Mid-Season Leaf Glutamine Predicts End-Season Maize Grain Yield and Nitrogen Content in Response to Nitrogen Fertilization under Field Conditions
Abstract
:1. Introduction
2. Results
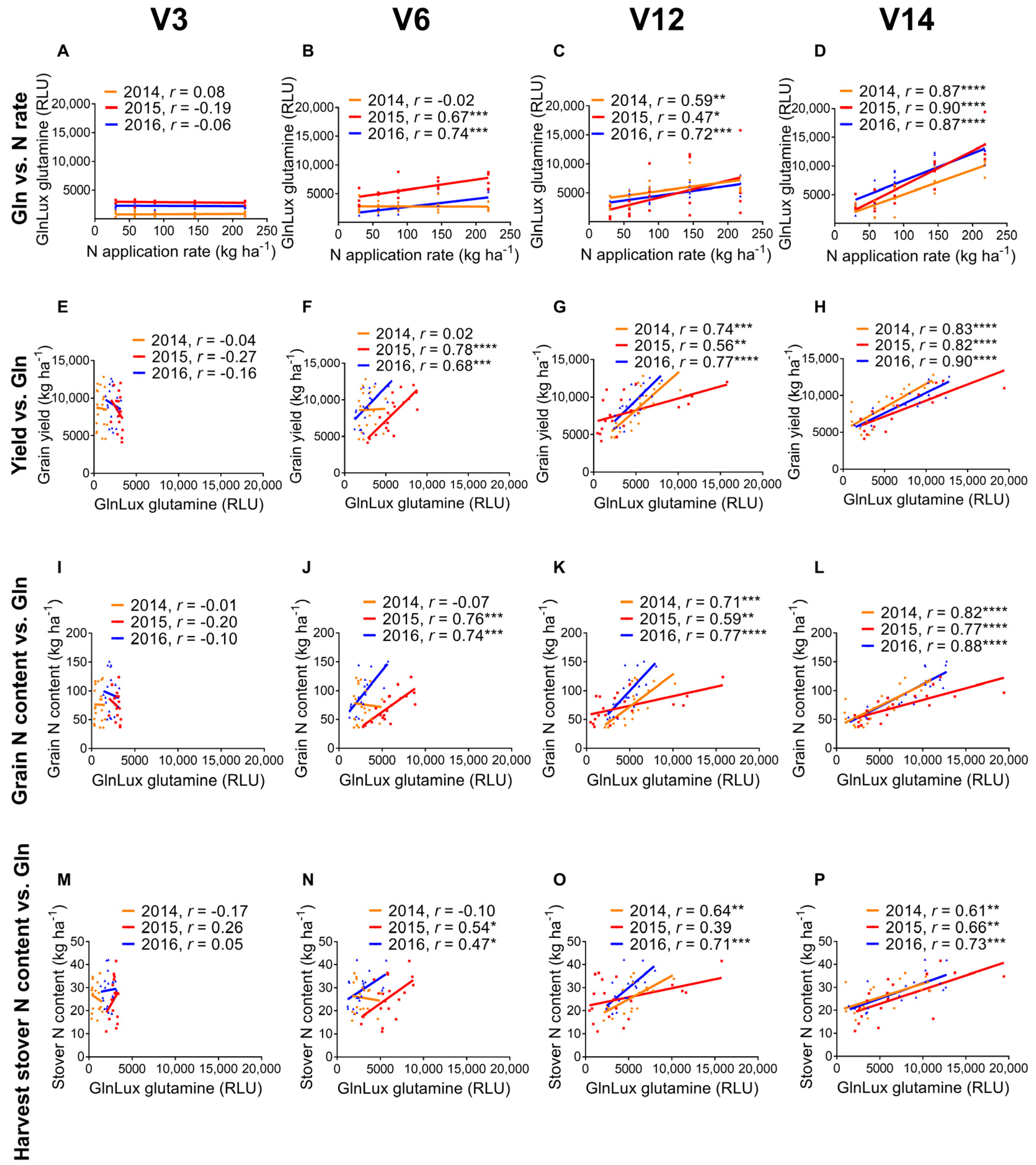
2.1. Correlation of Leaf GlnLux Glutamine and Nitrogen Application Rate
2.2. GlnLux Glutamine Correlation with End-Season Field Measurements
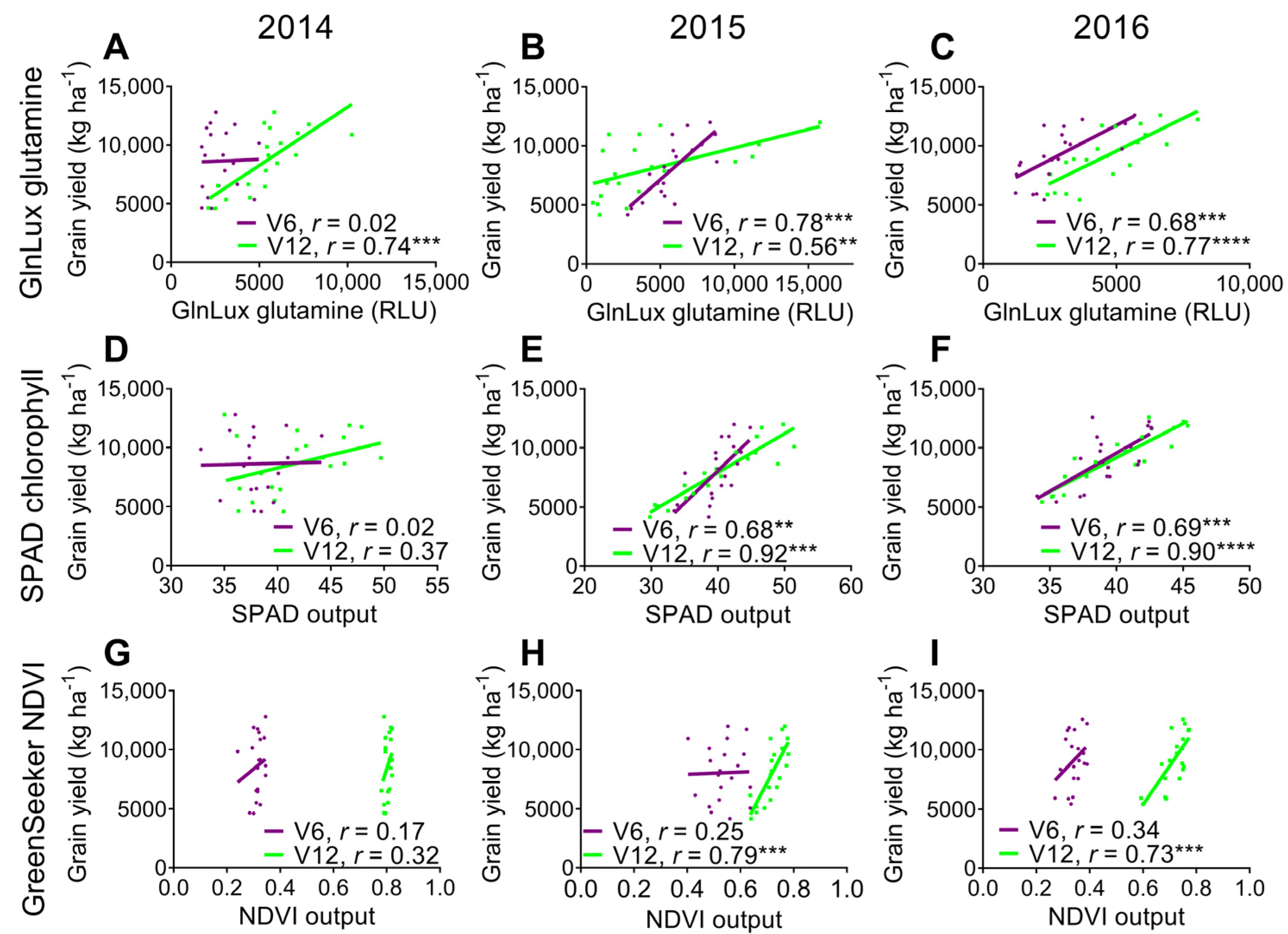
2.3. Comparison of the Yield-Predictive Potential of Vegetative GlnLux Glutamine, SPAD Chlorophyll, and GreenSeekerTM NDVI
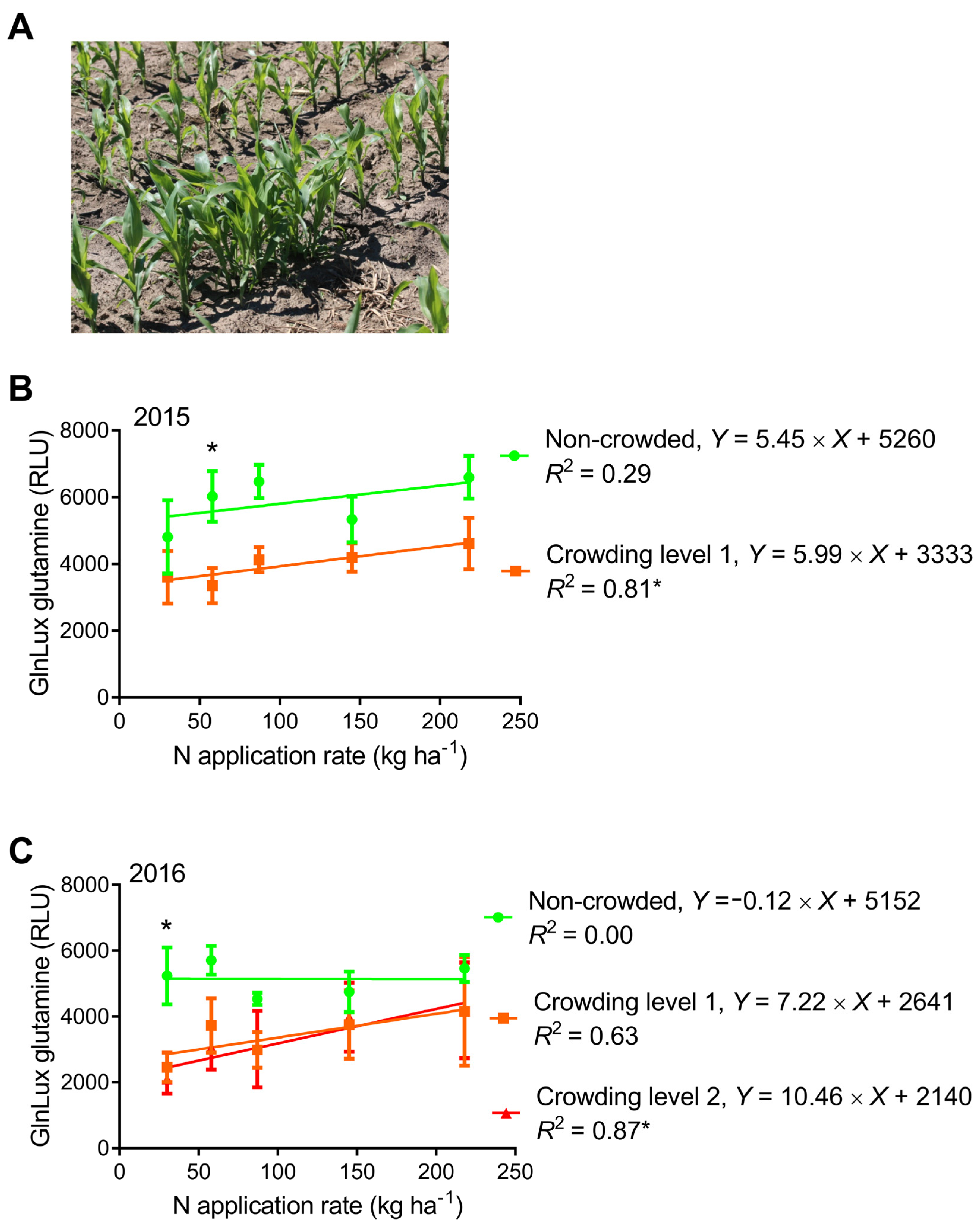
2.4. Comparison of GlnLux Glutamine Accumulation between Crowded and Non-Crowded Maize Plants
3. Discussion
3.1. GlnLux Glutamine Becomes a Reliable Indicator of N Application Rate as Vegetative Growth Progresses
3.2. Measurements of GlnLux Glutamine at Different Growth Stages Correlate with End-Season Grain Yield
3.3. Comparison to Commercially Available In-Season N Health Indicators
3.4. Can the Yield-Predictive Value of Leaf N-Health Indicators Be Improved by Creating Early-Crowding Subplots?
3.5. Potential of Leaf Gln and GlnLux as Tools for Field Research
4. Materials and Methods
4.1. Main Experiment: Site Description and Planting
4.2. Crowding Experiment: Planting
4.3. Fertilizer and Herbicide Treatments
4.4. End-Season Measurements
4.5. Relative Measurements of Glutamine from Leaf Disk Extracts
4.6. SPAD and GreenSeekerTM Measurements
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cairns, J.E.; Sonder, K.; Zaidi, P.H.; Verhulst, N.; Mahuku, G.; Babu, R.; Nair, S.; Das, B.; Govaerts, B.; Vinayan, M.; et al. Maize production in a changing climate: Impacts, adaptation, and mitigation strategies. In Advances in Agronomy; Elsevier: San Diego, CA, USA, 2012; Volume 114, pp. 1–58. [Google Scholar]
- Cassman, K.G.; Grassini, P. Can there be a green revolution in Sub-Saharan Africa without large expansion of irrigated crop production? Glob. Food Secur. 2013, 2, 203–209. [Google Scholar] [CrossRef]
- Cassman, K.G. Ecological intensification of cereal production systems: Yield potential, soil quality, and precision agriculture. Proc. Natl. Acad. Sci. USA 1999, 96, 5952–5959. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.E.; Miller, A.J. Transporters responsible for the uptake and partitioning of nitrogenous sources. Annu. Rev. Plant Biol. 2001, 52, 659–688. [Google Scholar] [CrossRef] [PubMed]
- Crawford, N.; Forde, B. Molecular and developmental biology of inorganic nitrogen nutrition. Arabidopsis Book 2002, 1, e0011. [Google Scholar] [CrossRef] [PubMed]
- Stanford, G. Rationale for optimum nitrogen fertilization in corn production. J. Environ. Qual. 1973, 2, 159–166. [Google Scholar] [CrossRef]
- Abbasi, M.K.; Tahir, M.M.; Rahim, N. Effect of N fertilizer source and timing on yield and N use efficiency of rainfed maize (Zea mays L.) in Kashmir-Pakistan. Geoderma 2013, 195, 87–93. [Google Scholar] [CrossRef]
