Volatile Semiochemical Mediated Plant Defense in Cereals: A Novel Strategy for Crop Protection
Abstract
:1. Introduction
2. Plant Volatile Semiochemicals
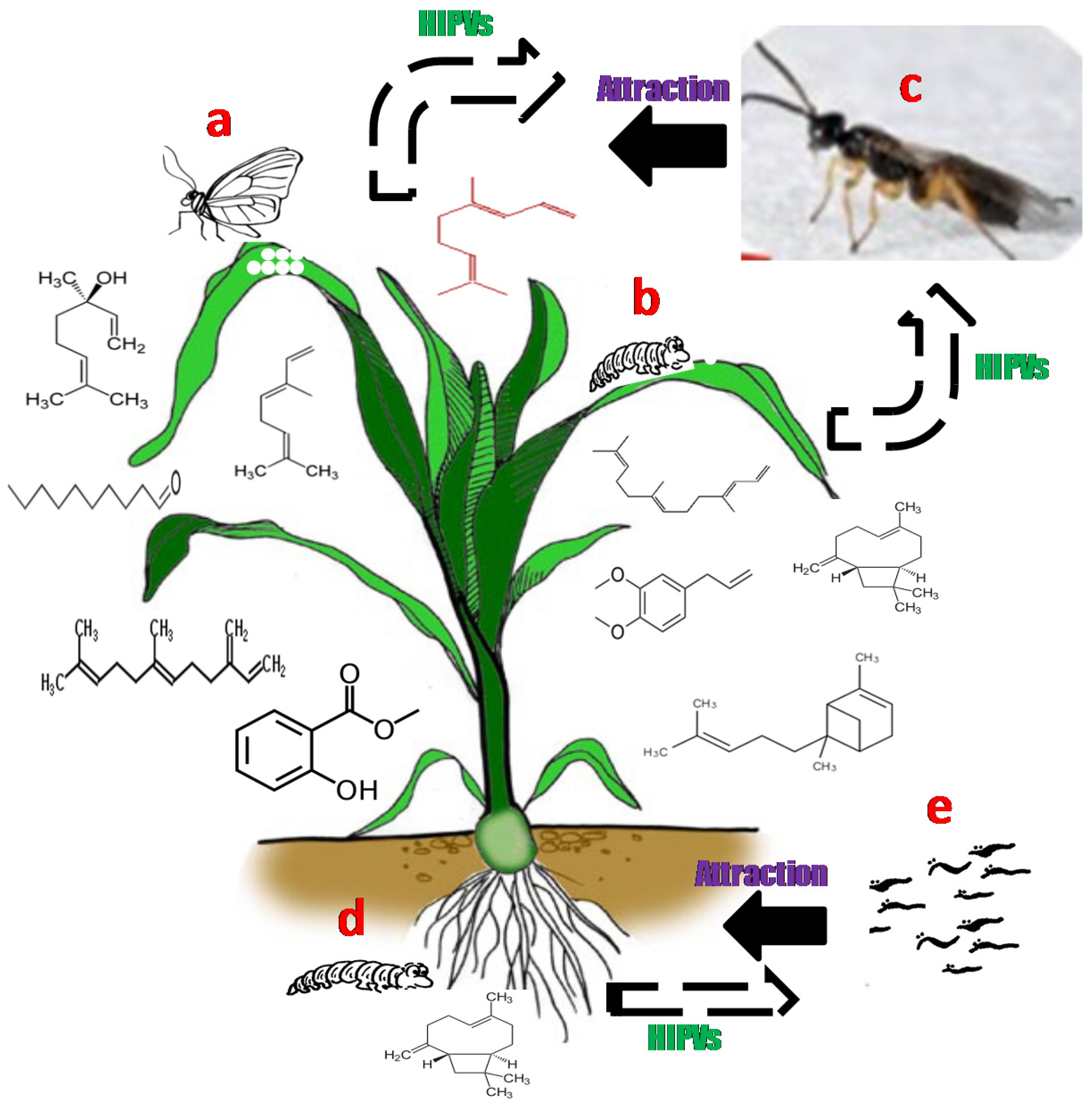
3. Herbivore Induced Plant Volatile Compounds (HIPVs)
4. Role of Induced and Constitutive Volatiles in Plant Defence
5. Genetic Engineering
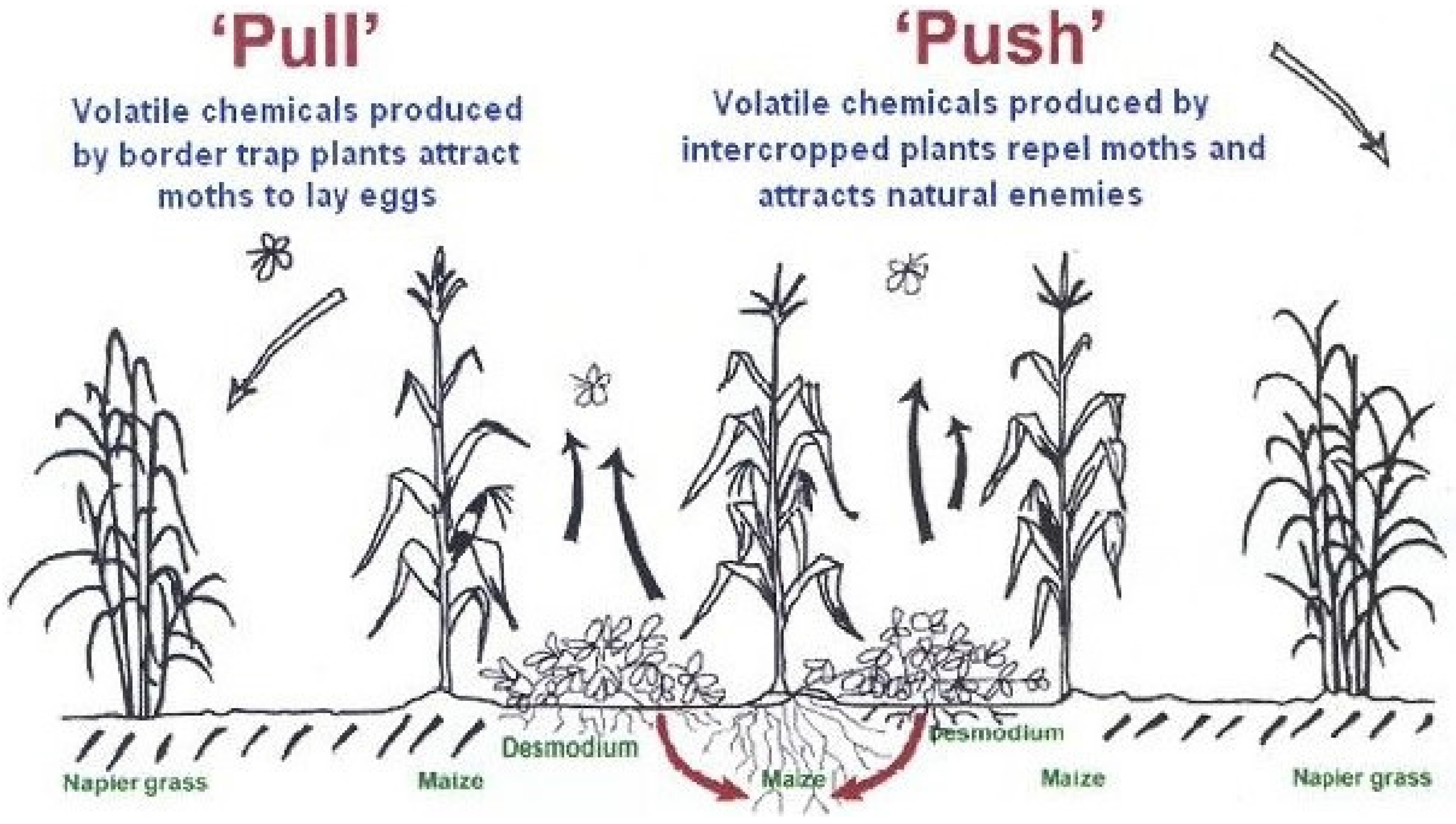
6. Stimulo-Deterrent Diversionary Strategy
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Law, J.H.; Regnier, F.E. Pheronomes. Annu. Rev. Biochem. 1971, 40, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of Push-Pull strategies in integrated pest management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Kost, C.; Heil, M. Herbivore-induced plant volatiles induce an indirect defence in neighbouring plants. J. Ecol. 2006, 94, 619–628. [Google Scholar] [CrossRef]
- Dicke, M.; van Loon, J.J.A.; Soler, R. Chemical complexity of volatiles from plants induced by multiple attack. Nat. Chem. Biol. 2009, 5, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, J. Indirect defence responses to herbivory in grasses. Plant Physiol. 2009, 149, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Büchel, K.; Malskies, S.; Mayer, M.; Fenning, T.M.; Gershenzon, J.; Hilker, M.; Meiners, T. How plants give early herbivore alert: Volatile terpenoids attract parasitoids to egg-infested elms. Basic Appl. Ecol. 2011, 12, 403–412. [Google Scholar] [CrossRef]
- De Moraes, C.M.; Mescher, M.C.; Tumlinson, J.H. Caterpillar-induced nocturnal plant volatiles repel nonspecific females. Nature 2001, 410, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Pickett, J.A.; Khan, Z.R. Plant volatile-mediated signalling and its application in agriculture: Successes and challenges. New Phytol. 2016, 212, 856–870. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, J.; Hiltpold, I.; Köllner, T.G.; Frey, M.; Gierl, A.; Gershenzon, J.; Hibbard, B.E.; Ellersieck, M.R.; Turlings, T.C.J. Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proc. Natl. Acad. Sci. USA. 2009, 32, 13213–13218. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.R.; Midega, C.A.O.; Hooper, A.M.; Pickett, J.A. Push-Pull: Chemical Ecology-Based Integrated Pest Management Technology. J. Chem. Ecol. 2016, 42, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.A. Tackling the threat to food security caused by crop pests in the new millennium. Food Secur. 2010, 2, 133–141. [Google Scholar] [CrossRef]
