1. Introduction
Biochar is the carbon rich co-product of pyrolysis or gasification of biomass. Biochar application to soils is of public and agricultural interest [
1]. The public interest in biochar application to soil is focused on the potential to decrease global net carbon dioxide emission by an increased soil storage of carbon [
2]. The agricultural interest is focused on a number of positive properties [
3], the most striking being plant growth stimulation by increased water storage [
4], increased nutrient supply [
5,
6], increased beneficial microbial life [
7,
8] and disease suppression [
7,
9]. Just as for agriculture, biochar application to horticultural rooting media (soilless substrates) is of public and agricultural interest. (1) The public interest is to use biochars from renewable organic residual streams to substitute part of the peat used in rooting media in greenhouse horticulture [
10,
11]. Peat bogs are important carbon (C) stocks and regulate the local water quality and water regime [
12]. In the light of environmental concerns, peat substitution by biochar will preserve peat bogs and lower global carbon dioxide emissions linked with the use of peat extraction and use [
13]; (2) The horticultural interest in biochar apart from peat substitution is the use and manipulation of bacterial communities for the protection of plants against diseases, either by direct protection or by induced plant resilience [
14,
15,
16]. In certain plant growth media, biochar amendment results in chemical responses in the plant as well as shifts in the rhizosphere microbiome [
17]. In greenhouse horticulture, the use of high input fertigation systems makes biochar related increases in water storage and nutrient supply of less economic consequence than for agricultural applications. An advantage of greenhouse testing is the improved control over climate effects including rain related water content and nutrient concentration fluctuations.
Several researchers have looked into the potential of biochar as a rooting medium for horticulture [
14,
18,
19], including the substitution of peat. The study presented in [
17] demonstrates the effect of oak wood pyrolysis biochar on strawberry grown in white peat and lettuce grown in field soil. In the strawberry bioassay, addition of 3% w/w biochar to peat resulted in (1) a higher fresh and dry plant weight; (2) a lower susceptibility for the fungal pathogen
Botrytis cinerea on both leaves and fruits; and (3) changes in the rhizosphere microbiology such as an increase of bacterial diversity and a shift in composition of the rhizosphere microbiota. Extra inorganic plant nutrition and lime added to the peat reduced these effects of biochar on the strawberry plants. In [
11] the authors reported that the hydrophysical properties of peat based growing media changed with the addition of various biochars up to 70% v/v. Lettuce (
Lactuca sativa) grown in the peat/biochar mixtures showed substantially higher yields than with peat alone. In addition, this study confirmed the importance of biochar production conditions on the product properties. In [
20] Dumroese et al. found that peat moss, amended with various ratios of pellets comprised of equal proportions of biochar and wood flour, generally had chemical and physical properties suitable for service as a rooting medium during nursery production of plants. A mixture of 75% v/v peat and 25% v/v pellets enhanced hydraulic conductivity and water availability at low (<−10 kPa) matric potentials. In [
21] it was demonstrated in rooting media used for container production of greenhouse crops that biochars from wood gasification were able to buffer peat acidity, eliminating the need for liming agents. Gasification biochars reduced shrinkage of peat acting as a stable skeleton, and reduced the ammonium/nitrate ratio in the peat after a fertilization event. Lastly, biochars added stable and high levels of potassium to rooting media. In [
22], the performance of tomato crop green-waste pyrolysis biochar as a rooting medium for hydroponic tomato production was compared with an existing, commercially acceptable rooting medium, pine sawdust. No peat was used in these rooting media. The EC of rooting media containing, or consisting entirely of biochar was reduced by rinsing with water before use, believing there is potential to capture and recycle nutrients flushed during this process. In terms of growth, yield, or fruit quality no differences were found among biochar and pine sawdust rooting media. In another attempt to completely replace peat [
23] as well as vermiculite, a dried anaerobic digestate remaining after the fermentation of potato processing wastes, was mixed with three biochars produced from either wood pellets, pelletized wheat straw or field pennycress press cake. All three biochars were acidified and combined in 50%/50% v/v ratios with the digestate before comparing with a 50%/50% v/v sphagnum peat moss/vermiculite control medium containing slow-release chemical fertilizers. A growth increase of tomato was observed for the mix containing the wood pellet biochar.
Horticultural interest in biochar also seems justified in the light of prior results with biochar-like materials such as charred rice husks [
24,
25] and torrefied Gramineae [
26,
27]. Torrefaction or carbonization is a process that happens at temperatures (250–400 °C) with the objective of creating an energy-dense, solid biofuel with gas and liquids as by-products. The term biochar usually describes the by-product of pyrolysis and gasification. Pyrolysis is carried out at temperatures of 400 °C to 650 °C, without oxygen, while gasification occurs at temperatures above 600 °C. Both pyrolysis and gasification have as main objective the production of syngas, liquid fuels and chemicals. Charred rice husks (synonymous with “burnt” rice husks) are used in mixes and on their own on a large scale in horticulture in South East Asia, notably Indonesia, as well as in South America [
28].
