Trade-Offs in Arbuscular Mycorrhizal Symbiosis: Disease Resistance, Growth Responses and Perspectives for Crop Breeding
Abstract
:1. Introduction
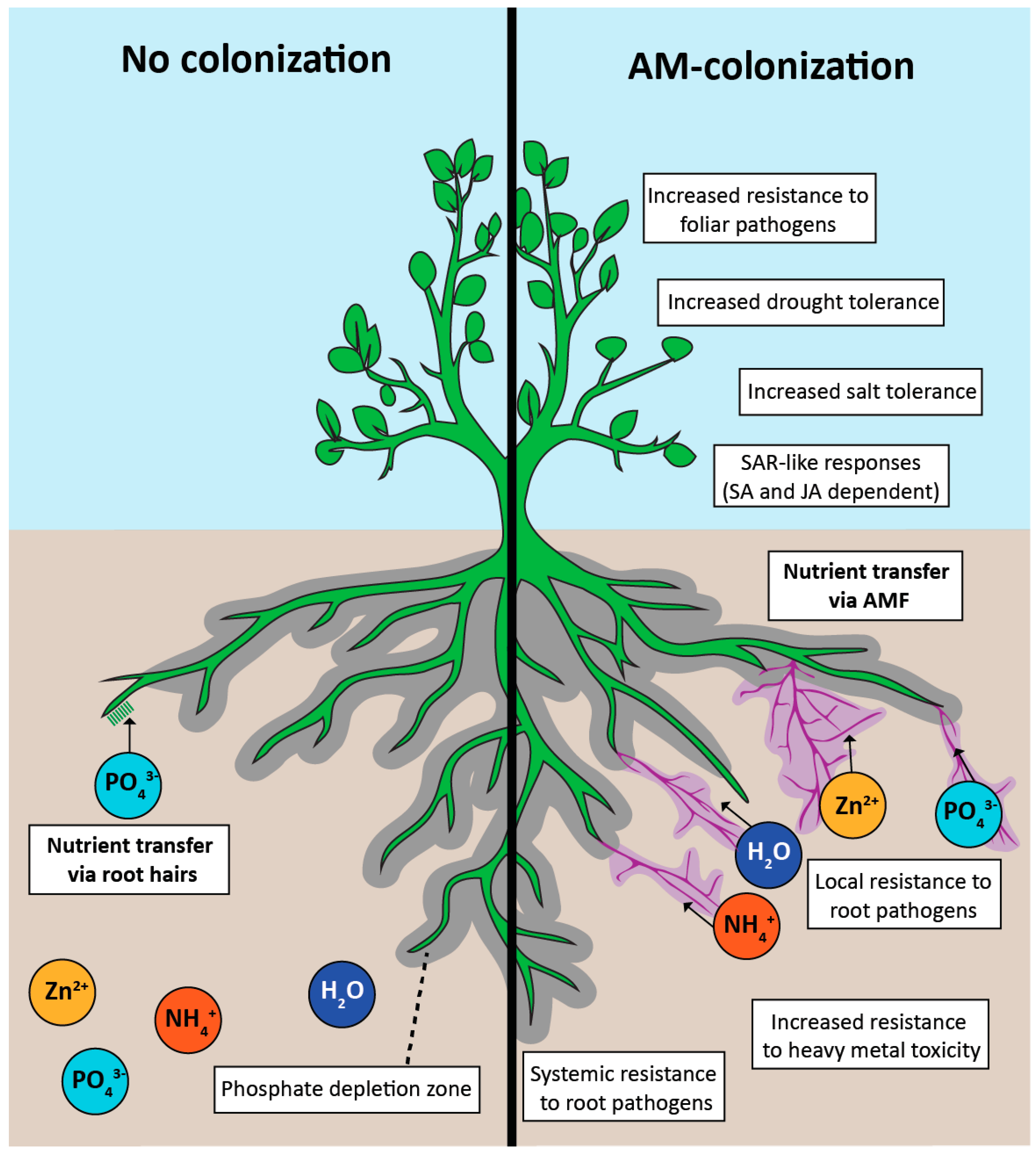
2. Arbuscular Mycorrhizal Symbiosis and Agronomic Traits
2.1. Positive Effects
2.2. Potential Trade-Offs
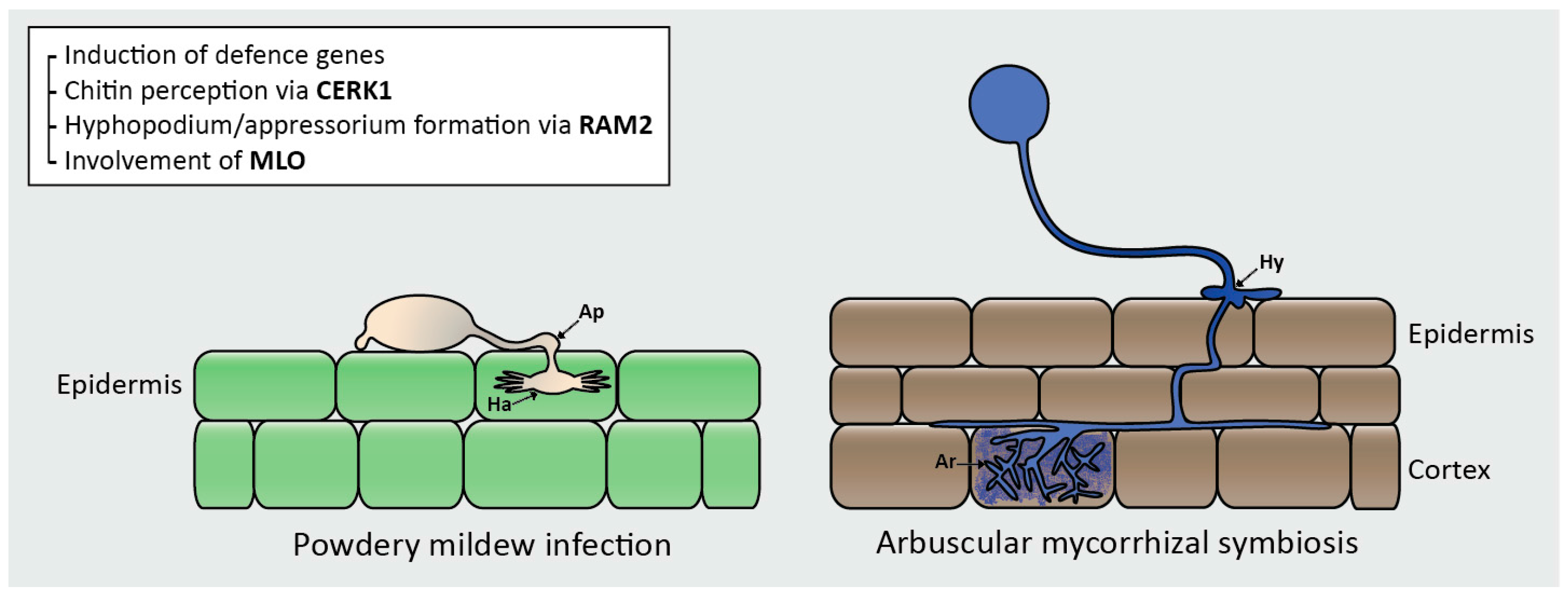
3. Arbuscular Mycorrhizal Symbiosis and Disease Resistance
3.1. Positive Effects
3.2. Potential Trade-Offs
4. Perspectives for Research and Crop Breeding
4.1. Growth Responses
4.2. Disease Resistance
4.3. Translational Research for Crop Breeding
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Wang, B.; Qiu, Y.-L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Smith, F.A.; Jakobsen, I. Functional diversity in arbuscular mycorrhizal (AM) symbioses: The contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol. 2004, 162, 511–524. [Google Scholar] [CrossRef]
- Cooper, K.M.; Tinker, P.B. Translocation and transfer of nutrients in vesicular arbuscular mycorrhizas: II. Uptake and translocation of phosphorus, zinc and sulphur. New Phytol. 1978, 81, 43–52. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Pfeffer, P.E.; Jin, H.; Abubaker, J.; Douds, D.D.; Allen, J.W.; Bücking, H.; Lammers, P.J.; Shachar-Hill, Y. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 2005, 435, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Lambert, D.H.; Baker, D.E.; Cole, H. The role of mycorrhizae in the interactions of phosphorus with zinc, copper and other elements. Soil Sci. Soc. Am. J. 1979, 43, 976–980. [Google Scholar] [CrossRef]
- Watts-Williams, S.J.; Cavagnaro, T.R. Arbuscular mycorrhizas modify tomato responses to soil zinc and phosphorus addition. Biol. Fertil. Soils 2012, 48, 285–294. [Google Scholar] [CrossRef]
- Paszkowski, U. A journey through signaling in arbuscular mycorrhizal symbioses. New Phytol. 2006, 172, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Dumbrell, A.J.; Ashton, P.D.; Aziz, N.; Feng, G.; Nelson, M.; Dytham, C.; Fitter, A.H.; Helgason, T. Distinct seasonal assemblages of arbuscular mycorrhizal fungi revealed by massively parallel pyrosequencing. New Phytol. 2011, 190, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Öpik, M.; Zobel, M.; Cantero, J.J.; Davison, J.; Facelli, J.M.; Hiiesalu, I.; Jairus, T.; Kalwij, J.M.; Koorem, K.; Leal, M.E.; et al. Global sampling of plant roots expands the described molecular diversity of arbuscular mycorrhizal fungi. Mycorrhiza 2013, 23, 411–430. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohmann, P.; Messmer, M.M. Breeding for mycorrhizal symbiosis: Focus on disease resistance. Euphytica 2017, 213, 1–11. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 1995; ISBN 0123849063. [Google Scholar]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Elsevier: Amsterdam, The Netherlands, 1997; ISBN 9780126528404. [Google Scholar]
