Detection and Response of Sugarcane against the Infection of Sugarcane Mosaic Virus (SCMV) in Indonesia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sugarcane Leaf Samples, Disease Assessment, and Plant Inoculation
2.2. Total Plant RNA Extraction and Reverse Transcriptase Polymerase Chain Reaction
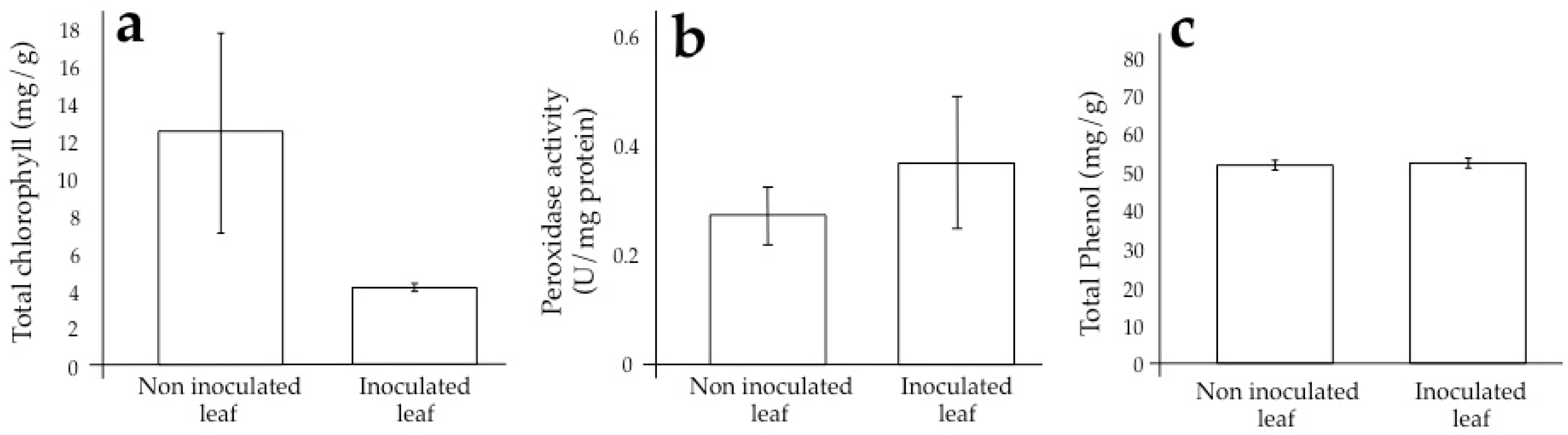
2.3. Estimation of Total Chlorophyll, Phenol, and Peroxidase Activity
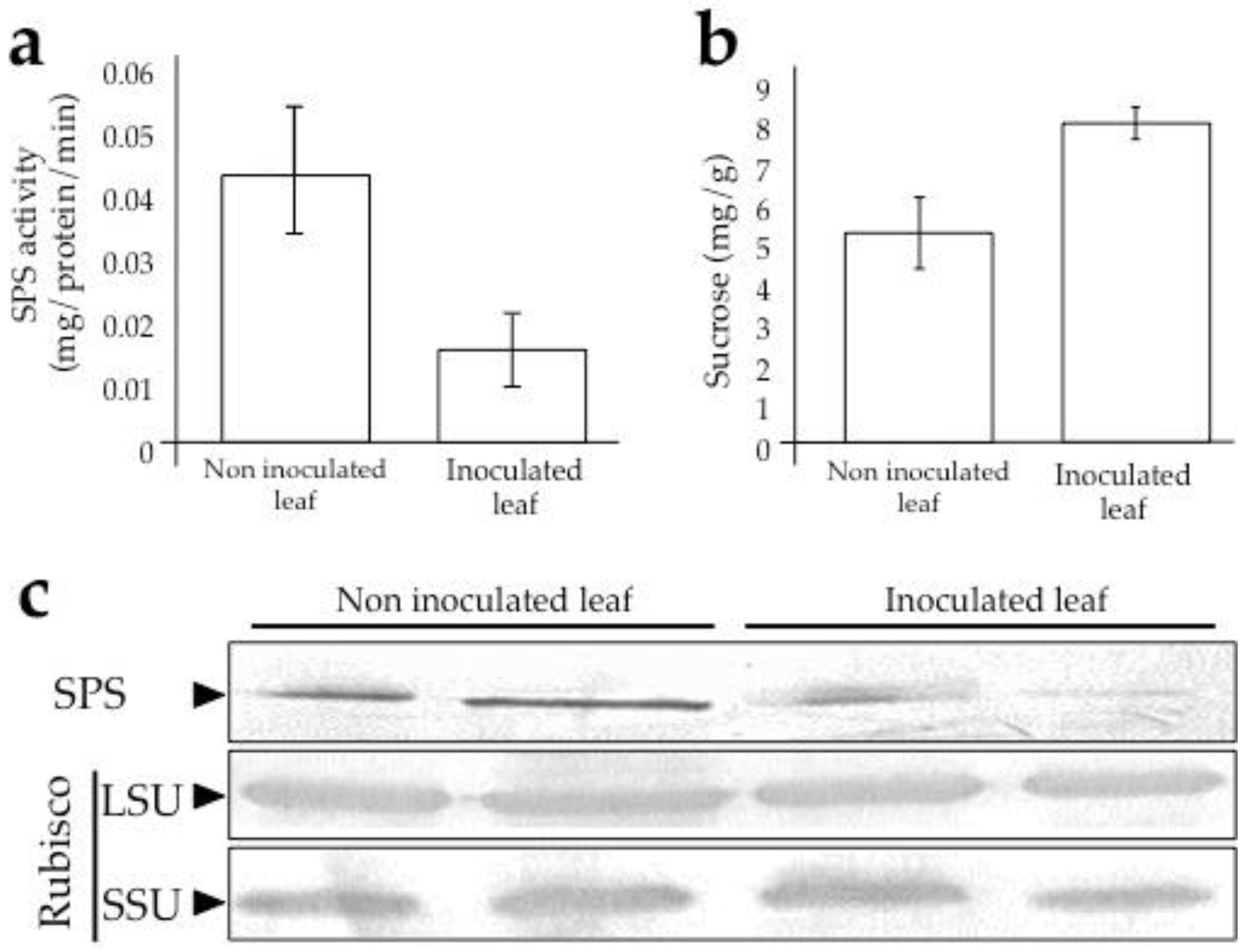
2.4. Analysis of Sucrose Phosphate Synthase, Rubisco, and Sucrose Accumulation in Leaves
3. Results
3.1. Mosaic Disease Incidence, Severity, Symptom Development, and Its Pathogen
3.2. Sugarcane Response and Its Alteration During Infection by SCMV
4. Discussion
5. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Putra, L.K.; Kristini, A.; Achadian, E.M.; Damayanti, T.A. Sugarcane streak mosaic virus in Indonesia: Distribution, characterization, yield losses and management approaches. Sugar Tech 2014, 16, 392–399. [Google Scholar] [CrossRef]
- Rao, G.R.; Chatenet, M.; Girard, J.G.; Rott, P. Distribution of Sugarcane mosaic and Sugarcane streak mosaic virus in India. Sugar Tech 2006, 8, 79–81. [Google Scholar] [CrossRef]
- Yahaya, A.; Dangora, D.B.; Khan, A.U.; Zangoma, M.A. Detection of Sugarcane mosaic disease (SCMV) in crops and weeds associated with sugarcane fields in Makarfi and Sabon Gari local government areas of Kaduna state, Nigeria. Int. J. Curr. Sci. 2014, 11, 99–104. [Google Scholar]
- Xu, D.L.; Park, J.W.; Mikrov, T.E.; Zhou, G.H. Viruses causing mosaic disease in sugarcane and their genetic diversity in Southern China. Arch. Virol. 2008, 153, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Ahmad, K.; Fu, H.Y.; Wang, J.D.; Chen, R.K.; Gao, S.J. Genetic diversity and population structure of Sorghum mosaic virus infecting Saccharum spp. hybrids. Ann. Appl. Biol. 2016, 169, 398–407. [Google Scholar] [CrossRef]
- Perera, M.F.; Filippone, M.P.; Ramallo, C.J.; Cuenya, M.I.; García, M.L.; Ploper, L.D.; Castagnaro, A.P. Genetic diversity among viruses associated with sugarcane mosaic disease in Tucumán, Argentina. Phytopathology 2009, 99, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Mollov, D.; Tahir, M.N.; Wei, C.; Kaye, C.; Lockhart, B.; Comstock, J.C.; Rott, P. First report of Sugarcane mosaic virus infecting Columbus grass (Sorghum almum) in the United States. Plant Dis. 2016, 100, 1510. [Google Scholar] [CrossRef]