In [
24] the study of gasified rice hull biochar (GRHB) on available nutrients in a rooting medium for containers revealed that GRHB provides sufficient P and K to support a production cycle of geranium, but lacked either the correct concentration or balance of micronutrients for healthy growth. In [
25] a number of crops were grown on standard commercial rooting medium composed of sphagnum peat moss/perlite (85%/15% v/v) and mixed with 0%, 5%, or 10% v/v GRHB. GRHB provided a source of readily available phosphate and potassium when incorporated at 5% or 10% v/v. The amount of available phosphate and potassium became depleted after a period of up to 6 weeks. Torrefied reed (
Phragmites australis) was successfully used in an experiment with up to 50% v/v as compared to peat [
27]. Torrefaction was used as low oxygen gasification of organic materials at temperatures of 150–400 °C [
26]. Both charred rice husks and torrefied reed indicated potential for charred products at dosages far beyond 25% by volume.
A large and growing body of literature has reported no beneficial, adverse or contradicting effects on plant growth when using biochar [
29,
30,
31]. The negative and neutral effects of biochar application have resulted in increased attention on methods to characterize biochars in general and for specific applications [
32,
33,
34]. A wide variety of growing media are already being used in horticulture and a set of specific quality parameters and measuring methods has been developed [
35]. Evaluating biochar for horticultural applications requires both, a material characterization with rooting media tests and a quantified link with results in field or container experiments. Such data will then fill the gap between biochar engineering and horticultural application results. Evidence of the importance of production factors has been reported for the nature of feedstocks [
36,
37]; the temperature of production [
34,
37,
38]; the supply of oxygen [
34,
39]; and the cooling procedure to prevent condensation of toxic substances [
14]. Gray et al., 2014, have reported a decreased hydrophobicity and related greater water entry at higher production temperatures [
40].
The objective of our paper is a follow up on an earlier study by Fryda and Visser [
41], who related the feedstock materials and thermal processes (pyrolysis and gasification) to the properties of the produced biochar. They concluded that biochars of widely different properties can be produced using the same feedstock under different production conditions. The authors further concluded that phytotoxic properties caused by condensation of tar loaded gas on the biochar particles can be avoided by using higher temperatures and early separation of gas from solids. They also reported increased internal particle porosity with gasification temperatures. In the present paper, we aim to first show the influence of feedstock and production parameters on a set of growth influencing properties, and second, to investigate the impact of two ways of disease suppression on rooting media. Our hypotheses are: (1) biochar is a potting soil constituent which can be used in growing medium blends in quantities of 20% v/v without negative growth effects; (2) biochar can induce disease suppression.
Our approach is to use well-defined materials for testing on horticultural properties, like water retention, water uptake rate, phytotoxicity, pH buffer values, nutrient and EC levels, cation exchange capacity (CEC), microbial stability and nitrate immobilization [
35]. In addition we use biochars in plant tests to find potentially positive effects of biochar addition on the suppression of powdery mildew and
Fusarium. Powdery mildew is selected based on possible biochar induced stimulation of plant hormones, which are related to induced plant resistance against biotrofic pathogens [
9].
Fusarium is selected based on possible biochar enhanced
Fusarium suppression by beneficial microorganisms [
7].
4. Discussion
4.1. Feedstock Related Effects of Biochar
Biochars produced from beech wood or mixed residual wood have a higher specific surface area compared to biochars produced from tomato or sweet pepper waste. This is expected to increase its water retention and to increase the cation exchange capacity (CEC). Increased water content is a positive property if mixtures of >35% v/v biochar are going to be used as these might become too dry on their own. A higher CEC is not valued in professional horticulture as interference of the CEC with the nutrient solutions used, changes the ratios of the elements in the nutrient solutions to undesirable values. The values for CEC measured here are low enough to allow them to be disregarded in mixtures with <50% v/v of biochar. CEC is thought to be proportional to surface area of the biochar but also the result of negatively charged carboxyl (COO−) groups. The amount of carboxyl groups depends initially on the feedstock, in which composted leaves are known to have more of these groups per unit weight than woody products.
Biochar produced from source materials containing nutrient-rich biomass (i.e., tomato leaves, sweet pepper waste) appears unsuitable for use as potting soil because of its high salt content and high pH. Even biochar produced from feedstock with only 20% v/v tomato leaves and 80% v/v wood chips is too saline to be used as an ingredient for potting soil. We interpret this as evidence that the cations in the feedstock oxidize into oxides (Na
2O, K
2O, CaO and MgO) during biochar production. As these oxides are readily soluble in water and highly alkaline this explains why feedstock influences EC and pH. It also explains how feedstock can be quantitatively tested prior to use on salinity and alkalinity, notably by measuring the content of alkali metals Na, K, Ca and Mg. Even so there are reports where high EC feedstocks are used for biochar for horticultural purposes [
23] in which case the EC was lowered by either prior washing with water or dilution with low EC biochar.
4.2. Production Setting Related Effects of Biochar
The temperature of the production process increases salinity in accordance with the mass loss i.e., the salt ions concentrate in the remaining solids. Conversely, to produce higher amounts of biochar from a mass unit of dry feedstock, the process temperature and oxygen levels should be reduced. Higher process temperatures (>500 °C) and higher process oxygen levels, including the oxygen in the process water vapor, will increase the degree of oxidation of feedstock cations which will increase the pH and pH buffer capacity of the material. The influence of temperature and oxidation level on alkalinity was observed before [
34], as was a smaller but still relevant relation of ash content with pH [
41]. Generally if nutrient poor feedstock is used the biochar qualities are closer to those of peat.