- Cameron, K.; Goh, K.; Sherlock, R. Mineral Nitrogen in the Plant-Soil System, 1st ed.; Haynes, R., Ed.; Academic Press Inc.: Sydney, Australia, 1986. [Google Scholar]
- Karlen, D.L.; Sadler, E.J.; Camp, C.R. Dry matter, nitrogen, phosphorus, and potassium accumulation rates by corn on Norfolk loamy sand. Agron. J. 1987, 79, 649–656. [Google Scholar] [CrossRef]
- Malzer, G.; Copeland, P.; Davis, J.; Lamb, J.; Robert, P.; Bruulsema, T. Spatial variability of profitability in site-specific N management. In Precision Agriculture; Robert, P., Rust, R., Larson, W., Eds.; ASA, CSSA, SSSA: Madison, WI, USA, 1996; pp. 967–975. [Google Scholar]
- Dhital, S.; Raun, W. Variability in optimum nitrogen rates for maize. Agron. J. 2016, 108, 2165–2173. [Google Scholar] [CrossRef]
- Shanahan, J.F.; Kitchen, N.R.; Raun, W.R.; Schepers, J.S. Responsive in-season nitrogen management for cereals. Comput. Electron. Agric. 2008, 61, 51–62. [Google Scholar] [CrossRef]
- Andraski, T.W.; Bundy, L.G. Using the presidedress soil nitrate test and organic nitrogen crediting to improve corn nitrogen recommendations. Agron. J. 2002, 94, 1411–1418. [Google Scholar] [CrossRef]
- Cui, Z.; Chen, X.; Miao, Y.; Zhang, F.; Sun, Q.; Schroder, J.; Zhang, H.; Li, J.; Shi, L.; Xu, J.; et al. On-farm evaluation of the improved soil Nmin-based nitrogen management for summer maize in North China Plain. Agron. J. 2008, 100, 517–525. [Google Scholar] [CrossRef]
- da Silva, A.N.; Schoninger, E.L.; Trivelin, P.C.O.; Dourado-neto, D.; Pinto, V.; Reichardt, K. Maize response to nitrogen: Timing, leaf variables and grain yield. J. Agric. Sci. 2017, 9, 85–95. [Google Scholar] [CrossRef]
- Olfs, H.; Blankenau, K.; Brentrup, F.; Jasper, J.; Link, A.; Lammel, J. Soil- and plant-based nitrogen-fertilizer recommendations in arable farming. J. Plant Nutr. Soil Sci. 2005, 168, 414–431. [Google Scholar] [CrossRef]
- Zebarth, B.J.; Drury, C.F.; Tremblay, N.; Cambouris, A.N. Opportunities for improved fertilizer nitrogen management in production of arable crops in eastern Canada: A review. Can. J. Soil Sci. 2009, 89, 113–132. [Google Scholar] [CrossRef]
- Millard, P. The accumulation and storage of nitrogen by herbaceous plants. Plant Cell Environ. 1988, 11, 1–8. [Google Scholar] [CrossRef]
- Wood, C.W.; Reeves, D.W.; Himelrick, D.G. Relationships between chlorophyll meter readings and leaf chlorophyll concentration, N status, and crop yield: A review. Proc. Agron. Soc. N. Z. 1993, 23, 1–9. [Google Scholar]
- Rostami, M.; Koocheki, A.; Mahallati, M.; Kafi, M. Evaluation of chlorophyll meter (SPAD) data for prediction of nitrogen status in corn (Zea mays L.). Am. J. Agric. Environ. Sci. 2008, 3, 79–85. [Google Scholar]