- Puchades, Y.; O, M.L.; Montalvan, J.; Carvajal, O.; Martinez, Y.; Zardon, M.A.; Mesa, J.M.; Lossbrant, S.; Arencibia, A.D. Genetic and symptomatic characterization of Sugarcane mosaic virus (SCMV) in Cuba. Sugar Tech 2016, 18, 184–191. [Google Scholar] [CrossRef]
- Arif, M.; Ali, M.; Rehman, A.; Fahim, M. Detection of Potato mop-top virus in soils and potato tubers using bait-plant bioassay, ELISA and RT-PCR. J. Virol. Methods 2014, 195, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.V.S.; Sreenivasulu, P.; Sekhar, G. Duplex-immunocapture-RT-PCR for detection and discrimination of two distinct potyviruses naturally infecting sugarcane (Saccharum spp. hybrid). Ind. J. Exp. Biol. 2011, 49, 68–73. [Google Scholar]
- Jiang, J.X.; Chen, Z.X.; Zhou, X.P. Production of a monoclonal antibody to sugarcane mosaic virus and its application for virus detection in China. J. Phytopathol. 2003, 151, 361–364. [Google Scholar] [CrossRef]
- Urcuqui-Inchima, S.; Haenni, A.L.; Bernardi, F. Potyvirus proteins: A wealth of functions. Virus Res. 2001, 74, 157–175. [Google Scholar] [CrossRef]
- Moradi, Z.; Mehrvar, M.; Nazifi, E.; Zakiaghl, M. The complete genome sequences of two naturally occurring recombinant isolates of Sugarcane mosaic virus from Iran. Virus Genes 2016, 52, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Shalitin, D.; Wolf, S. Cucumber mosaic virus infection affects sugar transport in melon plants. Plant Physiol. 2000, 123, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhang, C.; Hong, J.; Xiong, R.; Kasschau, K.D.; Zhou, X.; Carrington, J.C.; Wang, A. Formation of complexes at plasmodesmata for potyvirus intercellular movement is mediated by the viral protein P3N-PIPO. PLoS Pathog. 2010, 6, e1000962. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.A.; Davis, J.A.; Abad, J.A.; Cuellar, W.J.; Fuentes, S.; Kreuze, J.F.; Gibson, R.W.; Mukasa, S.B.; Tugume, A.K.; Tairo, F.D.; et al. Sweetpotato viruses: 15 years of progress of understanding and managing complex diseases. Plant Dis. 2012, 96, 168–185. [Google Scholar] [CrossRef]