The amount of carboxyl groups in the end product is influenced by the process parameters’ temperature and oxygen level. Higher temperatures and lower oxygen levels reduce the amount of carboxylic groups left in the biochar and therefore presumably reduce the cation exchange capacity.
The surface area of the feedstock is reduced by the level of clogging of smaller pores. Such surface area reduction supposedly by clogging is reported for condensation of volatile tar [
34] and may also have been caused by slagging [
41]. Slagging arguably is worse in materials with higher amounts of potassium and calcium salts which act as viscosity reductants for the slag.
Condensation of plant toxic volatile tar products is possible over the total temperature range of 200–900 °C [
36,
55,
56], as long as the volatiles are not immediately transported away from the biochar mass, especially during the cooling of the mass.
The particle size of the biochar is a production variable which affects physical and chemical properties of the materials as well as the properties of rooting media which are mixed with a biochar. (1) Physical properties affected are density, specific surface area (SSA) and water retention, which all increase with smaller particle size; and air content, which decreases with smaller particle size [
28,
56,
57]; (2) Chemical properties affected are pH, cation exchange capacity (CEC) and surface adsorption, which all increase with smaller particle size [
4,
34,
58]; (3) Rooting media properties require that the biochar particle size is tuned to an intended application such as coarse particles >4 mm to replace peat fractions or bark; 3–4 mm particles to replace milled peat; or powdered <1 mm particles to replace lime [