- Tinker, P.B.; Nye, P.H. Solute Movement in the Rhizosphere; Oxford University Press: Oxford, UK, 2000; ISBN 0195352319. [Google Scholar]
- Karandashov, V.; Bucher, M. Symbiotic phosphate transport in arbuscular mycorrhizas. Trends Plant Sci. 2005, 10, 22–29. [Google Scholar] [CrossRef] [PubMed]
- FAO. Use of phosphate rocks for sustainable agriculture. Fertil. Plant Nutr. Bull. 2009, 13. [Google Scholar] [CrossRef]
- Khasawneh, F.E. The Role of Phosphorus in Agriculture, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1980. [Google Scholar]
- Glendining, M.J.; Dailey, A.G.; Williams, A.G.; Evert, F.K.V.; Goulding, K.W.T.; Whitmore, A.P. Is it possible to increase the sustainability of arable and ruminant agriculture by reducing inputs? Agric. Syst. 2009, 99, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Thirkell, T.J.; Charters, M.D.; Elliott, A.J.; Sait, S.M.; Field, K.J. Are mycorrhizal fungi our sustainable saviours? Considerations for achieving food security. J. Ecol. 2017, 105, 921–929. [Google Scholar] [CrossRef]
- Akiyama, K.; Matsuzaki, K.-I.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 453, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Besserer, A.; Puech-Pagès, V.; Kiefer, P.; Gomez-Roldan, V.; Jauneau, A.; Roy, S.; Portais, J.C.; Roux, C.; Bécard, G.; Séjalon-Delmas, N. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 2006, 4, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Genre, A.; Chabaud, M.; Balzergue, C.; Puech-Pagès, V.; Novero, M.; Rey, T.; Fournier, J.; Rochange, S.; Bécard, G.; Bonfante, P.; et al. Short-chain chitin oligomers from arbuscular mycorrhizal fungi trigger nuclear Ca2+ spiking in Medicago truncatula roots and their production is enhanced by strigolactone. New Phytol. 2013, 198, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Kosuta, S.; Chabaud, M.; Lougnon, G. A diffusible factor from arbuscular mycorrhizal fungi induces symbiosis-specific MtENOD11 expression in roots of Medicago truncatula. Plant 2003, 131, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Stracke, S.; Kistner, C.; Yoshida, S.; Mulder, L.; Sato, S.; Kaneko, T.; Tabata, S.; Sandal, N.; Stougaard, J.; Szczyglowski, K.; et al. A plant receptor-like kinase required for both bacterial and fungal