Reducing Severity of Late Blight (Phytophthora infestans) and Improving Potato (Solanum tuberosum L.) Tuber Yield with Pre-Harvest Application of Calcium Nutrients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Growth Substrate
2.3. Experimental Design and Treatments
2.4. Data Collection
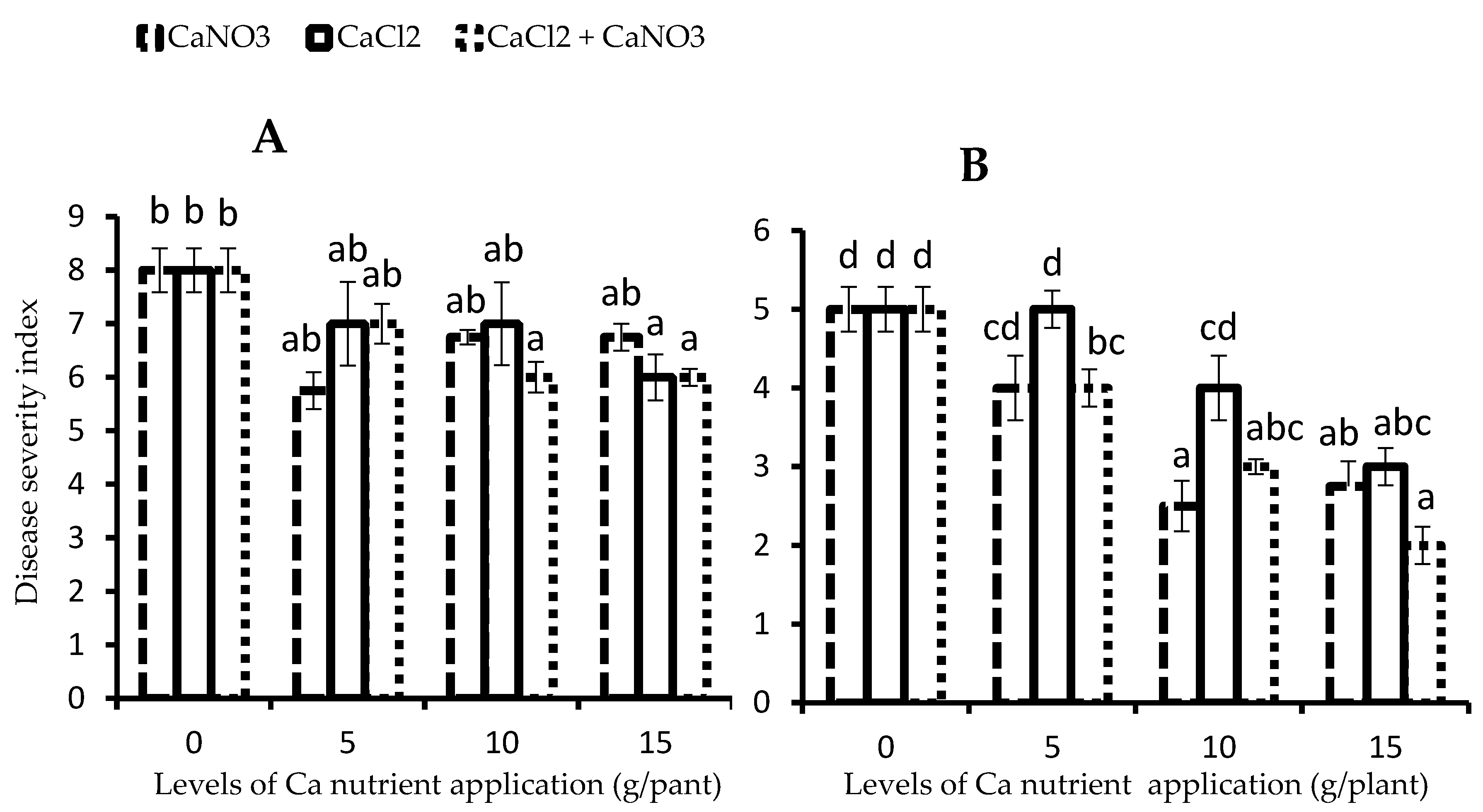
2.4.1. Severity of Late Blight Disease
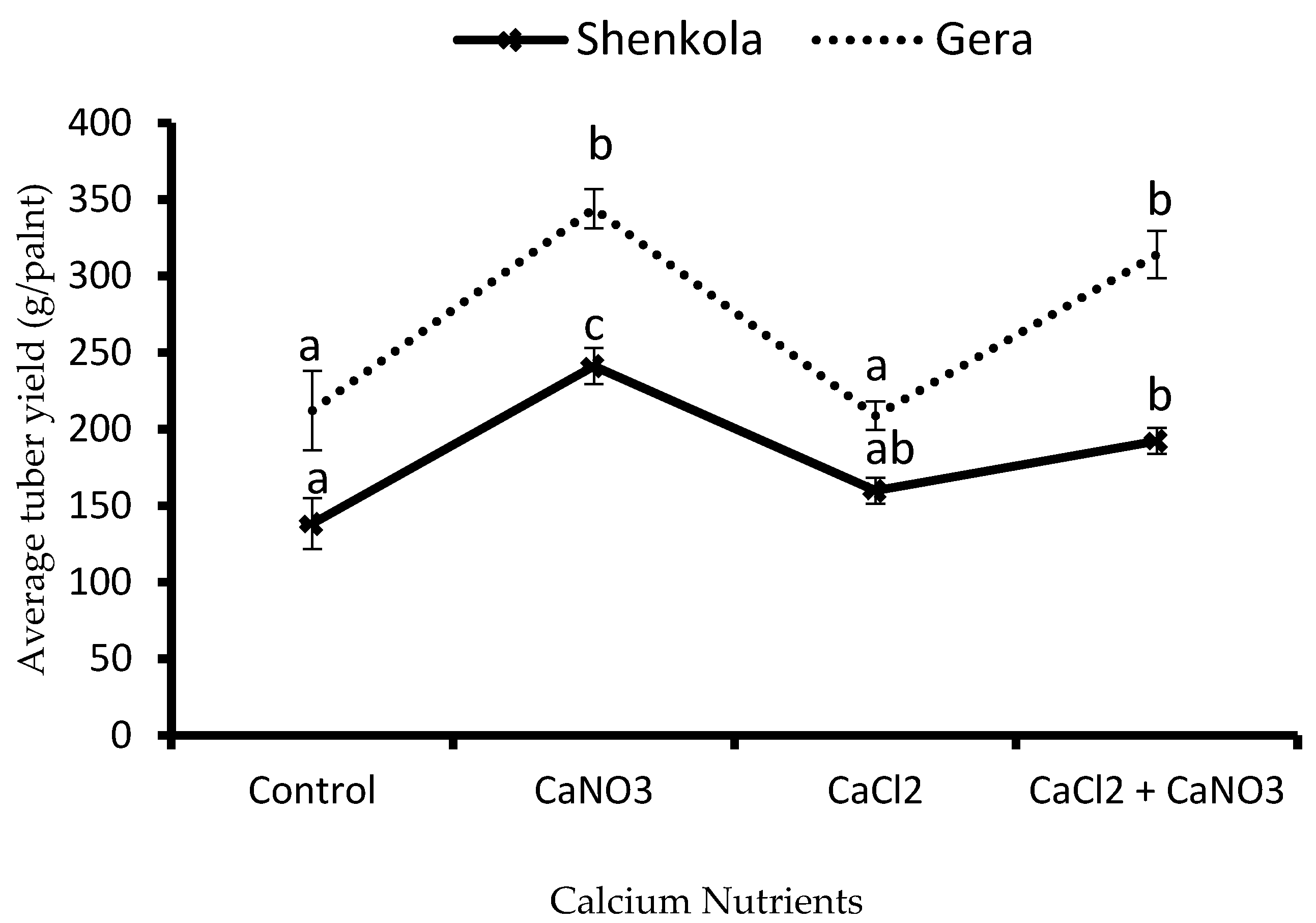
2.4.2. Tuber Yield
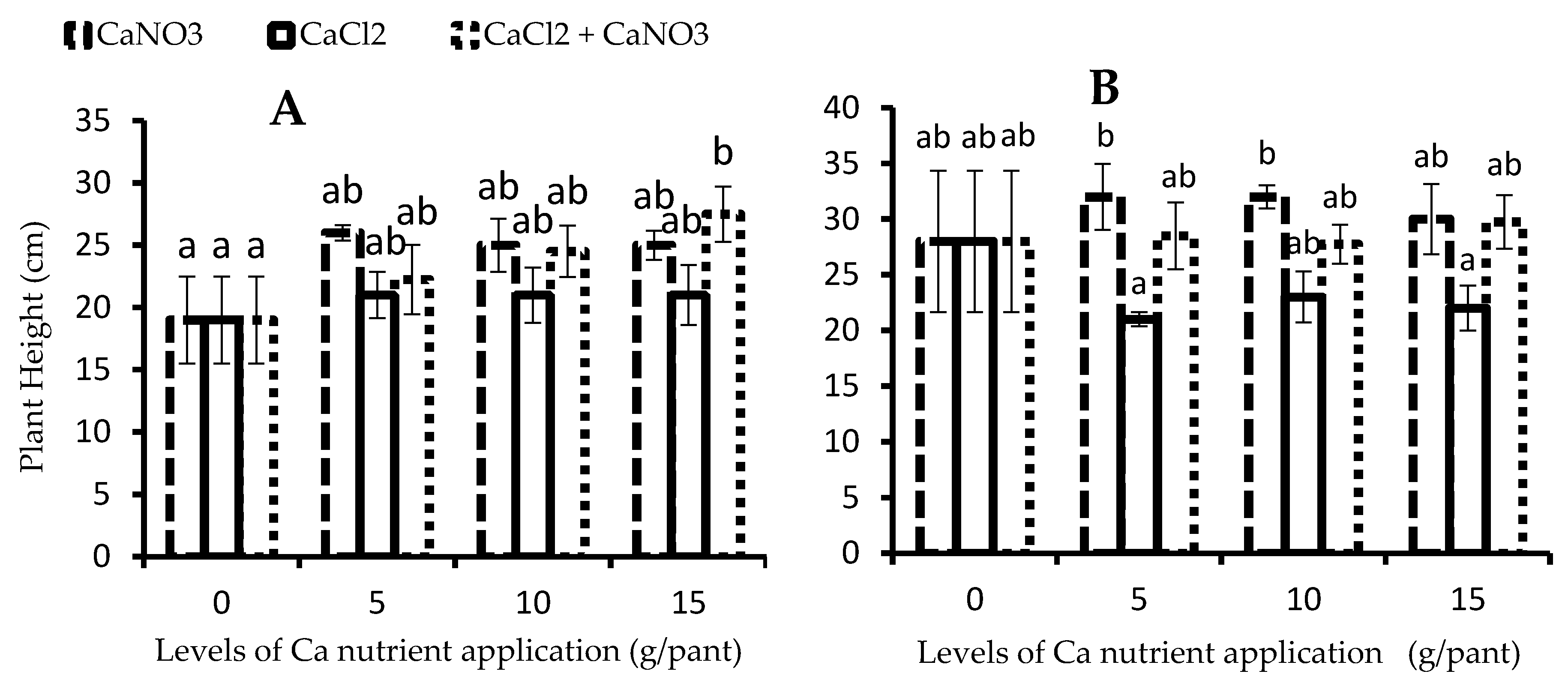
2.4.3. Plant Height, Number of Branches, and Number of Tubers
2.4.4. Tuber Tissue Analysis for the Determination of Calcium Content
2.5. Data Analysis
3. Results
3.1. Severity of Late Blight (P. infestans)
3.2. Tuber Yield
3.3. Plant Height
3.4. Number of Branches and Number of Tubers
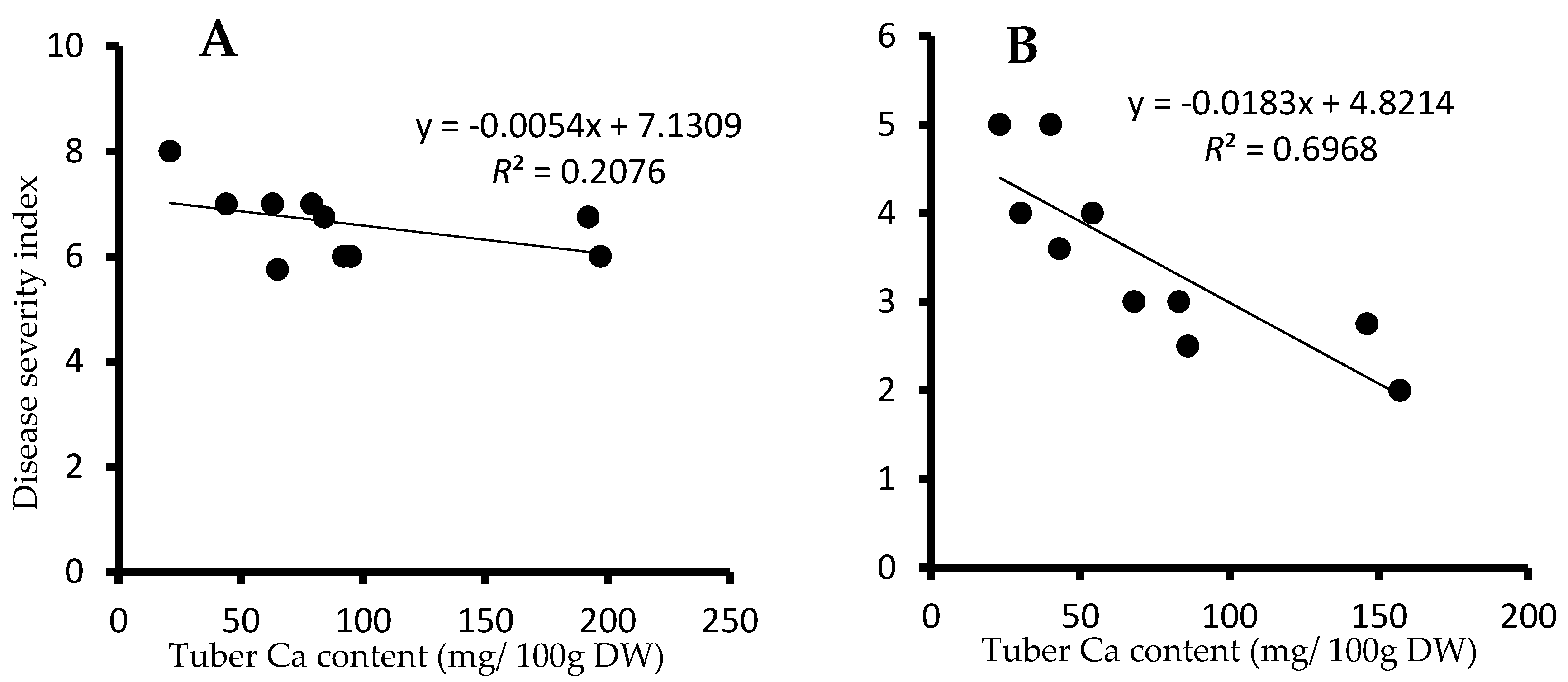
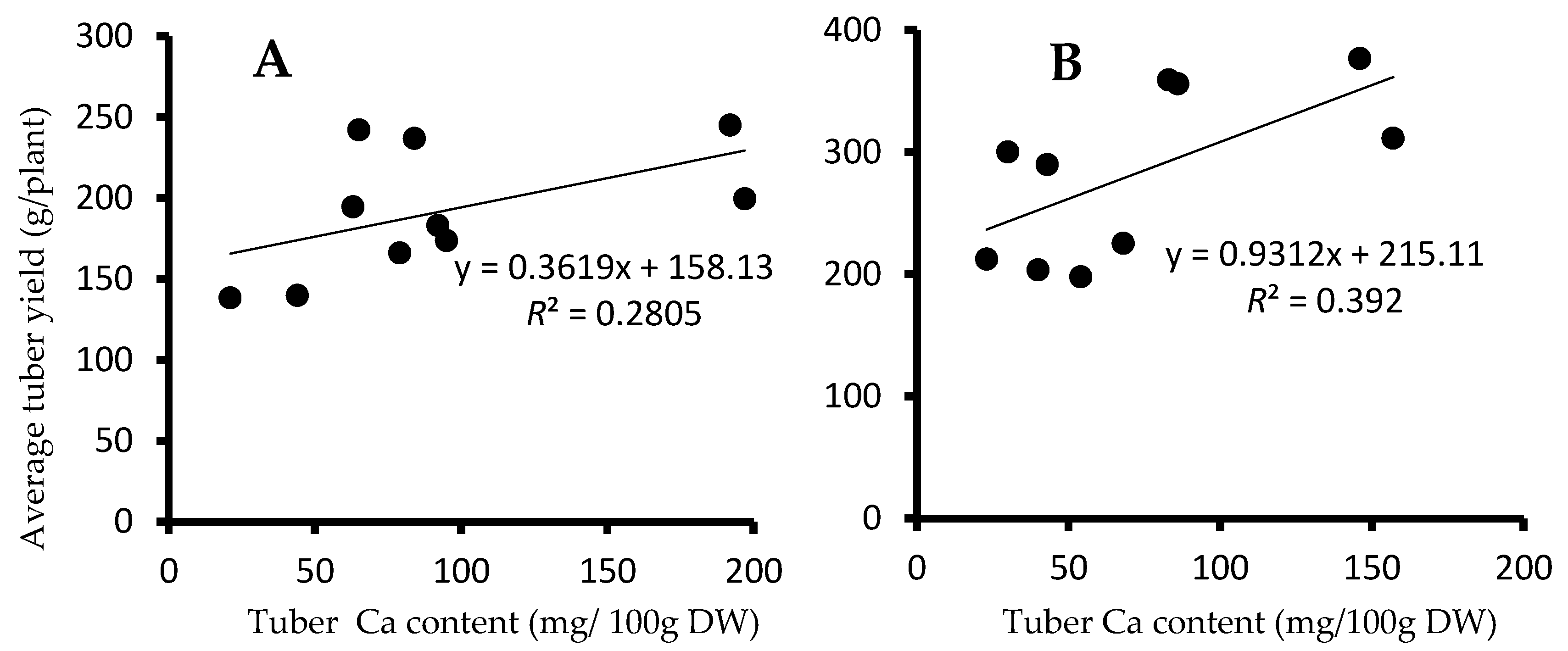
3.5. Correlation Study
4. Discussions
4.1. Severity of Late Blight Disease and Potato Tuber Yield
4.2. Plant Height, Number of Branches, and Number of Tubers
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Latijnhouwers, M.; Ligterink, W.; Vleeshouwers, V.G.; van West, P.; Govers, F.A. Galpha subunit controls zoospore motility and virulence in the potato late blight pathogen Phytophthora infestans. Mol. Microbiol. 2004, 51, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Tsedaley, B.; Hussen, T.; Tsegaw, T. Tuber yield loss assessment of potato (Solanum tuberosum L.) varieties due to late blight (Phytophthora infestans) and its management Haramaya, Eastern Ethiopia. J. Biol. Agric. Healthc. 2014, 4, 45–54. [Google Scholar]
