Effects on Water Management and Quality Characteristics of Ozone Application in Chicory Forcing Process: A Pilot System
Abstract
:1. Introduction
2. Results and Discussion
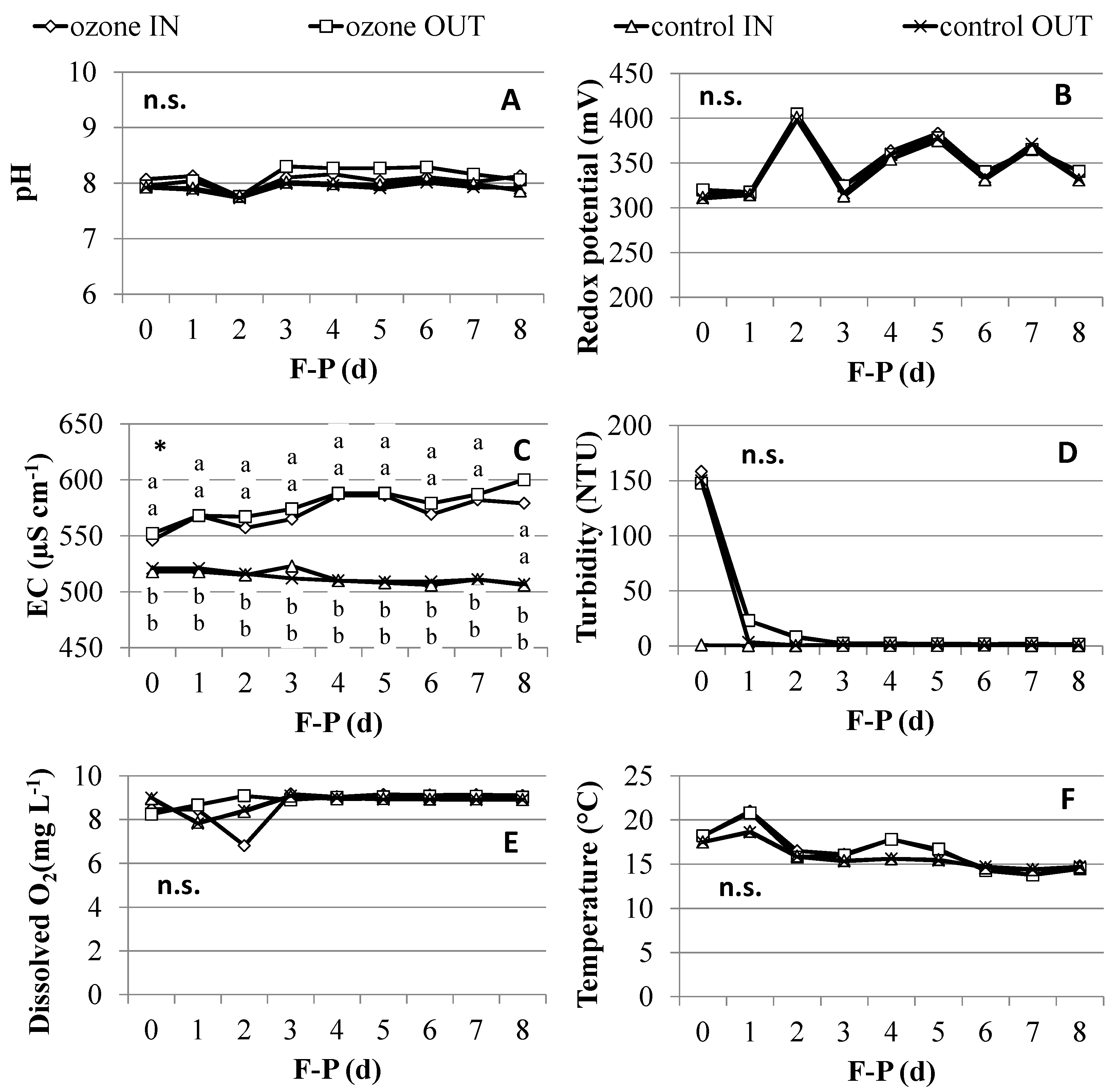
2.1. Water Quality
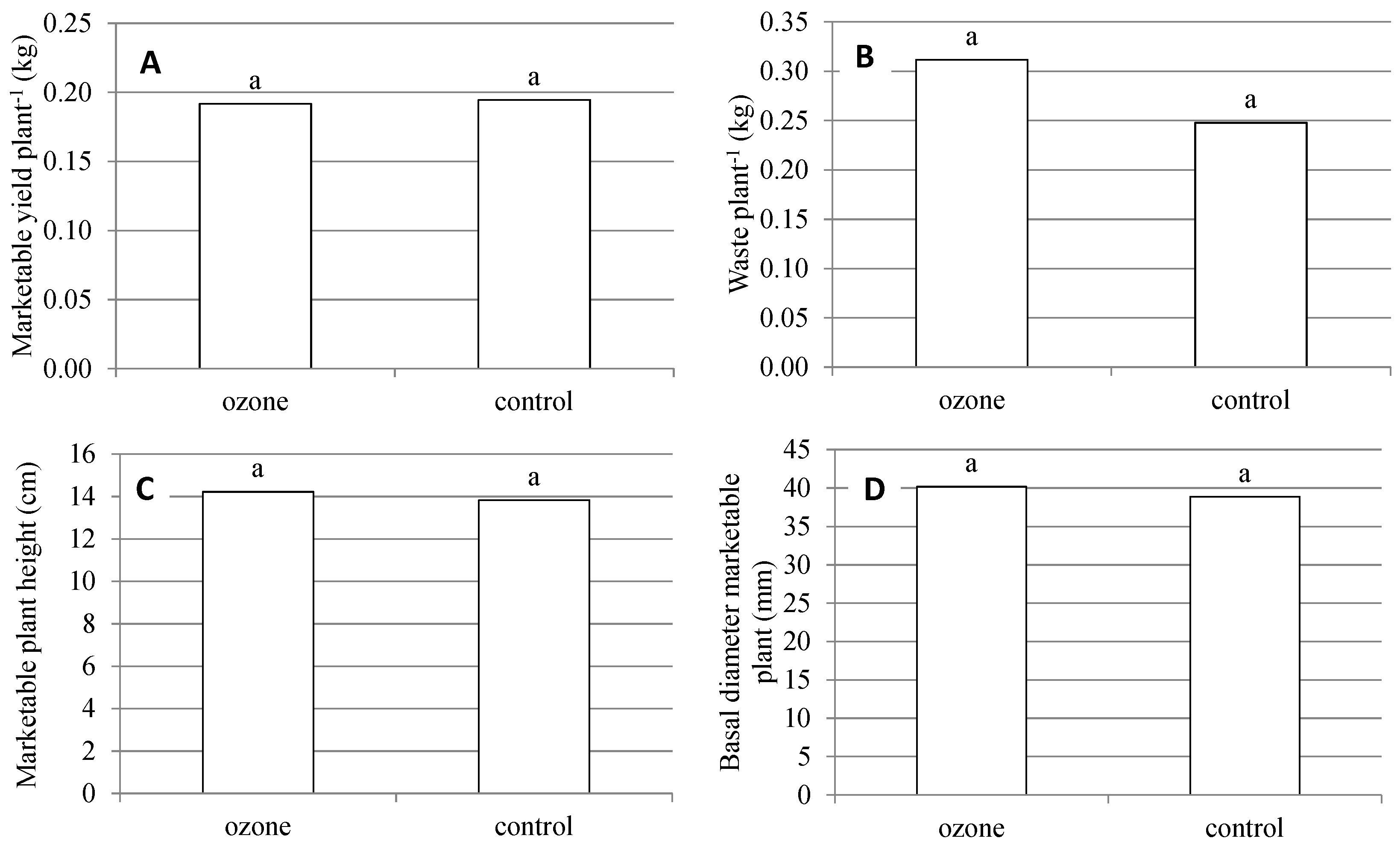
2.2. Production and Quality Aspects of RTT
2.3. Cost Considerations
3. Materials and Methods
3.1. Forcing Process Systems
3.2. Plant Material
3.3. Water Analysis and Plant Sampling
3.4. Extraction of Phenols for Analysis
3.5. Determination of Total Phenols by the Folin Ciocalteu Assay
3.6. Determination of Total Antioxidant Activity by Ferric Reducing Antioxidant Power
3.7. Separation and Analysis of Free Phenolic Acids by HPLC
3.8. Quantitative Determination of Sugars by HPLC
3.9. Extraction of Sesquiterpene Lactones (SLs)
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| RTT | Radicchio rosso di Treviso Tardivo |
| ANOVA | Analysis Of Variance |
| FRAP | Ferric Reducing Antioxidant Power |
| GAE | Gallic Acid Equivalent |
| FC | Folin Ciocalteau |
| SLs | Sesquiterpene Lactones |
| TP | Total Phenols |
| TAA | Total Antioxidant Activity |
| EC | Electrical Conductivity |
| NTU | Nephelometric Turbidity Units |
| DO | Dissolved Oxygen |
References
- Pisante, M.; Stagnari, F. L’agricoltura Sostenibile. 2013. Available online: http://hdl.handle.net/11575/12668 (accessed on 3 April 2017).