symbiosis. Nature 2002, 417, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Genre, A.; Chabaud, M.; Timmers, T.; Bonfante, P.; Barker, D.G. Arbuscular mycorrhizal fungi elicit a novel intracellular apparatus in Medicago truncatula root epidermal cells before infection. Plant Cell 2005, 17, 3489–3499. [Google Scholar] [CrossRef] [PubMed]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Smith, F.A.; Jakobsen, I. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol. 2003, 133, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Nelsen, C.E.; Safir, G.R. Increased drought tolerance of mycorrhizal onion plants caused by improved phosphorus nutrition. Planta 1982, 154, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Zhang, F.S.; Li, X.L.; Tian, C.Y.; Tang, C.; Rengel, Z. Improved tolerance of maize plants to salt stress by arbuscular mycorrhiza is related to higher accumulation of soluble sugars in roots. Mycorrhiza 2002, 12, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Bødker, L.; Kjøller, R.; Rosendahl, S. Effect of phosphate and the arbuscular mycorrhizal fungus Glomus intraradices on disease severity of root rot of peas (Pisum sativum) caused by Aphanomyces euteiches. Mycorrhiza 1998, 8, 169–174. [Google Scholar] [CrossRef]
- Sikes, B.A. When do arbuscular mycorrhizal fungi protect plant roots from pathogens? Plant Signal. Behav. 2010, 5, 763–765. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed]
- Weerasinghe, R.R.; Bird, D.M.; Allen, N.S. Root-knot nematodes and bacterial Nod factors elicit common signal transduction events in Lotus japonicus. Proc. Natl. Acad. Sci. USA 2005, 102, 3147–3152. [Google Scholar] [CrossRef] [PubMed]
- Güimil, S.; Chang, H.-S.; Zhu, T.; Sesma, A.; Osbourn, A.; Roux, C.; Ioannidis, V.; Oakeley, E.J.; Docquier, M.; Descombes, P.; et al. Comparative transcriptomics of rice reveals an ancient pattern of response to microbial colonization. Proc. Natl. Acad. Sci. USA 2005, 102, 8066–8070. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Schornack, S.; Marsh, J.F.; Gobbato, E.; Schwessinger, B.; Eastmond, P.; Schultze, M.; Kamoun, S.; Oldroyd, G.E.D. A common signaling process that promotes mycorrhizal and oomycete colonization of plants. Curr. Biol. 2012, 22, 2242–2246. [Google Scholar] [CrossRef] [PubMed]
- Ferrol, N.; Tamayo, E.; Vargas, P. The heavy metal paradox in arbuscular mycorrhizas: From mechanisms to biotechnological applications. J. Exp. Bot. 2016, 67, 6253–6265. [Google Scholar] [CrossRef] [PubMed]