- Berenguer, P.; Santiveri, F.; Boixadera, J.; Lloveras, J. Nitrogen fertilisation of irrigated maize under Mediterranean conditions. Eur. J. Agron. 2009, 30, 163–171. [Google Scholar] [CrossRef]
- Schmidt, J.; Beegle, D.; Zhu, Q.; Sripada, R. Improving in-season nitrogen recommendations for maize using an active sensor. Field Crops Res. 2011, 120, 94–101. [Google Scholar] [CrossRef]
- Shaver, T.; Khosla, R.; Westfall, D. Evaluation of two crop canopy sensors for nitrogen variability determination in irrigated maize. Precis. Agric. 2011, 12, 892–904. [Google Scholar] [CrossRef]
- Zhang, J.; Blackmer, A.M.; Blackmer, T.M.; Kyveryga, P.M.; Ellsworth, J.W. Nitrogen deficiency and recovery in sustainable corn production as revealed by leaf chlorophyll. Agron. Sustain. Dev. 2007, 27, 313–319. [Google Scholar] [CrossRef]
- Zhang, J.; Blackmer, A.M.; Ellsworth, J.W.; Kyveryga, P.M.; Blackmer, T.M. Luxury production of leaf chlorophyll and mid-season recovery from nitrogen deficiencies in corn. Agron. J. 2008, 100, 658–664. [Google Scholar] [CrossRef]
- Ma, B.L.; Subedi, K.D.; Costa, C. Comparison of crop-based indicators with soil nitrate test for corn nitrogen requirement. Agron. J. 2005, 97, 462–471. [Google Scholar] [CrossRef]
- Teal, R.K.; Tubana, B.; Girma, K.; Freeman, K.W.; Arnall, D.B.; Walsh, O.; Raun, W.R. In-season prediction of corn grain yield potential using normalized difference vegetation index. Agron. J. 2006, 98, 1488–1494. [Google Scholar] [CrossRef]
- Piekielek, W.P.; Fox, R.H. Use of a chlorophyll meter to predict sidedress nitrogen requirements for maize. Agron. J. 1992, 84, 59–65. [Google Scholar] [CrossRef]
- Schepers, J.S.; Francis, D.; Vigil, M.; Below, F. Comparison of corn leaf nitrogen concentratioin and chlorophyll meter readings. Commun. Soil Sci. Plant Anal. 1992, 23, 2173–2187. [Google Scholar] [CrossRef]
- Bullock, D.G.; Anderson, D.S. Evaluation of the Minolta SPAD-502 chlorophyll meter for nitrogen management in corn. J. Plant Nutr. 1998, 21, 741–755. [Google Scholar] [CrossRef]
- Poudel, M.; Paudel, H.; Yadav, B. Correlation of traits affecting grain yield in winter maize (Zea mays L.) genotypes. Int. J. Appl. Sci. Biotechnol. 2015, 3, 443–445. [Google Scholar] [CrossRef]
- Lindsey, A.J.; Steinke, K.; Rutan, J.; Thomison, P.R. Relationship of DGCI and SPAD values to corn grain yield in the Eastern corn belt. Crop Forage Turfgrass Manag. 2016, 2, 1–9. [Google Scholar] [CrossRef]
- Ma, B.L.; Morrison, M.J.; Dwyer, L.M. Canopy light reflectance and field greenness to assess nitrogen fertilization and yield of maize. Agron. J. 1996, 88, 915–920. [Google Scholar] [CrossRef]
- Bender, R.R.; Haegele, J.W.; Ruffo, M.L.; Below, F.E. Nutrient uptake, partitioning, and remobilization in modern, transgenic insect-protected maize hybrids. Agron. J. 2013, 105, 161–170. [Google Scholar] [CrossRef]