- Pisante, M. Agricoltura Sostenibile Principi. In Sistemi E Tecnologie Applicate All’agricoltura Produttiva Per La Salvaguardia Dell’ambiente E La Tutela Climatica; Edagricole-New Business Media Srl: Milano, Italy, 2013. [Google Scholar]
- Adinolfi, F.; Di Pasquale, J. L’impronta idrica dell’Italy. 2014. Available online: http://www.expo2015.org/magazine/it/cultura/il-buon-uso-dell-acqua-conviene-a-tutti.html (accessed on 3 April 2017).
- Falloon, P.; Betts, R. Climate impacts on European agriculture and water management in the context of adaptation and mitigation—The importance of an integrated approach. Sci. Total Environ. 2010, 408, 5667–5687. [Google Scholar] [CrossRef] [PubMed]
- Guzel-Seydim, Z.B.; Greene, A.K.; Seydim, A.C. Use of ozone in the food industry. Food Sci. Technol. 2004, 37, 453–460. [Google Scholar] [CrossRef]
- Kim, J.G.; Yousef, A.E.; Dave, S. Application of ozone for enhancing the microbiological safety and quality of foods: A review. J. Food Protect. 1999, 62, 1071–1087. [Google Scholar] [CrossRef]
- Kim, J.G.; Yousef, A.E.; Khadre, M.A. Ozone and its current and future application in the food industry. Adv. Food Nutr. Res. 2003, 45, 167–218. [Google Scholar] [PubMed]
- Soliva-Fortuny, R.C.; Martín-Belloso, O. New advances in extending the shelf-life of fresh-cut fruits: A review. Trends Food Sci. Technol. 2003, 14, 341–353. [Google Scholar] [CrossRef]
- Akata, I.; Torlak, E.; Erci, F. Efficacy of gaseous ozone for reducing microflora and foodborne pathogens on button mushroom. Postharvest Biol. Technol. 2015, 109, 40–44. [Google Scholar] [CrossRef]
- Wani, S.; Maker, J.K.; Thompson, J.R.; Barnes, J.; Singleton, I. Effect of ozone treatment on inactivation of Escherichia coli and Listeria sp. on Spinach. Agriculture 2015, 5, 155–169. [Google Scholar] [CrossRef]
- Bermúdez-Aguirre, D.; Barbosa-Cánovas, G.V. Disinfection of selected vegetables under nonthermal treatments: Chlorine, acid citric, ultraviolet light and ozone. Food Control 2013, 29, 82–90. [Google Scholar] [CrossRef]
- Camel, V.; Bermond, A. The use of ozone and associated oxidation processes in drinking water treatment. Water Res. 1998, 32, 3208–3222. [Google Scholar] [CrossRef]
- Kobayashi, F.; Ikeura, H.; Ohsato, S.; Goto, T.; Tamaki, M. Disinfection using ozone microbubbles to inactivate Fusarium. oxysporum f. sp. melonis and Pectobacterium. carotovorum subsp. carotovorum. Crop Protect. 2011, 30, 1510–1518. [Google Scholar]
- Msayleb, N.; Kanwar, R.; Robertson, A.; Wu, H. Ozone inactivation of Fusarium oxysporum conidia in hydroponic nutrient solutions. In Soil Ozonation as a Sustainable Alternative to Methyl Bromide Fumigation and Synthetic Pesticides, Graduate Theses and Dissertations; Iowa State University: Ames, IA, USA, 2014; pp. 74–95. [Google Scholar]
- Nicoletto, C.; Pimpini, F. Influence of the forcing process on some qualitative aspects in radicchio “Rosso di Treviso tardivo” (Cichorium. intybus L., group rubifolium). 1. Nitrate, nitrite and organic nitrogen. Italy J. Agron. 2009, 4, 137–146. [Google Scholar] [CrossRef]