- Tamiru, A.; Bruce, T.J.A.; Woodcock, C.M.; Caulfield, J.C.; Midega, C.A.O.; Ogol, C.K.P.O.; Mayon, P.; Birkett, M.A.; Pickett, J.A.; Khan, Z.R. Maize landraces recruit egg and larval parasitoids in response to egg deposition by a herbivore. Ecol. Lett. 2011, 14, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatile: Nature’s diversity and ingenuity. Science 2006, 311, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Stahl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1–120. [Google Scholar] [CrossRef]
- Arimura, G.; Matsui, K.; Takabayashi, J. Chemical and molecular ecology of herbivore-induced plant volatiles: Proximate factors and their ultimate functions. Plant Cell Physiol. 2009, 50, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Tamiru, A.; Bruce, T.J.A.; Woodcock, C.M.; Birkett, M.A.; Midega, C.A.O.; Pickett, J.A.; Khan, Z.R. Chemical cues modulating electrophysiological and behavioral responses in the parasitic wasp Cotesia sesamiae. Can. J. Zool. 2015, 93, 281–287. [Google Scholar] [CrossRef]
- Heil, M. Herbivore-induced plant volatiles: Targets, perception and unanswered questions. New Phytol. 2014, 204, 297–306. [Google Scholar] [CrossRef]
- Rasmann, S.; Köllner, T.G.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T.C.J. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Tamiru, A.; Bruce, T.J.A.; Richter, A.; Woodcock, C.M.; Midega, C.A.O.; Degenhardt, J.; Kelemu, S.; Pickett, J.A.; Khan, Z.R. A maize landrace that emits defense volatiles in response to herbivore eggs possesses a strongly inducible terpene synthase gene. Ecol. Evol. 2017, 7, 2835–2845. [Google Scholar] [CrossRef] [PubMed]
- Hilker, M.; Meiners, T. Early herbivore alert: Insect eggs induce plant defense. J. Chem. Ecol. 2006, 32, 1379–1397. [Google Scholar] [CrossRef] [PubMed]