- Lemaire, G.; Jeuffroy, M.H.; Gastal, F. Diagnosis tool for plant and crop N status in vegetative stage. Theory and practices for crop N management. Eur. J. Agron. 2008, 28, 614–624. [Google Scholar] [CrossRef]
- McMaster, G.S.; Ascough, J.C.; Edmunds, D.A.; Wagner, L.E.; Fox, F.A.; DeJonge, K.C.; Hansen, N.C. Simulating unstressed crop development and growth using the unified plant growth model (UPGM). Environ. Model. Assess. 2014, 19, 407–424. [Google Scholar] [CrossRef]
- Devienne-Barret, F.; Justes, E.; Machet, J.M.; Mary, B. Integrated control of nitrate uptake by crop growth rate and soil nitrate availability under field conditions. Ann. Bot. 2000, 86, 995–1005. [Google Scholar] [CrossRef]
- Gaudin, A.C.M.; Janovicek, K.; Deen, B.; Hooker, D.C. Wheat improves nitrogen use efficiency of maize and soybean-based cropping systems. Agric. Ecosyst. Environ. 2015, 210, 1–10. [Google Scholar] [CrossRef]
- Gaudin, A.C.M.; Janovicek, K.; Martin, R.C.; Deen, W. Approaches to optimizing nitrogen fertilization in a winter wheat-red clover (Trifolium pratense L.) relay cropping system. Field Crops Res. 2014, 155, 192–201. [Google Scholar] [CrossRef]
- Naud, C.; Makowski, D.; Jeuffroy, M.H. Application of an interacting particle filter to improve nitrogen nutrition index predictions for winter wheat. Ecol. Model. 2007, 207, 251–263. [Google Scholar] [CrossRef]
- Naud, C.; Makowski, D.; Jeuffroy, M. Is it useful to combine measurements taken during the growing season with a dynamic model to predict the nitrogen status of winter wheat? Eur. J. Agron. 2008, 28, 291–300. [Google Scholar] [CrossRef]
- Bagheri, S.; Sepaskhah, A.R.; Razzaghi, F.; Zand-Parsa, S. Developing a dynamic yield and growth model for maize under various water and nitrogen regimes. Arch. Agron. Soil Sci. 2014, 60, 1173–1191. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.J.; Leech, R.M. Changes in pool sizes of free amino acids and amides in leaves and plastids of Zea mays during leaf development. Plant Physiol. 1979, 63, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, J.R.; Ju, G.C.; Rich, P.J.; Rhodes, D. Kinetics of 15NH4+ Assimilation in Zea mays. Plant Physiol. 1990, 94, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ponnala, L.; Gandotra, N.; Wang, L.; Si, Y.; Tausta, S.L.; Kebrom, T.H.; Provart, N.; Patel, R.; Myers, C.R.; et al. The developmental dynamics of the maize leaf transcriptome. Nat. Genet. 2010, 42, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Czedik-Eysenberg, A.; Mertz, R.A.; Si, Y.; Tohge, T.; Nunes-Nesi, A.; Arrivault, S.; Dedow, L.K.; Bryant, D.W.; Zhou, W.; et al. Comparative analyses of C4 and C3 photosynthesis in developing leaves of maize and rice. Nat. Biotechnol. 2014, 32, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Prinsi, B.; Espen, L. Mineral nitrogen sources differently affect root glutamine synthetase isoforms and amino acid balance among organs in maize. BMC Plant Biol. 2015, 15, 96. [Google Scholar] [CrossRef] [PubMed]