58]. This demonstrates that even a biochar from one specific feedstock, produced at one set of specific production parameters still can produce disappointing results if the particle size does not match the application. On the other hand, particle size manipulation before the heating process is one of the easier ways to diversify biochar product applications.
4.3. The Survival Rate of Beneficial Microorganisms as Affected by Biochar
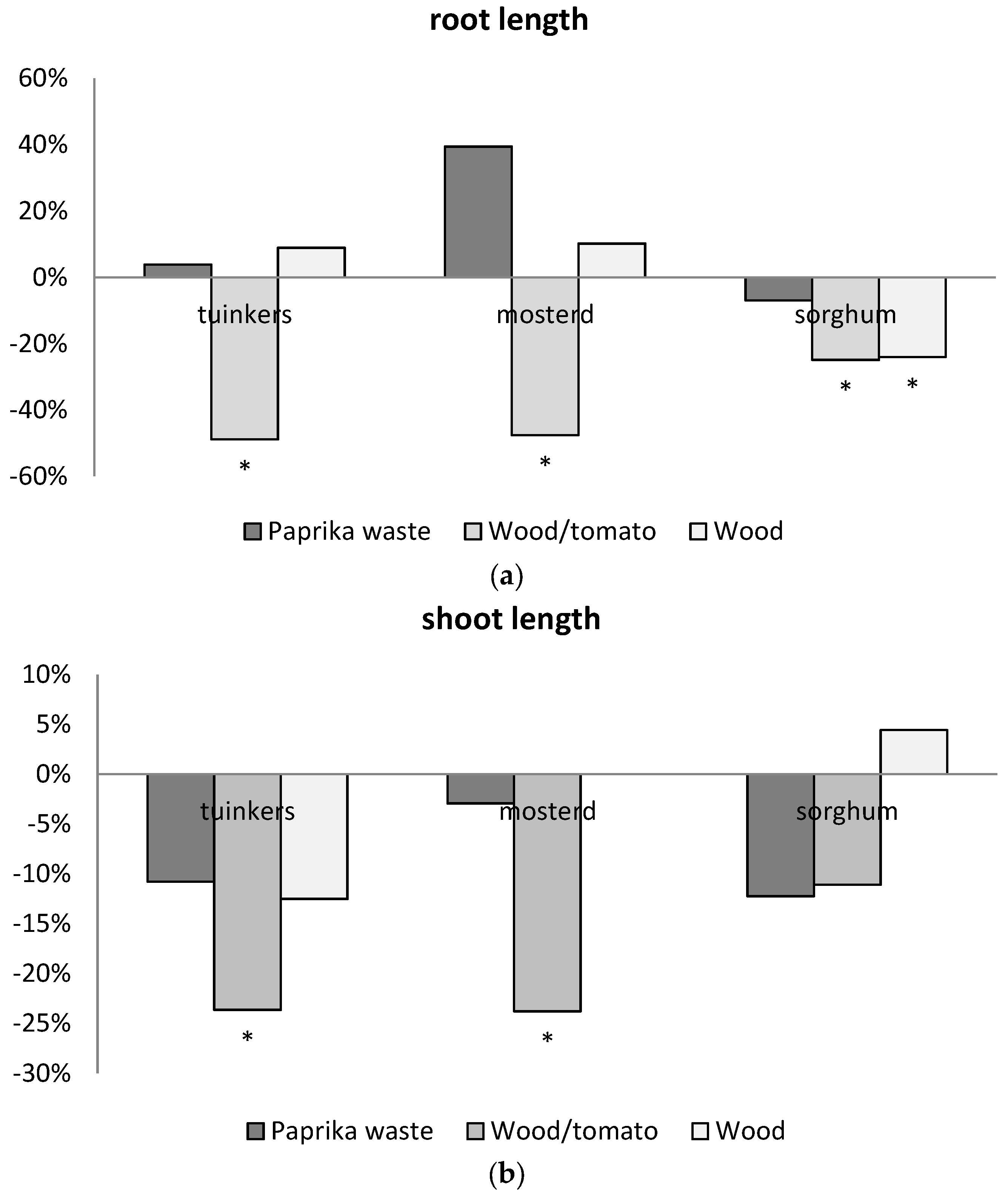
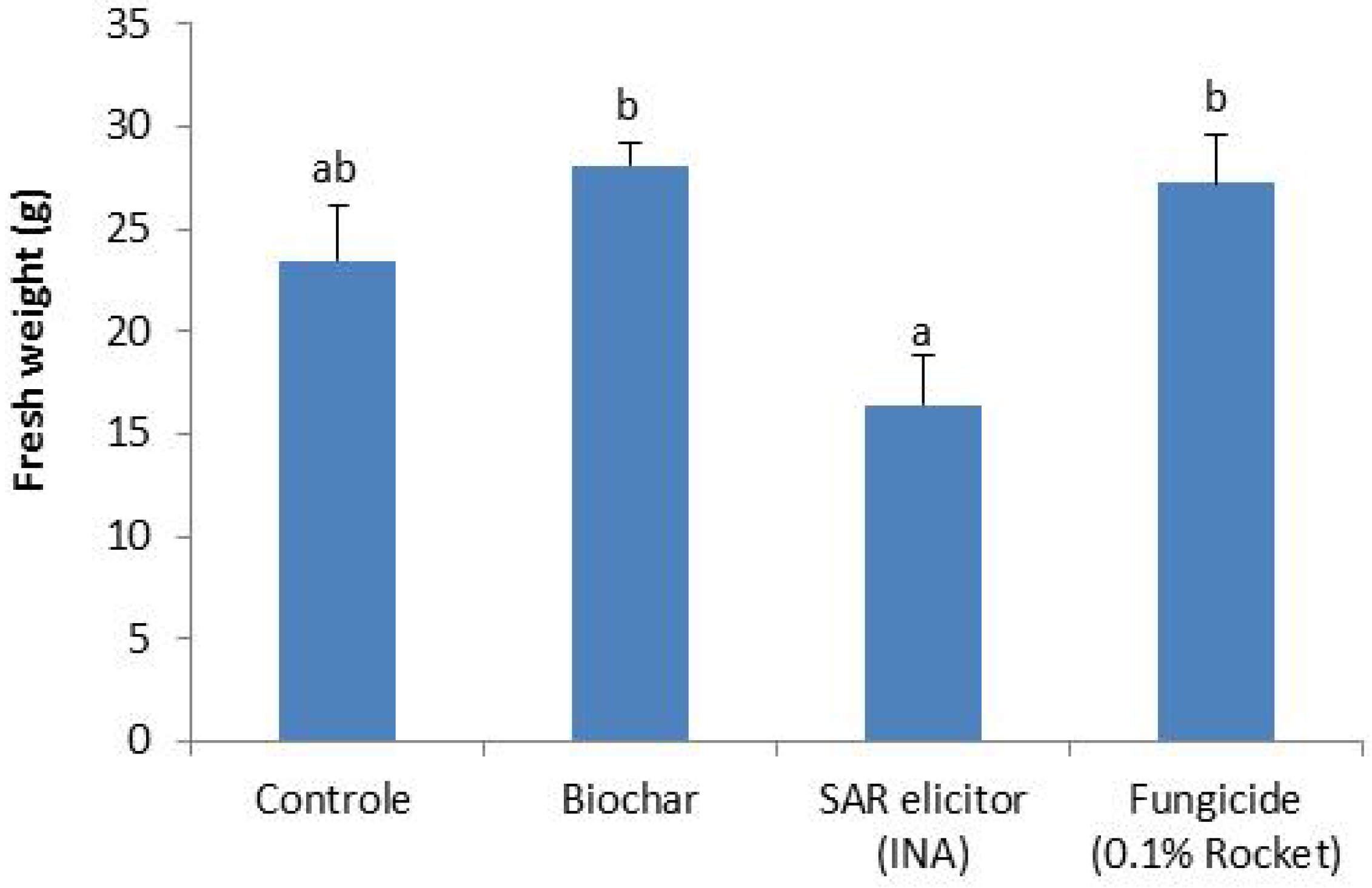
In two pot experiments, plants were grown on mixtures of peat and biochar (beech wood respectively residual wood). In both experiments, nutrient availability and plant growth were similar in the biochar treatment compared to the control treatment without biochar with the exemption of a slight decrease in nitrate availability. There were no signs of phytotoxic or growth reducing effects of biochar. Thus, the pot experiments confirm that peat/biochar media, with substantial biochar content (20% v/v), can be used in horticulture without yield reduction and without adjustment of the fertilization scheme, other than a preventive increase in nitrate to counteract nitrogen immobilization.
Results of the pot experiment with Gerbera show that the severity of powdery mildew infection is similar for Gerberas cultivated on rooting medium with 20% v/v biochar and the control treatment without biochar. Thus, biochar addition does neither decrease nor increase severity of powdery mildew infection in Gerberas and a systemic mode of action on disease resistance is not proven for the biochar used. In the experiments with chrysanthemums, no Fusarium incidence was found meaning that the effect of biochar addition on disease suppression could not be tested. More research is needed in order to elucidate if, and if so under which conditions, biochar additions can enhance disease suppression of diseases.