- Heil, M. Indirect defence via tritrophic interactions. New Phytol. 2008, 178, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.D. Ecological role of volatiles produced by plants in response to damage by herbivorous insects. Ann. Rev. Entomol. 2011, 56, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Sanchez-Moreiras, A.M.; Abel, C.; Sohrabi, R.; Lee, S.; Gershenzon, J.; Tholl, D. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-β-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 2012, 193, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, I.T.; Halitschke, R.; Paschold, A.; von Dahl, C.C.; Catherine, A.; Preston, C.A. Volatile Signaling in Plant-Plant Interactions: “Talking Trees” in the Genomics Era. Science 2006, 311, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Tamiru, A.; Bruce, T.J.A.; Midega, C.A.O.; Woodcock, C.M.; Birkett, M.A.; Pickett, J.A.; Khan, Z.R. Oviposition induced volatile emissions from African smallholder farmers’ maize varieties. J. Chem. Ecol. 2012, 38, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Tamiru, A.; Khan, Z.R.; Bruce, T.J.A. New directions for improving crop resistance to insects by breeding for egg induced defence. Curr. Opin. Insect Sci. 2015, 9, 51–55. [Google Scholar] [CrossRef]
- Glinwood, R.; Ahmed, E.; Qvarfordt, E.; Ninkovic, V.; Pettersson, J. Airborne interactions between undamaged plants of different cultivars affect insect herbivores and natural enemies. Arth. Plant Int. 2009, 3, 215–224. [Google Scholar] [CrossRef]
- Khan, Z.R.; Midega, C.A.O.; Bruce, T.J.A.; Hooper, A.M.; Pickett, J.A. Exploiting phytochemicals for developing a ‘push-pull’ crop protection strategy for cereal farmers in Africa. J. Exp. Bot. 2010, 61, 4185–4196. [Google Scholar] [CrossRef] [PubMed]
- Unsicker, S.B.; Kunert, G.; Gershenzon, J. Protective perfumes: The role of vegetative volatiles in plant defense against herbivores. Curr. Opin. Plant Biol. 2009, 12, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Mumm, R.; Dicke, M. Variation in natural plant products and the attraction of bodyguards involved in indirect plant defense. Can. J. Zool. 2010, 88, 628–667. [Google Scholar] [CrossRef]
- Åhman, I.; Glinwood, R.; Ninkovic, V. The potential for modifying plant volatile composition to enhance resistance to arthropod pests. CAB Rev. 2010, 5, 1–10. [Google Scholar] [CrossRef]