- Amiard, V.; Mueh, K.E.; Demmig-Adams, B.; Ebbert, V.; Turgeon, R.; Adams, W.W. Anatomical and photosynthetic acclimation to the light environment in species with differing mechanisms of phloem loading. Proc. Natl. Acad. Sci. USA 2005, 102, 12968–12973. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Guo, R.; Jie, F.; Nettleton, D.; Peng, J.; Carr, T.; Yeakley, J.M.; Fan, J.B.; Whitham, S.A. Spatial analysis of Arabidopsis thaliana gene expression in response to Turnip mosaic virus infection. Mol. Plant Microbe Interact. 2007, 20, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Putra, L.K.; Ogle, H.J.; James, A.P.; Whittle, P.J.L. Distribution of Sugarcane mosaic virus in sugarcane plants. Australasian Plant Pathol. 2003, 32, 305–307. [Google Scholar] [CrossRef]
- Fu, W.L.; Sun, S.R.; Fu, H.Y.; Chen, R.K.; Su, J.W.; Gao, S.J. A one-step real-time RT-PCR assay for the detection and quantitation of Sugarcane streak mosaic virus. Biomed. Res. Int. 2015, 2015, 569131. [Google Scholar] [CrossRef] [PubMed]
- Molazem, D.; Qurbanov, E.M.; Dunyamaliyev, S.A. Role of proline, Na and chlorophyll content in salt tolerance of corn (Zea mays) L. American-Eurasian J. Agric. Environ. Sci. 2010, 9, 319–324. [Google Scholar]
- Chlopicka, J.; Pasko, P.; Gorinstein, S.; Jedryas, A.; Zagrodzki, P. Total phenolic and total flavonoid content, antioxidant activity and sensory evaluation of pseudocereal breads. LWT– Food Sci. Tech. 2012, 46, 548–555. [Google Scholar] [CrossRef]
- Sawitri, W.D.; Narita, H.; Ishizaka-Ikeda, E.; Sugiharto, B.; Hase, T.; Nakagawa, A. Purification and characterization of recombinant sugarcane sucrose phosphate synthase expressed in E. coli and insect Sf9 cells: An importance of the N-terminal domain for an allosteric regulatory property. J. Biochem. 2016, 159, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zu, X.; Wang, S.; Chen, Y. Sugarcane mosaic virus—Long history but still a threat to industry. Crop Prot. 2012, 42, 74–78. [Google Scholar] [CrossRef]
- Gan, D.; Zhang, J.; Jiang, H.; Jiang, T.; Zhu, S.; Cheng, B. Bacterially expressed dsRNA protects maize against SCMV infection. Plant Cell Rep. 2010, 29, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Bedoya, G.; Espejel, F.; Alcalá-Briseño, R.I.; Hernández-Vela, J.; Silva-Rosales, L. Short distance movement of genomic negative strands in a host and nonhost for Sugarcane mosaic virus (SCMV). Virol. J. 2011, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Gemechu, A.L.; Chiemsombat, P.; Attathom, S.; Reanwarakorn, K.; Lersrutaiyotin, R. Cloning and sequence analysis of coat protein gene for characterization of Sugarcane mosaic virus isolated from sugarcane and maize in Thailand. Arch. Virol. 2006, 151, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Chen, W.; Fu, Q.; Zhang, P.; An, T.; Cui, A.; An, D. Molecular variability and distribution of Sugarcane mosaic virus in Shanxi, China. PLoS ONE 2016, 11, e0151549. [Google Scholar] [CrossRef] [PubMed]