- Cheshire, M.V.; Bick, W.; Dekock, P.; Inkson, R. The effect of copper and nitrogen on the amino acid composition of oat straw. Plant Soil 1982, 66, 139–147. [Google Scholar] [CrossRef]
- Millard, P.; Sharp, G.; Scott, N. The effect of sulphur deficiency on the uptake and incorporation of nitrogen in ryegrass. J. Agric. Sci. 1985, 105, 501–504. [Google Scholar] [CrossRef]
- Angell, A.R.; Mata, L.; de Nys, R.; Paul, N.A. Variation in amino acid content and its relationship to nitrogen content and growth rate in Ulva ohnoi (chlorophyta). J. Phycol. 2014, 50, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Coruzzi, G.M.; Zhou, L. Carbon and nitrogen sensing and signaling in plants: Emerging “matrix effects”. Curr. Opin. Plant Biol. 2001, 4, 247–253. [Google Scholar] [CrossRef]
- Tessaro, M.J.; Soliman, S.S.M.; Raizada, M.N. Bacterial whole-cell biosensor for glutamine with applications for quantifying and visualizing glutamine in plants. Appl. Environ. Microbiol. 2012, 78, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Goron, T.L.; Raizada, M.N. Biosensor-based spatial and developmental mapping of maize leaf glutamine at vein- level resolution in response to different nitrogen rates and uptake/assimilation durations. BMC Plant Biol. 2016, 16, 230. [Google Scholar] [CrossRef] [PubMed]
- Historical Data. Available online: http://climate.weather.gc.ca/historical_data/search_historic_data_e.html (accessed on 11 March 2017).
- Peng, Y.; Niu, J.; Peng, Z.; Zhang, F.; Li, C. Shoot growth potential drives N uptake in maize plants and correlates with root growth in the soil. Field Crops Res. 2010, 115, 85–93. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Vyn, T.J. Nutrient sufficiency concepts for modern corn hybrids: Impacts of management practices and yield levels. Crop Manag. 2014, 47, 38–44. [Google Scholar]
- Buchanan-Wollaston, V. The molecular biology of leaf senescence. J. Exp. Bot. 1997, 48, 181–199. [Google Scholar] [CrossRef]
- Lipson, D.A.; Bowman, W.D.; Monson, R.K. Luxury uptake and storage of nitrogen in the rhizomatous alpine herb, Bistorta bistortoides. Ecology 1996, 77, 1277–1285. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. Ecological significance and complexity of N-source preference in plants. Ann. Bot. 2013, 112, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Goron, T.L.; Raizada, M.N. Genetic diversity and genomic resources available for the small millet crops to accelerate a New Green Revolution. Front. Plant Sci. 2015, 6. Article 127. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, M.S.; Raizada, M.N. A review of nutrient management studies involving finger millet in the semi-arid tropics of Asia and Africa. Agronomy 2015, 5, 262–290. [Google Scholar] [CrossRef]