- Nicoletto, C.; Pimpini, F. Influence of the forcing process on some qualitative aspects in radicchio “Rosso di Treviso Tardivo” (Cichorium. intybus L., group rubifolium). 2. Antioxidant capacity, phenols and ascorbic acid. Ital. J. Agron. 2010, 5, 43–52. [Google Scholar] [CrossRef]
- Vertregt, N.; Van Kruistum, G. Redistribution of dry matter and carbohydrates in witloof chicory during forcing. Sci. Hortic. 1989, 39, 271–278. [Google Scholar] [CrossRef]
- Fouldrin, K.; Limami, A. The influence of nitrogen (15NO3) supply to chicory (Cichorium. intybus L.) plants during forcing on the uptake and remobilization of N reserves for chicon growth. J. Exp. Bot. 1993, 44, 1313–1319. [Google Scholar] [CrossRef]
- De Jonghe, K.; De Dobbelaere, I.; Sarrazyn, R.; Höfte, M. Control of brown root rot caused by Phytophthora cryptogea in the hydroponic forcing of witloof chicory (Cichorium. intybus var. foliosum) by means of a nonionic surfactant. Crop Protect. 2005, 24, 771–778. [Google Scholar]
- Siddiqui, M.S.; Amy, G.L.; Murphy, B.D. Ozone enhanced removal of natural organic matter from drinking water sources. Water Res. 1997, 31, 3098–3106. [Google Scholar] [CrossRef]
- Gottschalk, C.; Libra, J.A.; Saupe, A. Ozonation of Water and Waste Water: A Practical Guide to Understanding Ozone and Its Applications; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Kainulainen, T.K.; Tuhkanen, T.A.; Vartainen, T.K.; Kalliokoski, P.J. Removal of residual organics from drinking water by ozonation and activated carbon filtration: A pilot plant study. Ozone Sci. Eng. 1995, 17, 449–462. [Google Scholar] [CrossRef]
- Elovitz, M.S.; von Gunten, U.; Kaiser, H.P. Hydroxyl radical/ozone ratios during ozonation processes. II. The effect of temperature, pH, alkalinity, and DOM properties. Ozone Sci. Eng. 2000, 22, 123–150. [Google Scholar] [CrossRef]
- Restaino, L.; Frampton, E.W.; Hemphill, J.B.; Palnikar, P. Efficacy of ozonated water against various food-related microorganisms. Appl. Environ. Microb. 1995, 61, 3471–3475. [Google Scholar]
- Khadre, M.A.; Yousef, A.E.; Kim, J.G. Microbiological aspects of ozone applications in food: A review. J. Food Sci. 2001, 66, 1242–1252. [Google Scholar] [CrossRef]
- Karaca, H.; Velioglu, Y.S. Effects of ozone treatments on microbial quality and some chemical properties of lettuce, spinach, and parsley. Postharvest Biol. Technol. 2014, 88, 46–53. [Google Scholar] [CrossRef]
- Pérez, A.; Poznyak, T.; Chairez, I. Microorganism inactivation by ozone dissolved in aqueous solution: A kinetic study based on bacterial culture lipid unsaturation. Ozone Sci. Eng. 2015, 37, 119–126. [Google Scholar] [CrossRef]
- Al-Hashimi, A.M.; Mason, T.J.; Joyce, E.M. Combined effect of ultrasound and ozone on bacteria in water. Environ. Sci. Technol. 2015, 49, 11697–11702. [Google Scholar] [CrossRef] [PubMed]
- Skog, C.L.; Chu, L.J. Effect of ozone on qualities of fruits and vegetables in cold storage. Can. J. Plant Sci. 2001, 81, 773–778. [Google Scholar] [CrossRef]
- Glowacz, M.; Rees, D. The practicality of using ozone with fruit and vegetables. J. Sci. Food Agric. 2016, 96, 4637–4643. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, C.; Moraes, K.; Perez-Gago, M.B.; Palou, L.; Maraschin, M.; Monteiro, A.R. Effect of active modified atmosphere and cold storage on the postharvest quality of cherry tomatoes. Postharvest Biol. Technol. 2015, 109, 73–81. [Google Scholar] [CrossRef]