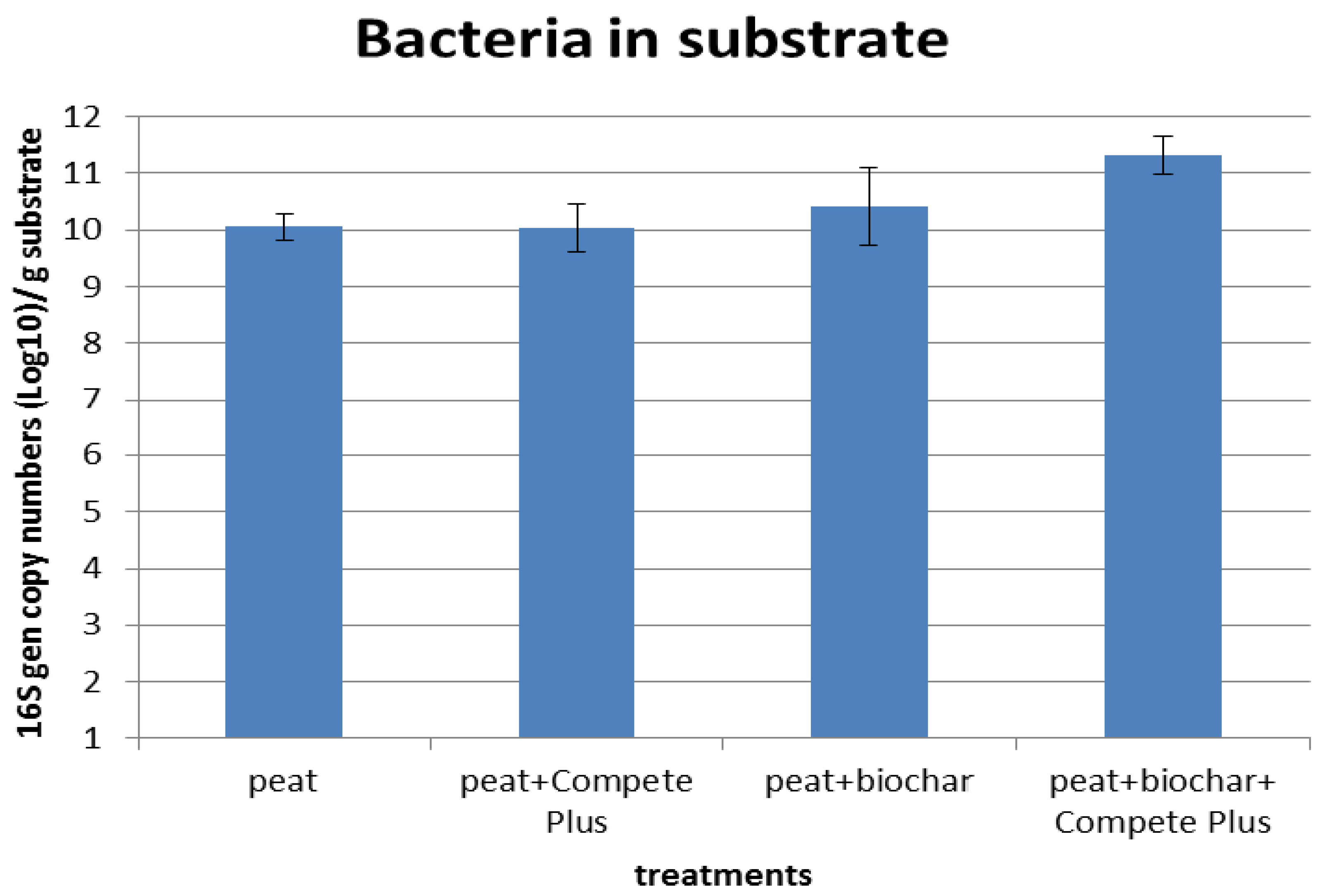
The addition of biochar did not increase the overall numbers of micro-organisms in the potting soil. A reason the level of micro-organisms did not respond to biochar addition could be the low level of degradation (OUR < 3), showing biochar particles to be a poor environment for micro-organisms needing carbon for growth. Possibly, colonization of biochar can be enhanced by loading the biochar with biodegradable organic acids, which would make biochar a more attractive environment for microbes. This is made more plausible by the observation that 20% v/v biochar can halve the amount of free organic carbon in a peat sample, presumably by absorbing the organic acids. Thus, it may be possible to pre-treat biochar with small organic molecules which can then feed a population of micro-organisms establishing themselves on the biochar particles. This will be the topic for further work.