- Coninx, L.; Martinova, V.; Rineau, F. Mycorrhiza-assisted phytoremediation. Adv. Bot. Res. 2017, 83, 127–188. [Google Scholar] [CrossRef]
- Rajtor, M.; Piotrowska-Seget, Z. Prospects for arbuscular mycorrhizal fungi (AMF) to assist in phytoremediation of soil hydrocarbon contaminants. Chemosphere 2016, 162, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Feddermann, N.; Boller, T.; Salzer, P.; Elfstrand, S.; Wiemken, A.; Elfstrand, M. Medicago truncatula shows distinct patterns of mycorrhiza-related gene expression after inoculation with three different arbuscular mycorrhizal fungi. Planta 2008, 227, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Monzon, A.; Azcón, R. Relevance of mycorrhizal fungal origin and host plant genotype to inducing growth and nutrient uptake in Medicago species. Agric. Ecosyst. Environ. 1996, 60, 9–15. [Google Scholar] [CrossRef]
- Burleigh, S.H.; Cavagnaro, T.; Jakobsen, I. Functional diversity of arbuscular mycorrhizas extends to the expression of plant genes involved in P nutrition. J. Exp. Bot. 2002, 53, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Hayman, D.S.; Mosse, B. Plant growth responses to vesicular-arbuscular mycorrhiza III. Increased uptake of labile P from soil. New Phytol. 1972, 71, 41–47. [Google Scholar] [CrossRef]
- Hetrick, B.A.D.; Wilson, G.W.T.; Cox, T.S. Mycorrhizal dependence of modern wheat-varieties, landraces, and ancestors. Can. J. Bot. Can. Bot. 1992, 70, 2032–2040. [Google Scholar] [CrossRef]
- Wani, S.P.; McGill, W.B.; Tewari, J.P. Mycorrhizal and common root-rot infection, and nutrient accumulation in barley grown on breton loam using N from biological fixation or fertilizer. Biol. Fertil. Soils 1991, 12, 46–54. [Google Scholar] [CrossRef]
- Abbott, L.K.; Robson, A.D. Growth of subterranean clover in relation to formation of endomycorrhizas by introduced and indigenous fungi in a field soil. New Phytol. 1978, 81, 575–585. [Google Scholar] [CrossRef]
- Graham, J.H.; Abbott, L.K. Wheat responses to aggressive and non-aggressive arbuscular mycorrhizal fungi. Plant Soil 2000, 220, 207–218. [Google Scholar] [CrossRef]
- Li, H.; Smith, S.E.; Holloway, R.E.; Zhu, Y.; Smith, F.A. Arbuscular mycorrhizal fungi contribute to phosphorus uptake by wheat grown in a phosphorus-fixing soil even in the absence of positive growth responses. New Phytol. 2006, 172, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Hetrick, B.A.D.; Wilson, G.W.T.; Cox, T.S. Mycorrhizal dependence of modern wheat cultivars and ancestors: A synthesis. Can. J. Bot. 1993, 71, 512–518. [Google Scholar] [CrossRef]