- Oliveira, M.; Abadias, M.; Usall, J.; Torres, R.; Teixidó, N.; Viñas, I. Application of modified atmosphere packaging as a safety approach to fresh-cut fruits and vegetables–A review. Trends Food Sci. Technol. 2015, 46, 13–26. [Google Scholar] [CrossRef]
- Zhang, M.; Meng, X.; Bhandari, B.; Fang, Z. Recent developments in film and gas research in modified atmosphere packaging of fresh foods. Crit. Rev. Food Sci. 2016, 56, 2174–2182. [Google Scholar] [CrossRef] [PubMed]
- Lima, G.P.P.; Machado, T.M.; Oliveira, L.M.D.; Borges, L.D.S.; Pedrosa, V.D.A.; Vanzani, P.; Vianello, F. Ozonated water and chlorine effects on the antioxidant properties of organic and conventional broccoli during postharvest. Sci. Agric. 2014, 71, 151–156. [Google Scholar] [CrossRef]
- Koukounaras, A.; Papachristodoulou, M.; Chatzidimos, C.; Tsouvaltzis, P.; Gerasopoulos, D.; Siomos, A.S. The effects of ozone treatment on quality and biochemical parameters of fresh-cut lettuce. Acta Hortic. 2016, 1142, 349–354. [Google Scholar] [CrossRef]
- Bortolin, R.C.; Caregnato, F.F.; Junior, A.M.D.; Zanotto-Filho, A.; Moresco, K.S.; de Oliveira Rios, A.; Gelain, D.P. Chronic ozone exposure alters the secondary metabolite profile, antioxidant potential, anti-inflammatory property, and quality of red pepper fruit from Capsicum baccatum. Ecotox. Environ. Saf. 2016, 129, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Li, J.; Yu, W.; Lu, X.; Kou, X. Effects of nonthermal preservation technologies on antioxidant activity of fruits and vegetables: A review. Food Sci. Technol. Int. 2016, 22, 440–458. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M. Ozone sterilizing for the plant pathogenic fungi in the solution of soilless culture: The case of microconidia of Fusarium. oxysporum cucumerinum. J. Jpn. Soc. Agric. Mach. 1993, 55, 105–111. [Google Scholar]
- Graziani, G.; Ferracane, R.; Sambo, P.; Santagata, S.; Nicoletto, C.; Fogliano, V. Profiling chicory sesquiterpene lactones by high resolution mass spectrometry. Food Res. Int. 2015, 67, 193–198. [Google Scholar] [CrossRef]
- Jaiswal, R.; Kiprotich, J.; Kuhnert, N. Determination of the hydroxycinnamate profile of 12 members of the Asteraceae. family. Phytochemistry 2011, 72, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Carazzone, C.; Mascherpa, D.; Gazzani, G.; Papetti, A. Identification of phenolic constituents in red chicory salads (Cichorium. intybus) by high-performance liquid chromatography with diode array detection and electrospray ionisation tandem mass spectrometry. Food Chem. 2013, 138, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, M.; Gallori, S.; Giaccherini, C.; Ieri, F.; Vincieri, F.F.; Mulinacci, N. Evaluation of the phenolic content in the aerial parts of different varieties of Cichorium. intybus L. J. Agric. Food Chem. 2005, 53, 6497–6502. [Google Scholar] [CrossRef] [PubMed]
- Speroni, E.; Covoni, P.; Guizzardi, S.; Renzulli, C.; Guerra, M.C. Anti-inflammatory and cicatrizing activity of Echinacea pallida Nutt. root extract. J. Ethnopharmacol. 2002, 79, 265–272. [Google Scholar] [CrossRef]