- Keizerweerd, A.T.; Chandra, A.; Grisham, M.P. Development of a reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay for the detection of Sugarcane mosaic virus and Sorghum mosaic virus in sugarcane. J. Virol. Methods 2015, 212, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Grisham, M.P.; Pan, Y.B. A genetic shift in the virus strains that cause mosaic in Louisiana sugarcane. Plant Dis. 2007, 91, 453–458. [Google Scholar] [CrossRef]
- Tang, W.; Xu, X.H.; Sun, H.W.; Li, F.; Gao, R.; Yang, S.K.; Lu, X.B.; Li, X.D. First report of Sugarcane mosaic virus infecting Canna spp. in China. Plant Dis. 2016, 100, 2541. [Google Scholar] [CrossRef]
- Hull, R. Comparative Plant Virology, 2nd ed.; Elsevier Academic Press: British, UK, 2009. [Google Scholar]
- Zhao, J.; Zhang, X.; Hong, Y.; Liu, Y. Chloroplast in plant-virus interaction. Front Microbiol. 2016, 7, 1565. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.U.; Jyothsna, Y. Biochemical and enzymatic changes in rice as a mechanism of defense. Acta Physiol. Plant. 2010, 32, 695–701. [Google Scholar] [CrossRef]
- Passardi, F.; Penel, C.; Dunand, C. Performing the paradoxical: How plant peroxidases modify the cell wall. Trends Plant Sci. 2004, 9, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, K.S.; Josh, R.D.; Srivastava, G.P. Catalase and peroxidase activity in sugarcane infected with Sugarcane mosaic virus. Experientia 1970, 26, 216–217. [Google Scholar] [CrossRef] [PubMed]
- Catoni, M.; Miozzi, L.; Fiorilli, V.; Lanfranco, L.; Accotto, G.P. Comparative analysis of expression profiles in shoots and roots of tomato systemically infected by Tomato spotted wilt virus reveals organ-specific transcriptional responses. Mol. Plant Microbe Interact. 2009, 33, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hajirezaei, M.; Bornke, F. Differential expression of sucrose-phosphate synthase isoenzymes in tobacco reflects their functional specialization during dark-governed starch mobilization in source leaves. Plant Physiol. 