.; Tremblay, G.F.; Berthiaume, R.; Allard, G. Increasing alfalfa non structural carbohydrates through genetic selection and cutting management. In Ruminants Physiology Digestion, Metabolisms and Effects of Nutrition on Reproduction and Welfare; Wagennigen Academic Publishers: Wagennigen, The Netherlands, 2009; pp. 138–139. [Google Scholar]
- Claessens, A.; Castonguay, Y.; Bertrand, A.; Bélanger, G.; Tremblay, G. Breeding for improved nonstructural carbohydrates in alfalfa. In Breeding in a World of Scarcity; Springer: Cham, CH, Switzerland, 2016; pp. 231–235. [Google Scholar]
- Zeeman, S.C.; Kossmann, J.; Smith, A.M. Starch: Its metabolism, evolution, and biotechnological modification in plants. Annu. Rev. Plant Biol. 2010, 61, 209–234. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Hatfield, R.; Muck, R. Characterization of proteolysis in alfalfa and red clover. Crop Sci. 1995, 35, 537–541. [Google Scholar] [CrossRef]
- Kötting, O.; Santelia, D.; Edner, C.; Eicke, S.; Marthaler, T.; Gentry, M.S.; Comparot-Moss, S.; Chen, J.; Smith, A.M.; Steup, M. Starch-excess4 is a laforin-like phosphoglucan phosphatase required for starch degradation in Arabidopsis thaliana. Plant Cell 2009, 21, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Zeeman, S.C. Starch turnover: Pathways, regulation and role in growth. Curr. Opin. Plant Biol. 2012, 15, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Feike, D.; Seung, D.; Graf, A.; Bischof, S.; Ellick, T.; Coiro, M.; Soyk, S.; Eicke, S.; Mettler-Altmann, T.; Lu, K.-J. The starch granule-associated protein early starvation1 (esv1) is required for the control of starch degradation in Arabidopsis thaliana leaves. Plant Cell 2016. [Google Scholar] [CrossRef]
- Seung, D.; Soyk, S.; Coiro, M.; Maier, B.A.; Eicke, S.; Zeeman, S.C. Protein targeting to starch is required for localising granule-bound starch synthase to starch granules and for normal amylose synthesis in arabidopsis. PLoS Biol. 2015, 13, e1002080. [Google Scholar] [CrossRef] [PubMed]
- Stettler, M.; Eicke, S.; Mettler, T.; Messerli, G.; Hörtensteiner, S.; Zeeman, S.C. Blocking the metabolism of starch breakdown products in arabidopsis leaves triggers chloroplast degradation. Mol. Plant 2009, 2, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.-S.; Kofler, H.; Häusler, R.E.; Hille, D.; Flügge, U.-I.; Zeeman, S.C.; Smith, A.M.; Kossmann, J.; Lloyd, J.; Ritte, G. The arabidopsis sex1 mutant is defective in the r1 protein, a general regulator of starch degradation in plants, and not in the chloroplast hexose transporter. Plant Cell 2001, 13, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Calenge, F.; Saliba-Colombani, V.; Mahieu, S.; Loudet, O.; Daniel-Vedele, F.; Krapp, A. Natural variation for carbohydrate content in arabidopsis. Interaction with complex traits dissected by quantitative genetics. Plant Physiol. 2006, 141, 1630–1643. [Google Scholar] [CrossRef] [PubMed]
- Gibon, Y.; Blaesing, O.E.; Hannemann, J.; Carillo, P.; Höhne, M.; Hendriks, J.H.; Palacios, N.; Cross, J.; Selbig, J.; Stitt, M. A robot-based platform to measure multiple enzyme activities in arabidopsis using a set of cycling assays: Comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. Plant Cell 2004, 16, 3304–3325. [Google Scholar] [CrossRef] [PubMed]
- Vriet, C.; Welham, T.; Brachmann, A.; Pike, M.; Pike, J.; Perry, J.; Parniske, M.; Sato, S.; Tabata, S.; Smith, A.M. A suite of lotus japonicus starch mutants reveals both conserved and novel features of starch metabolism. Plant Physiol. 2010, 154, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Wiese, A.; Christ, M.; Virnich, O.; Schurr, U.; Walter, A. Spatio-temporal leaf growth patterns of Arabidopsis thaliana and evidence for sugar control of the diel leaf growth cycle. New Phytol. 2007, 174, 752–761. [Google Scholar] [CrossRef]
- Mielewczik, M.; Friedli, M.; Kirchgessner, N.; Walter, A. Diel leaf growth of soybean: A novel method to analyze two-dimensional leaf expansion in high temporal resolution based on a marker tracking approach (martrack leaf). Plant Methods 2013, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Sulpice, R.; Pyl, E.-T.; Ishihara, H.; Trenkamp, S.; Steinfath, M.; Witucka-Wall, H.; Gibon, Y.; Usadel, B.; Poree, F.; Piques, M.C. Starch as a major integrator in the regulation of plant growth. Proc. Natl. Acad. Sci. USA 2009, 106, 10348–10353. [Google Scholar] [CrossRef] [PubMed]