- Pallavi, C.; Joseph, B.; Aariff Khan, M.A.; Hemalatha, S. Economic evaluation of finger millet under different nutrient management practices. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 690–698. [Google Scholar] [CrossRef]
- Goron, T.L.; Bhosekar, V.K.; Shearer, C.R.; Watts, S.; Raizada, M.N. Whole plant acclimation responses by finger millet to low nitrogen stress. Front. Plant Sci. 2015, 6, 652. [Google Scholar] [CrossRef] [PubMed]
- Ta, C.T.; Weiland, R.T. Nitrogen partitioning in maize during ear development. Crop Sci. 1992, 32, 443–451. [Google Scholar] [CrossRef]
- Huber, S.C.; Sugiyama, T.; Alberte, R.S. Photosynthetic determinants of growth in maize plants: Effects of nitrogen nutrition on growth, carbon fixation and photochemical features. Plant Cell Physiol. 1989, 30, 1063–1072. [Google Scholar] [CrossRef]
- Muchow, R.C.; Sinclair, T.R. Nitrogen response of leaf photosynthesis and canopy radiation use efficiency in field-grown maize and sorghum. Crop Sci. 1994, 34, 721–727. [Google Scholar] [CrossRef]
- Uhart, S.A.; Andrade, F.H. Nitrogen deficiency in maize: I. Effects on crop growth, development, dry matter partitioning, and kernel set. Crop Sci. 1995, 35, 1376–1383. [Google Scholar] [CrossRef]
- Lee, E.A.; Tollenaar, M. Physiological basis of successful breeding strategies for maize grain yield. Crop Sci. 2007, 47, S202–S215. [Google Scholar] [CrossRef]
- Borrell, A.K.; Hammer, G.L. Nitrogen dynamics and the physiological basis of stay-green in sorghum. Crop Sci. 2000, 40, 1295–1307. [Google Scholar] [CrossRef]
- Dhugga, K.S. Maize biomass yield and composition for biofuels. Crop Sci. 2007, 47, 2211–2227. [Google Scholar] [CrossRef]
- Debruin, J.; Messina, C.D.; Munaro, E.; Thompson, K.; Conlon-Beckner, C.; Fallis, L.; Sevenich, D.M.; Gupta, R.; Dhugga, K.S. N distribution in maize plant as a marker for grain yield and limits on its remobilization after flowering. Plant Breed. 2013, 132, 500–505. [Google Scholar] [CrossRef]
- Ning, P.; Fritschi, F.B.; Li, C. Temporal dynamics of post-silking nitrogen fluxes and their effects on grain yield in maize under low to high nitrogen inputs. Field Crops Res. 2017, 204, 249–259. [Google Scholar] [CrossRef]
- Borrell, A.; Hammer, G.; Van Oosterom, E. Stay-green: A consequence of the balance between supply and demand for nitrogen during grain filling? Ann. Appl. Biol. 2001, 138, 91–95. [Google Scholar] [CrossRef]
- Subedi, K.D.; Ma, B.L. Nitrogen uptake and partitioning in stay-green and leafy maize hybrids. Crop Sci. 2005, 45, 740–747. [Google Scholar] [CrossRef]
- Coque, M.; Gallais, A. Genetic variation for nitrogen remobilization and postsilking nitrogen uptake in maize recombinant inbred lines: Heritabilities and correlations among traits. Crop Sci. 2007, 47, 1787–1796. [Google Scholar] [CrossRef]