2005, 139, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Lunn, J.E.; Rees, T.A. Apparent equilibrium constant and mass-action ratio for sucrose-phosphate synthase in seeds of Pisum sativum. Biochem. J. 1990, 267, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Upadhyay, S.K.; Verma, P.C.; Solomon, S.; Singh, S.B. Functional analysis of sucrose phosphate synthase (SPS) and sucrose synthase (SS) in sugarcane (Saccharum) cultivars. Plant Biol. 2011, 13, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Salerno, G.L.; Pontis, H.G. Studies on sucrose phosphate synthetase: The inhibitory action of sucrose. FEBS Lett. 1978, 86, 263–267. [Google Scholar] [CrossRef]
- Reimholz, R.; Geigenberger, P.; Stitt, M. Sucrose-phosphate synthase is regulated via metabolites and protein phosphorylation in potato tubers, in a manner analogous to the enzyme in leaves. Planta 1994, 192, 480–488. [Google Scholar] [CrossRef]
- Schulz, A.; Beyhl, D.; Marten, I.; Wormit, A.; Neuhaus, E.; Poschet, G.; Büttner, M.; Schneider, S.; Sauer, N.; Hedrich, R. Proton-driven sucrose symport and antiport are provided by the vacuolar transporters SUC4 and TMT1/2. Plant J. 2011, 68, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Hofius, D.; Herbers, K.; Melzer, M.; Omid, A.; Tacke, E.; Wolf, S.; Sonnewald, U. Evidence for expression level-dependent modulation of carbohydrate status and viral resistance by the Potato leafroll virus movement protein in transgenic tobacco plants. Plant J. 2001, 28, 529–543. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Addy, H.S.; Nurmalasari; Wahyudi, A.H.S.; Sholeh, A.; Anugrah, C.; Iriyanto, F.E.S.; Darmanto, W.; Sugiharto, B. Detection and Response of Sugarcane against the Infection of Sugarcane Mosaic Virus (SCMV) in Indonesia. Agronomy 2017, 7, 50. https://doi.org/10.3390/agronomy7030050
Addy HS, Nurmalasari, Wahyudi AHS, Sholeh A, Anugrah C, Iriyanto FES, Darmanto W, Sugiharto B. Detection and Response of Sugarcane against the Infection of Sugarcane Mosaic Virus (SCMV) in Indonesia. Agronomy. 2017; 7(3):50. https://doi.org/10.3390/agronomy7030050
Chicago/Turabian StyleAddy, Hardian Susilo, Nurmalasari, Agus Heri Setyo Wahyudi, Ahmil Sholeh, Cahya Anugrah, Febrian Eka Shandy Iriyanto, Win Darmanto, and Bambang Sugiharto. 2017. "Detection and Response of Sugarcane against the Infection of Sugarcane Mosaic Virus (SCMV) in Indonesia" Agronomy 7, no. 3: 50. https://doi.org/10.3390/agronomy7030050