- Judelson, H.S.; Blanco, F.A. The spores of Phytophthora: Weapons of the plant destroyer. Microbiology 2005, 3, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Turkensteen, L.J.; Flier, W.G.; Wanningen, R.; Mulder, A. Production, survival and infectivity of oospores of Phytophthora infestans. Plant Pathol. 2000, 49, 688–696. [Google Scholar] [CrossRef]
- Chindi, A.; Giorgis, G.W.; Solomon, A.; Tessama, L.; Negash, K. Rapid Multiplication Techniques (RMTs): A Tool for the Production of Quality Seed Potato (Solanum tuberosum L.) in Ethiopia. Asian J. Crop Sci. 2014, 6, 176–185. [Google Scholar] [CrossRef]
- Soylu, E.M.; Soylu, S.; Kurt, S. Antimicrobial activities of the essential oils of various plants against tomato late blight disease agent Phytophthora infestans. Mycopathologia 2006, 161, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Haverkort, A.J.; Struik, P.C.; Visser, R.G.F.; Jacobsen, E. Applied Biotechnology to Combat Late Blight in Potato Caused by Phytophthora Infestans. Potato Res. 2009, 52, 249–264. [Google Scholar] [CrossRef]
- Sanju, S.; Siddappa, S.; Thakur, A.; Shukla, P.K.; Srivastava, N.; Pattanayak, D.; Sharma, S.; Singh, B.P. Host-mediated gene silencing of a single effector gene from the potato pathogen Phytophthora infestans imparts partial resistance to late blight disease. Funct. Integr. Genom. 2015, 15, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Habtamu, K.; CHala, A.; Kassa, B.; Pananjay, G.B.G.; Tiwari, K. Evaluation of different potato variety and fungicide combinations for the management of potato late blight (Phytophthora infestans) in Southern Ethiopia. Int. J. Life Sci. 2012, 1, 8–15. [Google Scholar]
- Hussain, S.; Siddique, T.; Saleem, M.; Arshad, M.; Khalid, A. Impact of pesticides on soil microbial diversity, enzymes, and biochemical reactions. Adv. Agron. 2009, 102, 159–200. [Google Scholar]
- Aghofack-Nguemezi, J.; Noumbo, G.T.; Nkumbe, C.N. Influence of calcium and magnesium based fertilizers on fungal diseases, plant growth parameters and fruit quality of three varieties of tomato (Solanum lycopersicum). J. Sci. Technol. 2014, 34, 9–20. [Google Scholar] [CrossRef]
- Bain, R.A.; Millard, P.; Perombelon, M.C.M. The resistance of potato plants to Erwinia carotovora subsp. atroseptica in relation to their calcium and magnesium content. Potato Res. 1996, 39, 185–193. [Google Scholar] [CrossRef]
- Ippolito, A.; Schena, L.; Pentimone, I.; Nigro, F. Control of postharvest rots of sweet cherries by pre-and postharvest applications of Aureobasidium pullulans in combination with calcium chloride or sodium bicarbonate. Postharvest Biol. Technol. 2005, 36, 245–252. [Google Scholar] [CrossRef]