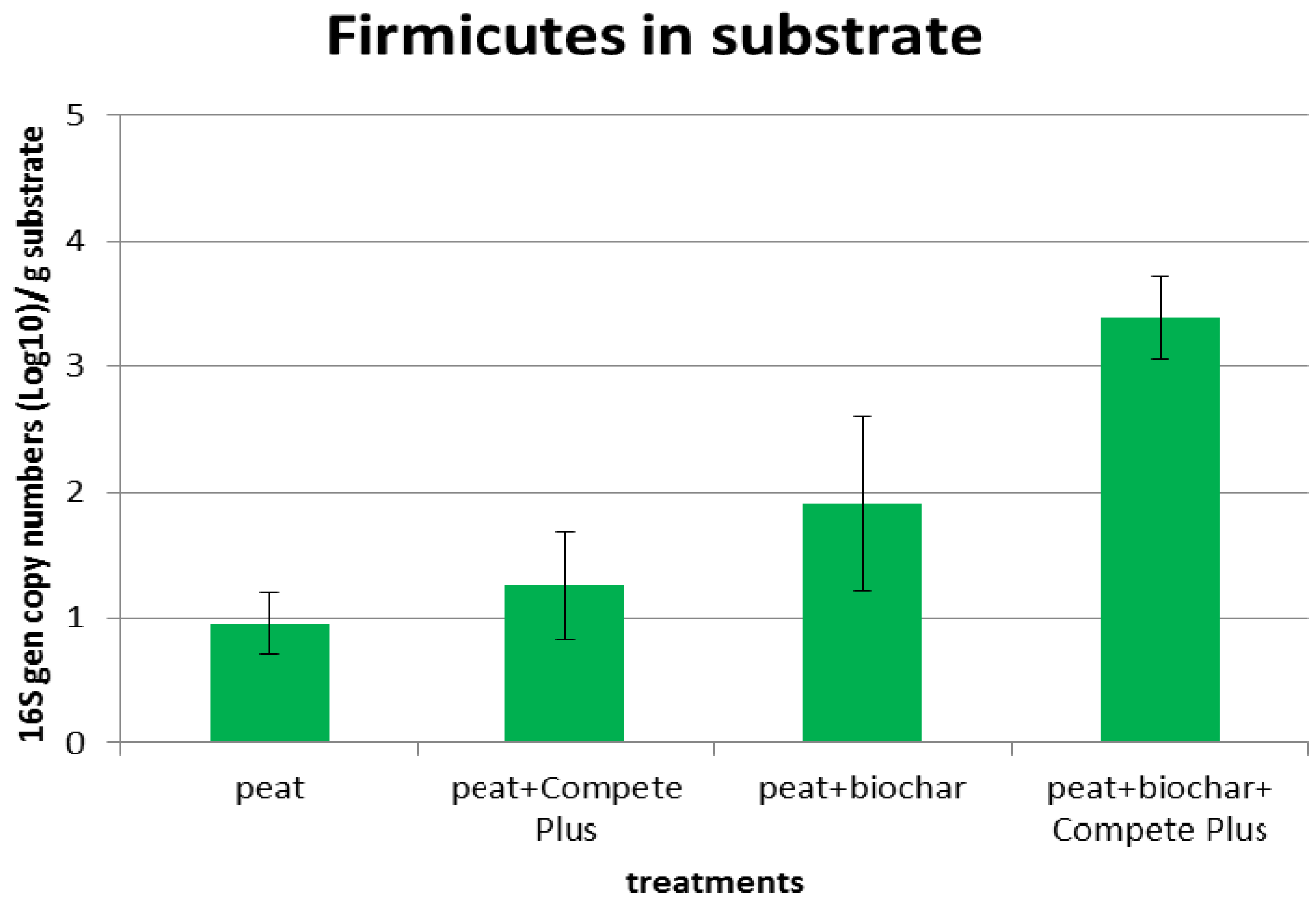
Our results showed that biochar offered some temporary or more permanent protection to Firmicutes, the bacteria which include the plant health supporting Bacilli. Whether the level of Firmicutes is high enough to influence disease suppression remains to be seen and merits further research.
4.4. Horticultural Perspectives of Biochar
Biochar dry bulk density and total pore space (100 kg·m−3 and 93% v/v) are well within the range required for horticultural rooting media.
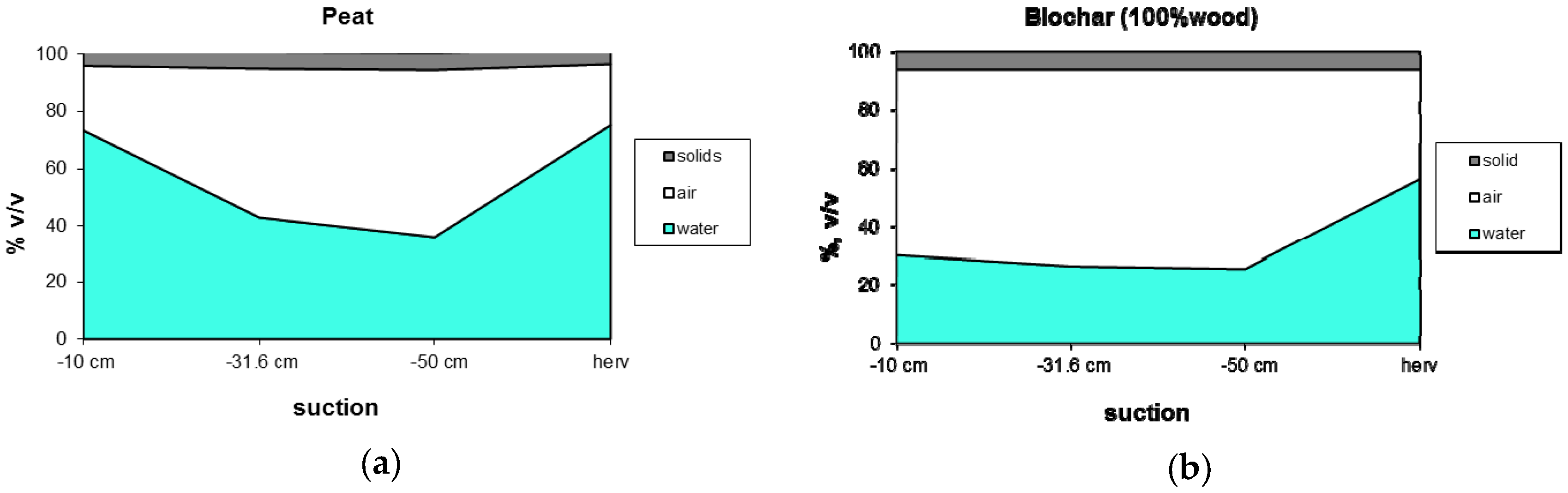
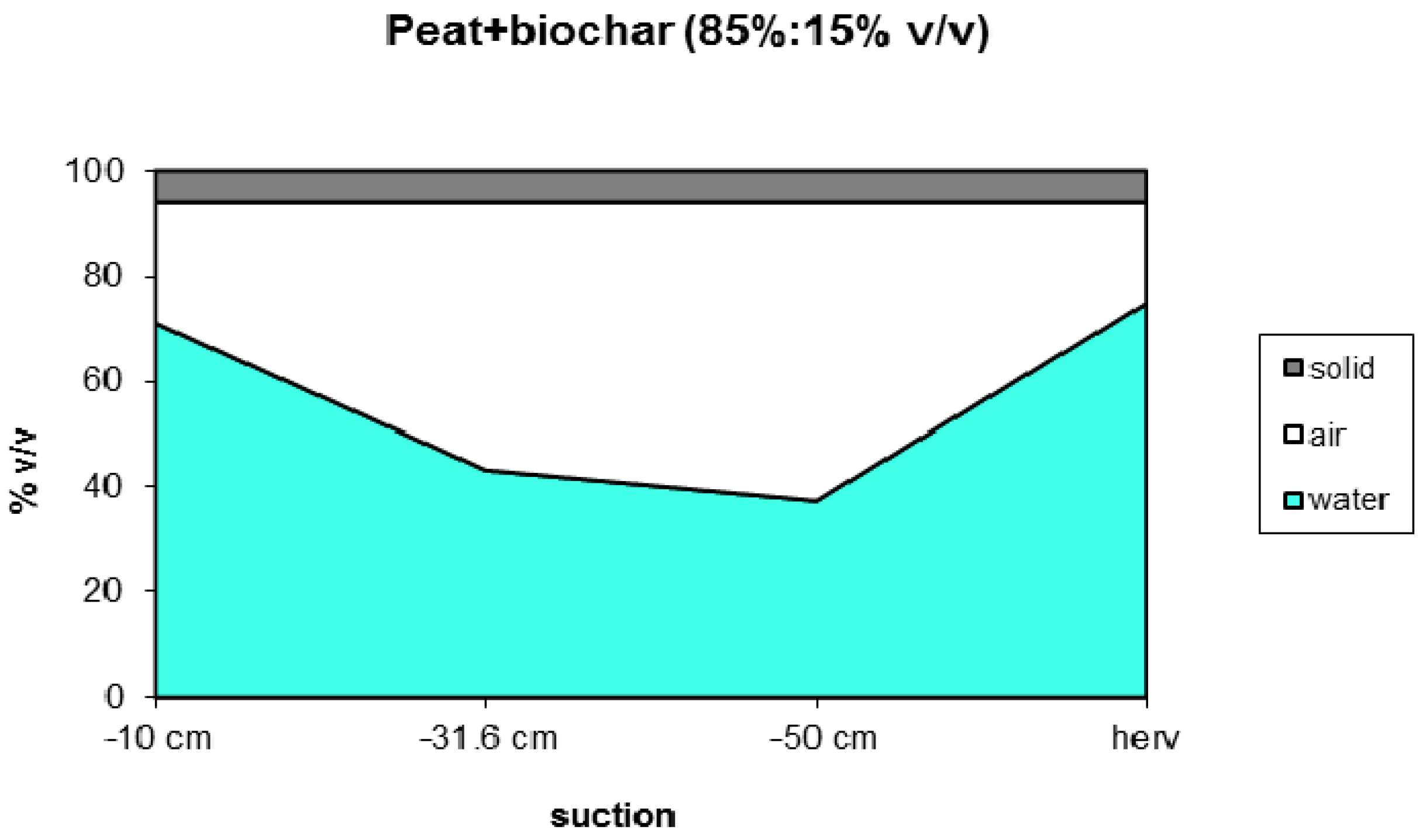
Peat/biochar mixtures, containing 20% v/v biochar, still have properties (EC, pH, water holding capacity, nutrient availability) similar to standard peat and therefore can be qualified as a proper peat alternative. The water retention of biochars (45% v/v at −10 cm) is about 20% v/v lower than for peat but by no means too dry for optimum growth. The water holding capacity can be improved by using relatively wet rooting materials like peat or coir pith but the water holding capacity of biochar can also be improved by mixing with a finer grade of biochar. The wettability of biochar can be increased by increasing the specific surface area by choosing a feedstock low in nutrients and/or increasing process temperature [
34]. It may be of practical interest to note that biochars with higher specific surface areas will reduce the effectivity of some crop protection agents by adsorption [
30].