- Weise, S.E.; Aung, K.; Jarou, Z.J.; Mehrshahi, P.; Li, Z.; Hardy, A.C.; Carr, D.J.; Sharkey, T.D. Engineering starch accumulation by manipulation of phosphate metabolism of starch. Plant Biotechnol. J. 2012, 10, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Atkin, O.K.; Tjoelker, M.G. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 2003, 8, 343–351. [Google Scholar] [CrossRef]
- Boller, B.C.; Nösberger, J. Effects of temperature and photoperiod on stolon characteristics, dry matter partitioning, and nonstructural carbohydrate concentration of two white clover ecotypes. Crop Sci. 1983, 23, 1057–1062. [Google Scholar] [CrossRef]
- Warrington, I.; Peet, M.; Patterson, D.; Bunce, J.; Haslemore, R.; Hellmers, H. Growth and physiological responses of soybean under various thermoperiods. Funct. Plant Biol. 1977, 4, 371–380. [Google Scholar] [CrossRef]
- Weston, E.; Thorogood, K.; Vinti, G.; López-Juez, E. Light quantity controls leaf-cell and chloroplast development in Arabidopsis thaliana wild type and blue-light-perception mutants. Planta 2000, 211, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.; Tremblay, G.; Lapierre, H.; Bertrand, A.; Castonguay, Y.; Bélanger, G.; Michaud, R.; Benchaar, C.; Ouellet, D.; Berthiaume, R. Alfalfa cut at sundown and harvested as baleage increases bacterial protein synthesis in late-lactation dairy cows. J. Dairy Sci. 2009, 92, 1092–1107. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.; Tremblay, G.; Bertrand, A.; Castonguay, Y.; Bélanger, G.; Michaud, R.; Lapierre, H.; Benchaar, C.; Petit, H.; Ouellet, D. Alfalfa cut at sundown and harvested as baleage improves milk yield of late-lactation dairy cows. J. Dairy Sci. 2008, 91, 3968–3982. [Google Scholar] [CrossRef] [PubMed]
- Lowell, M.E. Post-harvest physiological changes in forage plants. In Post-Harvest Physiology and Preservation of Forages; Crop Science Society of America: Madison, WI, USA, 1995; pp. 1–19. [Google Scholar]
- Li, R.; Volenec, J.; Joern, B.; Cunningham, S. Seasonal changes in nonstructural carbohydrates, protein, and macronutrients in roots of alfalfa, red clover, sweetclover, and birdsfoot trefoil. Crop Sci. 1996, 36, 617–623. [Google Scholar] [CrossRef]
- De Vega, J.J.; Ayling, S.; Hegarty, M.; Kudrna, D.; Goicoechea, J.L.; Ergon, Å.; Rognli, O.A.; Jones, C.; Swain, M.; Geurts, R. Red clover (Trifolium pratense L.) draft genome provides a platform for trait improvement. Sci. Rep. 2015, 5, 17394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ištvánek, J.; Jaroš, M.; Křenek, A.; Řepková, J. Genome assembly and annotation for red clover (trifolium pratense; fabaceae). Am. J. Bot. 2014, 101, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, A.G.; Barrett, B.A.; Simon, D.; Khan, A.K.; Bickerstaff, P.; Anderson, C.B.; Franzmayr, B.K.; Hancock, K.R.; Jones, C.S. An integrated genetic linkage map for white clover (Trifolium repens L.) with alignment to medicago. BMC Genom. 2013, 14, 388. [Google Scholar] [CrossRef] [PubMed]
- Annicchiarico, P.; Nazzicari, N.; Brummer, E. Alfalfa genomic selection: Challenges, strategies, transnational cooperation. In Breeding in a World of Scarcity; Springer: Cham, CH, Switzerland, 2016; pp. 145–149. [Google Scholar]
- Li, X.; Wei, Y.; Acharya, A.; Jiang, Q.; Kang, J.; Brummer, E.C. A saturated genetic linkage map of autotetraploid alfalfa (Medicago sativa L.) developed using genotyping-by-sequencing is highly syntenous with the medicago truncatula genome. G3: Genes Genomes Genet. 2014, 4, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, J.A.; Fu, F.; Bucciarelli, B.; Yang, S.S.; Samac, D.A.; Lamb, J.F.; Monteros, M.J.; Graham, M.A.; Gronwald, J.W.; Krom, N. The Medicago sativa gene index 1.2: A web-accessible gene expression atlas for investigating expression differences between Medicago sativa subspecies. BMC Genom. 2015, 16, 502. [Google Scholar] [CrossRef] [PubMed]