- Sugimoto, T.; Watanabe, K.; Yoshida, S.; Aino, M.; Irie, K.; Matoh, T.; Biggs, A.R. Select calcium compounds reduce the severity of Phytophthora stem rot of soybean. Plant Dis. 2008, 92, 1559–1565. [Google Scholar] [CrossRef]
- Elmer, P.A.G.; Spiers, T.M.; Wood, P.N. Effects of pre-harvest foliar calcium sprays on fruit calcium levels and brown rot of peaches. Crop Prot. 2006, 26, 11–18. [Google Scholar] [CrossRef]
- Nadia, G.; El-Gamal, F.; Kareem, A.; Fotouh, Y.O.; El-Mougy, N.S. Induction of systemic resistance in potato plants against late and early blight diseases using chemical inducers under greenhouse and field conditions. Res. J. Agric. Biol. Sci. 2007, 3, 73–81. [Google Scholar]
- Lobato, M.C.; Olivieri, F.P.; González Altamiranda, E.A.; Wolski, E.A.; Daleo, G.R.; Caldiz, D.O.; Andreu, A.B. Phosphite compounds reduce disease severity in potato seed tubers and foliage. Eur. J. Plant Pathol. 2008, 122, 349–358. [Google Scholar] [CrossRef]
- Subhani, M.N.; Sahi, S.T.; Rehman, A.; Wakil, W. Effect of late blight caused by Phytophthora infestans (Mont.) de Bary on Calcium content in leaves of advanced potato lines/cultivars. Acad. Res. J. Agric. Sci. Res. 2015, 3, 107–110. [Google Scholar]