- Rajcan, I.; Tollenaar, M. Source: Sink ratio and leaf senescence in maize: II. Nitrogen metabolism during grain filling. Field Crops Res. 1999, 60, 255–265. [Google Scholar] [CrossRef]
- Kosgey, J.R.; Moot, D.J.; Fletcher, A.L.; McKenzie, B.A. Dry matter accumulation and post-silking N economy of “stay-green” maize (Zea mays L.) hybrids. Eur. J. Agron. 2013, 51, 43–52. [Google Scholar] [CrossRef]
- Rambo, L.; Ma, B.; Xiong, Y.; da Silvia, P.R.F. Leaf and canopy optical characteristics as crop-N-status indicators for field nitrogen management in corn. J. Plant Nutr. Soil Sci. 2010, 173, 434–443. [Google Scholar] [CrossRef]
- Sharma, L.; Franzen, D. Use of corn height to improve the relationship between active optical sensor readings and yield estimates. Precis. Agric. 2014, 15, 331–345. [Google Scholar] [CrossRef]
- Martin, D.E.; Lopez, J.D.; Lan, Y. Laboratory evaluation of the GreenSeekerTM handheld optical sensor to variations in orientation and height above canopy. Int. J. Agric. Biol. Eng. 2012, 5, 43–47. [Google Scholar]
- Bernard, S.M.; Habash, D. The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytol. 2009, 182, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Moll, R.H.; Kamprath, E.J.; Jackson, W.A. Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization. Agron. J. 1982, 74, 562–564. [Google Scholar] [CrossRef]
- Clark, R. Plant genotype differences in the uptake, translocation, accumulation, and use of mineral elements required for plant growth. Plant Soil 1983, 72, 175–196. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed]
- Lillo, C. Diurnal variations of nitrite reductase, glutamine synthetase, glutamate synthase, alanine aminotraosferase and aspartate aminotransferase in barley leaves. Physiol. Plant 1984, 61, 214–219. [Google Scholar] [CrossRef]
- Valadier, M.H.; Yoshida, A.; Grandjean, O.; Morin, H.; Kronenberger, J.; Boutet, S.; Raballand, A.; Hase, T.; Yoneyama, T.; Suzuki, A. Implication of the glutamine synthetase⁄glutamate synthase pathway in conditioning the amino acid metabolism in bundle sheath and mesophyll cells of maize leaves. FEBS J. 2008, 275, 3193–3206. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Okumoto, S.; Zhang, X.; Ervin, E. Circadian patterns of the major nitrogen metabolism-related enzymes and metabolites in creeping bentgrass and the influence of cytokinin and nitrate. Crop Sci. 2011, 51, 2145–2154. [Google Scholar] [CrossRef]
- Hoffman, D.; Matthews, B.; Wicklund, R. Soil Survey of Wellington County Ontario; Report No. 35 of Ontario Soil Survey; Department of Agriculture and the Ontario Agriculture College: Guelph, ON, Canada, 1968. [Google Scholar]
- Fiedler, R.; Proksch, G.; Koepf, A. The determination of total nitrogen in plant materials with an automatic nitrogen analyser. Anal. Chim. Acta 1973, 63, 435–443. [Google Scholar] [CrossRef]