- Yates, S.A.; Swain, M.T.; Hegarty, M.J.; Chernukin, I.; Lowe, M.; Allison, G.G.; Ruttink, T.; Abberton, M.T.; Jenkins, G.; Skøt, L. De novo assembly of red clover transcriptome based on rna-seq data provides insight into drought response, gene discovery and marker identification. BMC Genom. 2014, 15, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzanares, C.; Yates, S.; Ruckle, M.; Nay, M.; Studer, B. Tilling in forage grasses for gene discovery and breeding improvement. New Biotechnol. 2016, 33, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.; van Eeuwijk, F.A.; Hammer, G.L.; Podlich, D.W.; Messina, C. Modeling qtl for complex traits: Detection and context for plant breeding. Curr. Opin. Plant Biol. 2009, 12, 231–240. [Google Scholar] [CrossRef]
- Hammer, G.; Messina, C.; van Oosterom, E.; Chapman, S.; Singh, V.; Borrell, A.; Jordan, D.; Cooper, M. Molecular breeding for complex adaptive traits: How integrating crop ecophysiology and modelling can enhance efficiency. In Crop Systems Biology; Springer: Cham, CH, Switzerland, 2016; pp. 147–162. [Google Scholar]
- Reymond, M.; Muller, B.; Leonardi, A.; Charcosset, A.; Tardieu, F. Combining quantitative trait loci analysis and an ecophysiological model to analyze the genetic variability of the responses of maize leaf growth to temperature and water deficit. Plant Physiol. 2003, 131, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.R.; Kossmann, J.; Ritte, G. Leaf starch degradation comes out of the shadows. Trends Plant Sci. 2005, 10, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Shu, Q.; Forster, B.P.; Nakagawa, H.; Nakagawa, H. Plant Mutation Breeding and Biotechnology; CABI: Wallingford, UK, 2012. [Google Scholar]
- Tsai, H.; Howell, T.; Nitcher, R.; Missirian, V.; Watson, B.; Ngo, K.J.; Lieberman, M.; Fass, J.; Uauy, C.; Tran, R.K. Discovery of rare mutations in populations: Tilling by sequencing. Plant Physiol. 2011, 156, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.L.; Quesenberry, K.H. Clover, red (Trifolium pratense). Agrobact. Protoc. 2015, 1, 237–254. [Google Scholar]
- Fu, C.; Hernandez, T.; Zhou, C.; Wang, Z.-Y. Alfalfa (Medicago sativa L.). Agrobact. Protoc. 2015, 1, 213–221. [Google Scholar]
- Svitashev, S.; Young, J.K.; Schwartz, C.; Gao, H.; Falco, S.C.; Cigan, A.M. Targeted mutagenesis, precise gene editing, and site-specific gene insertion in maize using cas9 and guide rna. Plant Physiol. 2015, 169, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Dreccer, M.F.; Barnes, L.R.; Meder, R. Quantitative dynamics of stem water soluble carbohydrates in wheat can be monitored in the field using hyperspectral reflectance. Field Crops Res. 2014, 159, 70–80. [Google Scholar] [CrossRef]
- Bowley, S.; Taylor, N.; Dougherty, C. Physiology and morphology of red clover. Adv. Agron. 1984, 37, 317–347. [Google Scholar]
- Huntington, G.B. Starch utilization by ruminants: From basics to the bunk. J. Anim. Sci. 1997, 75, 852–867. [Google Scholar] [CrossRef]
- Benchaar, C.; Pomar, C.; Chiquette, J. Evaluation of dietary strategies to reduce methane production in ruminants: A modelling approach. Can. J. Anim. Sci. 2001, 81, 563–574. [Google Scholar] [CrossRef]
- Reis, R.; Combs, D. Effects of increasing levels of grain supplementation on rumen environment and lactation performance of dairy cows grazing grass-legume pasture. J. Dairy Sci. 2000, 83, 2888–2898. [Google Scholar] [CrossRef]
- Van Dorland, H.; Wettstein, H.-R.; Leuenberger, H.; Kreuzer, M. Effect of supplementation of fresh and ensiled clovers to ryegrass on nitrogen loss and methane emission of dairy cows. Livest. Sci. 2007, 111, 57–69. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruckle, M.E.; Meier, M.A.; Frey, L.; Eicke, S.; Kölliker, R.; Zeeman, S.C.; Studer, B. Diurnal Leaf Starch Content: An Orphan Trait in Forage Legumes. Agronomy 2017, 7, 16. https://doi.org/10.3390/agronomy7010016
Ruckle ME, Meier MA, Frey L, Eicke S, Kölliker R, Zeeman SC, Studer B. Diurnal Leaf Starch Content: An Orphan Trait in Forage Legumes. Agronomy. 2017; 7(1):16. https://doi.org/10.3390/agronomy7010016
Chicago/Turabian StyleRuckle, Michael E., Michael A. Meier, Lea Frey, Simona Eicke, Roland Kölliker, Samuel C. Zeeman, and Bruno Studer. 2017. "Diurnal Leaf Starch Content: An Orphan Trait in Forage Legumes" Agronomy 7, no. 1: 16. https://doi.org/10.3390/agronomy7010016