- Glynn, C.P.; Ian, H. The influence of calcium sprays to reduce fungicide inputs against apple scab [Venturia inaequalis(Cooke) G. Wint.]. Arboric. Urban For. 2009, 35, 263–270. [Google Scholar]
- Ozgen, S.; Palta, J.P.; Kleinhenz, M.D. Influence of supplemental calcium fertilization on potato tuber size and tuber number. Acta Hortic. 2003, 619, 329–336. [Google Scholar] [CrossRef]
- Hamdi, W.; Helali, L.; Beji, R.; Zhani, K.; Ouertatani, S.; Gharbi, A. Effect of levels calcium nitrate addition on potatoes fertilizer. Int. Res. J. Eng. Technol. 2015, 2, 2006–2013. [Google Scholar]
- Sugiura, R.; Tsuda, S.; Tamiya, S.; Nuske, S. Field phenotyping system for the assessment of potato late blight resistance using RGB imagery from an unmanned aerial vehicle. Biosyst. Eng. 2016, 148, 1–10. [Google Scholar] [CrossRef]
- Andrivon, D.; Pelle, R.; Ellisseche, D. Assessing resistance types and levels to epidemic diseases from the analysis of disease progress curves. Am. J. Potato Res. 2006, 83, 455–461. [Google Scholar] [CrossRef]
- Karlsson, B.H.; Palta, J.P. Enhancing Tuber Calcium Concentration May Reduce Incidence of Blackspot Bruise injury in Potatoes. HortScience 2006, 41, 1213–1221. [Google Scholar]
- Tobias, R.B.; Conway, W.S.; Sams, C.E.; Gross, K.C.; Whitaker, B.D. Cell wall composition of calcium treated apples inoculated with Botrytis cinerea. Phytochemistry 1993, 32, 35–39. [Google Scholar] [CrossRef]