- Maffei Facino, R.; Carini, M.; Aldini, G.; Saibene, L.; Pietta, P.; Mauri, P. Echinacoside and caffeoyl conjugates protect collagen from free radical-induced degradation: A potent use of Echinacea extacts in the prevention of skin photodamage. Planta Med. 1995, 61, 510–514. [Google Scholar] [CrossRef]
- Lin, Z.; Neamati, N.; Zhao, H.; Kiryu, Y.; Turpin, J.A.; Aberham, C.; Strebel, K.; Kohn, K.; Witvrouw, M.; Pannacoque, C.; et al. Chicoric acid analogues as HIV-1 integrase inhibitors. J. Med. Chem. 1999, 42, 1401–1414. [Google Scholar] [CrossRef] [PubMed]
- Stanghellini, M.E.; Rasmussen, S.L.; Kim, D.H.; Rorabaugh, P.A. Efficacy of nonionic surfactants in the control of zoospore spread of Pythium. aphanidermatum in a recirculating hydroponic system. Plant. Dis. 1996, 80, 422–428. [Google Scholar] [CrossRef]
- Stanghellini, M.E.; Rasmussen, S.L. Hydroponics—A solution for zoosporic pathogens. Plant Dis. 1994, 78, 1129–1138. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]

| pH | EC | TDS | Titratable Acidity | Antioxidant Activity | Total Phenols | |
|---|---|---|---|---|---|---|
| (mS cm−1) | (°Brix) | (% Citric Acid) | (mg Fe2+E kg−1 dw) | (mg GAE kg−1 dw) | ||
| Ozone | 4.94 ± 0.12 a | 5.64 ± 0.90 a | 4.97 ± 0.46 a | 0.28 ± 0.05 a | 44,554 ± 348 a | 4757 ± 193 a |
| Control | 4.83 ±0.15 a | 6.45 ± 0.95 a | 5.30 ± 0.38 a | 0.31 ± 0.08 a | 39,042 ± 681 b | 4245 ± 142 a |
| 1 | 2 | 3 | 4 | 5 | Total | |
|---|---|---|---|---|---|---|
| Ozone | 4.12 ± 0.43 a | 3.28 ± 0.31 a | 6.53 ± 0.62 a | 4.29 ± 0.45 a | 2.38 ± 0.29 a | 20.6 ± 1.3 a |
| Control | 3.78 ± 0.47 a | 3.00 ± 0.27 a | 5.98 ± 0.71 a | 3.93 ± 0.50 a | 2.17 ± 0.22 a | 18.9 ± 1.6 a |
| Chicoric Acid | Chlorogenic Acid | Glucose | Fructose | Sucrose | |
|---|---|---|---|---|---|
| Ozone | 25,036 ± 365 a | 17,758 ± 1687 a | 102,068 ± 4298 a | 94,137 ± 1828 a | 13,965 ± 2415 a |
| Control | 19,473 ± 482 b | 16,249 ± 1734 a | 106,213 ± 4337 a | 92,453 ± 1749 a | 11,618 ± 2531 a |
| Crop Management Practice | Date | Note |
|---|---|---|
| Fertilization | 15 July | 65 kg N ha−1, 130 kg P2O5 ha−1, 1 kg B ha−1 |
| Field transplant | 21 July | 7.4 (plants m−2) |
| Irrigation | 21–27 July; 5 August | 100 m3 ha−1 (sprinkling method) |
| Mechanical weeding | 25 August | Inter row tillage |
| Harvest | 21 January | Uprooting |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicoletto, C.; Maucieri, C.; Sambo, P. Effects on Water Management and Quality Characteristics of Ozone Application in Chicory Forcing Process: A Pilot System. Agronomy 2017, 7, 29. https://doi.org/10.3390/agronomy7020029
Nicoletto C, Maucieri C, Sambo P. Effects on Water Management and Quality Characteristics of Ozone Application in Chicory Forcing Process: A Pilot System. Agronomy. 2017; 7(2):29. https://doi.org/10.3390/agronomy7020029
Chicago/Turabian StyleNicoletto, Carlo, Carmelo Maucieri, and Paolo Sambo. 2017. "Effects on Water Management and Quality Characteristics of Ozone Application in Chicory Forcing Process: A Pilot System" Agronomy 7, no. 2: 29. https://doi.org/10.3390/agronomy7020029