- Kappers, I.F.; Aharoni, A.; van Herpen, T.W.J.M.; Luckerhoff, L.L.P.; Dicke, M.; Bouwmeester, H.J. Genetic engineering of terpenoid metabolism attracts, bodyguards to Arabidopsis. Science 2005, 309, 2070–2072. [Google Scholar] [CrossRef] [PubMed]
- Schnee, C.; Köllner, T.G.; Held, M.; Turlings, T.C.J.; Gershenzon, J.; Degenhardt, J. The products of a single maize sesquiterpene synthase form a volatile defence signal that attracts natural enemies of maize herbivores. Proc. Natl. Acad. Sci. USA 2006, 103, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Beale, M.H.; Birkett, M.A.; Bruce, T.J.A.; Chamberlain, K.; Field, L.M.; Huttly, A.K.; Martin, J.L.; Parker, R.; Phillips, A.L.; Pickett, J.A.; et al. Aphid alarm pheromone produced by transgenic plants affects aphid and parasitoid behavior. Proc. Natl. Acad. Sci. USA 2006, 103, 10509–10513. [Google Scholar] [CrossRef] [PubMed]
- Kos, M.; van Loon, J.J.A.; Dicke, M.; Vet, L.E.M. Transgenic plants as vital components of integrated pest management. Trends Biotechnol. 2009, 27, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.A. GM as a route for delivery of sustainable crop protection. J. Exp. Bot. 2012, 63, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, B.; Hiltpold, I.; Turlings, T.C.J.; Kuhlmann, U.; Toepfer, S. Comparative susceptibility of larval instars and pupae of the western corn rootworm to infection by three entomopathogenic nematodes. Biocontrol 2009, 54, 255–262. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Aradottir, G.I.; Smart, L.E.; Martin, J.L.; Caulfield, J.C.; Doherty, A.; Sparks, C.A.; Woodcock, C.M.; Birkett, M.A.; Naipier, J.A.; et al. The first crop plant genetically engineered to release an insect pheromone for defence. Sci. Rep. 2015, 5, 118–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konermann, S.; Brigham, M.D.; Trevino, A.E.; Joung, J.; Abudayyeh, O.O.; Barcena, C.; Hsu, P.D.; Habib, N.; Gootenberg, J.S.; Nishimasu, H.; et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 2014, 517, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Voytas, D.F.; Gao, C. Precision genome engineering and agriculture: Opportunities and regulatory challenges. PLoS Biol. 2014, 12, e1001877. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.R.; Ampong-Nyarko, K.; Chilishwa, P.; Hassanali, A.; Kimani, S.; Lwande, W.; Overholt, W.A.; Pickett, J.A.; Smart, L.E.; Wadhams, L.J.; et al. Intercropping increases parasitism of pests. Nature 1997, 388, 631–632. [Google Scholar] [CrossRef]