- Ozgen, S.; Palta, J.P. Supplemental Calcium Application Influences Potato Tuber Number and Size. Hortic. Sci. 2004, 40, 102–105. [Google Scholar]
- EL-Beltagy, M.S.; FAbou-Hadid, A.; Singer, S.M.; Abdel-Naby, A. Response of fall season potato crop to different calcium levels. Acta Hortic. 2002, 579, 289–293. [Google Scholar] [CrossRef]
- Hirschi, K.D. The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol. 2004, 136, 2438–2442. [Google Scholar] [CrossRef] [PubMed]
- Abdur, R.A.B.; Ihsan-ul, H.A.Q. Foliar application of calcium chloride and borax influences plant growth, yield, and quality of tomato (Lycopersicon esculentum Mill.) fruit. Turk. J. Agric. For. 2012, 36, 695–701. [Google Scholar]
- Banjare, S.; Sharma, G.; Verma, S.K. Potato crop growth and yield response to different levels of nitrogen under chhattisgarh plains agro-climatic zone. Indian J. Sci. Technol. 2014, 7, 1504–1508. [Google Scholar]
- Ravichandran, G.; Venkatasalam, E.P.; Muthuraj, R.; Manorama, K. A method to use very small size potato (Solanum tuberosum L.) tubers as seed. Afr. J. Plant Sci. 2015, 9, 352–359. [Google Scholar]
- Mihovilovich, E.; Carli, C.; de Mendiburu, F.; Hualla, V.; Bonierbale, M. Tuber bulking maturity assessment of elite and advanced potato clones protocol. Lima (Peru). Int. Potato Cent. 2014, 43. [Google Scholar] [CrossRef]
| Potato Varieties | Ca Nutrient Type (Treatment Label) | Ca Nutrient Levels (g/plant) | Numbers of Tubers | Numbers of Branches |