Examples in literature show it is possible to grow plants just as successfully as on standard peat mixtures with 50%–100% v/v rice husk and reed based biochar [
24,
27]. It is, therefore, expected that the wood based biochars used in this work may be used in ratios up to 50% v/v if the pH is properly neutralized.
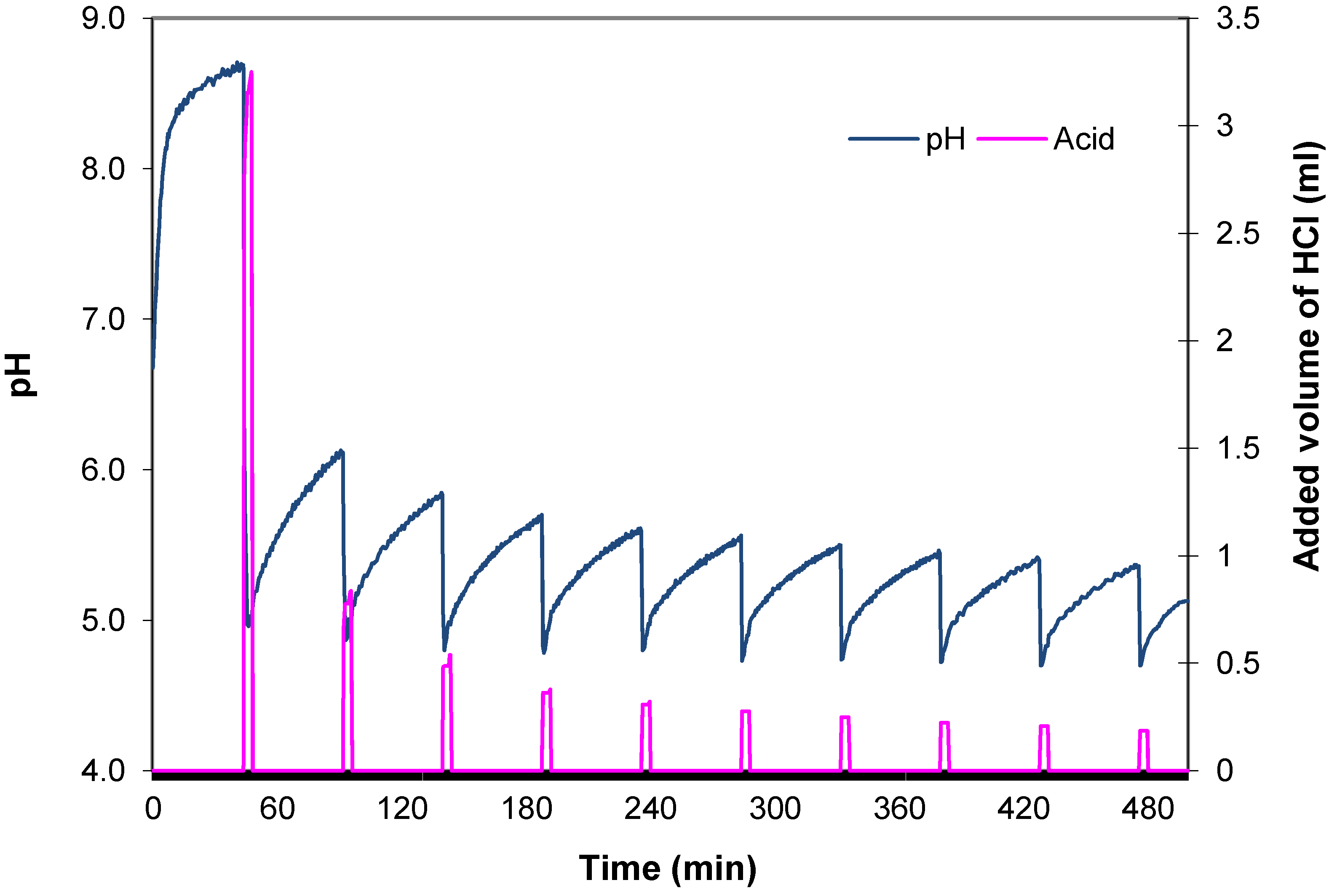
Because the pH of most biochars is too high, those biochars have to be acidified to obtain an optimal potting mixture. Most rooting media in their natural i.e., unlimed and unfertilized state are neutral to slightly alkaline (pH 6–7.5). The only exception is peat which can be harvested at pHs as low as pH 3.0–4.0. This leads to this paradox: in order to use biochar as a peat alternative one needs unlimed and acid peat. The mixing ratio differs depending on the biochar as well as on the peat used but expected maximum biochar levels range from 20%–50% v/v. The pH buffer method described is effective but the method needs to be adapted by an increase of the 16 hours measuring period to allow for the slow reaction of biochar with added acids.
The very low oxygen uptake rate (OUR) of biochar is a positive property for horticultural media since it indicates that biochar is poorly degradable, reducing the need for additional nitrate and reducing the risks of shrinkage of the rooting medium due to microbial degradation of biochar.
However, the presence of some phytotoxic compounds in biochar from wood chips in these experiments, despite that the problem was reported as technically solvable [
41], remains a point of concern that needs further attention in process design.