- Motulsky, H.J.; Brown, R.E. Detecting outliers when fitting data with nonlinear regression—A new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 2006, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Zar, J. The arcsine transformation. In Biostatistical Analysis; Prentice Hall: Upper Saddle River, NJ, USA, 1984; pp. 239–324. [Google Scholar]
- Sidak, Z. Rectangular confidence regions for the means of multivariate normal distributions. J. Am. Stat. Assoc. 1967, 62, 626–633. [Google Scholar] [CrossRef]
| Growth Stage | V3 | V6 | V12 | V14 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2014 | 2015 | 2016 | 2014 | 2015 | 2016 | 2014 | 2015 | 2016 | 2014 | 2015 | 2016 |
| N rate | 0.08 | −0.19 | −0.06 | −0.02 | 0.70 *** | 0.74 *** | 0.59 ** | 0.47 * | 0.72 *** | 0.87 **** | 0.90 **** | 0.87 **** |
| Grain yield y | −0.04 | −0.27 | −0.16 | 0.02 | 0.78 **** | 0.68 *** | 0.74 *** | 0.56 ** | 0.77 **** | 0.83 **** | 0.82 **** | 0.90 **** |
| Ear dry wt | 0.12 | −0.17 | −0.17 | −0.3 | 0.60 ** | 0.76 *** | 0.65 ** | 0.47 * | 0.62 ** | 0.66 ** | 0.62 ** | 0.84 **** |
| Grain dry wt | 0.11 | −0.18 | −0.17 | −0.23 | 0.59 ** | 0.76 *** | 0.65 ** | 0.46 * | 0.61 ** | 0.66 ** | 0.62 ** | 0.84 **** |
| Stover dry wt | 0.25 | −0.18 | −0.14 | −0.39 | 0.54 * | 0.62 ** | 0.56 * | 0.43 | 0.42 | 0.48 * | 0.54 * | 0.62 ** |
| Grain N% | 0.06 | 0.19 | 0.07 | −0.22 | 0.27 | 0.69 *** | 0.58 ** | 0.36 | 0.64 ** | 0.67 ** | 0.21 | 0.68 *** |
| Grain NC | −0.01 | −0.2 | −0.1 | −0.07 | 0.76 **** | 0.74**** | 0.71 *** | 0.59 ** | 0.77 **** | 0.82 **** | 0.77 **** | 0.88 **** |
| Stover N% | −0.45 | 0.4 | 0.25 | 0.23 | 0.06 | 0.33 | 0.42 | 0.03 | 0.60 ** | 0.4 | 0.24 | 0.58 ** |
| Stover NC | −0.17 | 0.26 | 0.05 | −0.1 | 0.54 * | 0.47 * | 0.64 ** | 0.39 | 0.71 ** | 0.61 ** | 0.66 ** | 0.73 *** |
| HI | −0.01 | −0.28 | −0.15 | −0.21 | 0.50 * | 0.64 ** | 0.68 ** | 0.33 | 0.62 ** | 0.71 *** | 0.58 ** | 0.84 **** |
| NHI | −0.04 | −0.4 | −0.22 | 0.13 | 0.25 | 0.75 *** | 0.65 ** | 0.21 | 0.55 * | 0.67 ** | 0.12 | 0.76 *** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goron, T.; Nederend, J.; Stewart, G.; Deen, B.; Raizada, M. Mid-Season Leaf Glutamine Predicts End-Season Maize Grain Yield and Nitrogen Content in Response to Nitrogen Fertilization under Field Conditions. Agronomy 2017, 7, 41. https://doi.org/10.3390/agronomy7020041
Goron T, Nederend J, Stewart G, Deen B, Raizada M. Mid-Season Leaf Glutamine Predicts End-Season Maize Grain Yield and Nitrogen Content in Response to Nitrogen Fertilization under Field Conditions. Agronomy. 2017; 7(2):41. https://doi.org/10.3390/agronomy7020041
Chicago/Turabian StyleGoron, Travis, Jacob Nederend, Greg Stewart, Bill Deen, and Manish Raizada. 2017. "Mid-Season Leaf Glutamine Predicts End-Season Maize Grain Yield and Nitrogen Content in Response to Nitrogen Fertilization under Field Conditions" Agronomy 7, no. 2: 41. https://doi.org/10.3390/agronomy7020041