|---|---|---|---|---|
| Shenkola | Control | 0 | 16 | 16 |
| CaNO3 | 5 | 13 | 13 | |
| 10 | 17 | 17 | ||
| 15 | 17 | 17 | ||
| CaCl2 | 5 | 14 | 14 | |
| 10 | 17 | 17 | ||
| 15 | 17 | 17 | ||
| CaNO3 + CaCl2 | 5 | 15 | 15 | |
| 10 | 14 | 14 | ||
| 15 | 17 | 17 | ||
| Gera | Control | 0 | 15 | 15 |
| CaNO3 | 5 | 17 | 17 | |
| 10 | 16 | 16 | ||
| 15 | 13 | 13 | ||
| CaCl2 | 5 | 15 | 15 | |
| 10 | 14 | 14 | ||
| 15 | 15 | 15 | ||
| CaNO3 + CaCl2 | 5 | 16 | 16 | |
| 10 | 17 | 17 | ||
| 15 | 13 | 13 | ||
| p value (α = 0.05) | 0.984 | 0.707 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seifu, Y.W. Reducing Severity of Late Blight (Phytophthora infestans) and Improving Potato (Solanum tuberosum L.) Tuber Yield with Pre-Harvest Application of Calcium Nutrients. Agronomy 2017, 7, 69. https://doi.org/10.3390/agronomy7040069
Seifu YW. Reducing Severity of Late Blight (Phytophthora infestans) and Improving Potato (Solanum tuberosum L.) Tuber Yield with Pre-Harvest Application of Calcium Nutrients. Agronomy. 2017; 7(4):69. https://doi.org/10.3390/agronomy7040069
Chicago/Turabian StyleSeifu, Yewubnesh Wendimu. 2017. "Reducing Severity of Late Blight (Phytophthora infestans) and Improving Potato (Solanum tuberosum L.) Tuber Yield with Pre-Harvest Application of Calcium Nutrients" Agronomy 7, no. 4: 69. https://doi.org/10.3390/agronomy7040069