- Khan, Z.R.; Pickett, J.A.; Van Den Berg, J.; Wadhams, L.J.; Woodcock, C.M. Exploiting chemical ecology and species diversity: Stemborer and Striga control for maize and sorghum in Africa. Pest Manag. Sci. 2000, 56, 957–962. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Wäckers, F. Recruitment of predators and parasitoids by herbivore-injured plants. In Advances in Insect Chemical Ecology; Carde, R.T., Millar, J.G., Eds.; Cambridge University Press: Cambridge, UK, 2004; pp. 21–75. [Google Scholar]
- A novel farming system for ending hunger and poverty in sub-Sanharan Africa. Available online: http://www.push-pull.net/works.shtml (accessed on 20 July 2017).
- Labandeira, C.C. A paleobiologic perspective on plant–insect interactions. Curr. Opin. Plant Biol. 2013, 16, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Moles, A.T.; Peco, B.; Wallis, I.R.; Foley, W.J.; Poore, A.G.; Seabloom, E.W.; Vesk, P.A.; Bisigato, A.J.; Cella-Pizarro, L.; Clark, C.J. Correlations between physical and chemical defences in plants: Tradeoffs, syndromes, or just many different ways to skin a herbivorous cat? New Phytol. 2013, 198, 252–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seifi, A.; Visser, R.G.F.; Bai, Y. How to effectively deploy plant resistances to pests and pathogens in crop breeding. Euphytica 2013, 190, 321–334. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamiru, A.; Khan, Z.R. Volatile Semiochemical Mediated Plant Defense in Cereals: A Novel Strategy for Crop Protection. Agronomy 2017, 7, 58. https://doi.org/10.3390/agronomy7030058
Tamiru A, Khan ZR. Volatile Semiochemical Mediated Plant Defense in Cereals: A Novel Strategy for Crop Protection. Agronomy. 2017; 7(3):58. https://doi.org/10.3390/agronomy7030058
Chicago/Turabian StyleTamiru, Amanuel, and Zeyaur R. Khan. 2017. "Volatile Semiochemical Mediated Plant Defense in Cereals: A Novel Strategy for Crop Protection" Agronomy 7, no. 3: 58. https://doi.org/10.3390/agronomy7030058