- Grace, E.J.; Cotsaftis, O.; Tester, M.; Smith, F.A.; Smith, S.E. Arbuscular mycorrhizal inhibition of growth in barley cannot be attributed to extent of colonization, fungal phosphorus uptake or effects on expression of plant phosphate transporter genes. New Phytol. 2009, 181, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.Z.; An, Z.Q.; Hendrix, J.W. A mycorrhizal pathogen (Glomus macrocarpum Tul. & Tul.) of tobacco: Effects of long- and short-term cropping on the mycorrhizal fungal community and stunt disease. Appl. Soil Ecol. 1994, 1, 269–276. [Google Scholar] [CrossRef]
- Modjo, H.; Hendrix, J.W.; Nesmith, W. Mycorrhizal fungi in relation to control of tobacco stunt disease with soil fumigants. Soil Biol. Biochem. 1987, 19, 289–295. [Google Scholar] [CrossRef]
- Modjo, H.S.; Hendrix, J.W. The mycorrhizal fungus Glomus macrocarpum as a cause of tobacco stunt disease. Phytopathology 1986, 76, 688–691. [Google Scholar] [CrossRef]
- Rausch, C.; Daram, P.; Brunner, S.; Jansa, J.; Laloi, M.; Leggewie, G.; Amrhein, N.; Bucher, M. A phosphate transporter expressed in arbuscule-containing cells in potato. Nature 2001, 414, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.J.; Dewbre, G.R.; Liu, J.Y. A phosphate transporter from Medicago truncatula involved in the acquisiton of phosphate released by arbuscular mycorrhizal fungi. Plant Cell 2002, 14, 2413–2429. [Google Scholar] [CrossRef] [PubMed]
- Sawers, R.J.H.; Svane, S.F.; Quan, C.; Grønlund, M.; Wozniak, B.; Gebreselassie, M.N.; González-Muñoz, E.; Chávez Montes, R.A.; Baxter, I.; Goudet, J.; et al. Phosphorus acquisition efficiency in arbuscular mycorrhizal maize is correlated with the abundance of root-external hyphae and the accumulation of transcripts encoding PHT1 phosphate transporters. New Phytol. 2017, 214, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Manjarrez, M.; Stonor, R.; McNeill, A.; Smith, F.A. Indigenous arbuscular mycorrhizal (AM) fungi contribute to wheat phosphate uptake in a semi-arid field environment, shown by tracking with radioactive phosphorus. Appl. Soil Ecol. 2015, 96, 68–74. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Grønlund, M.; Jakobsen, I.; Grotemeyer, M.S.; Rentsch, D.; Miyao, A.; Hirochika, H.; Kumar, C.S.; Sundaresan, V.; Salamin, N.; et al. Nonredundant regulation of rice arbuscular mycorrhizal symbiosis by two members of the PHOSPHATE TRANSPORTER1 gene family. Plant Cell 2012, 24, 4236–4251. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.A.; Grace, E.J.; Smith, S.E. More than a carbon economy: Nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbioses. New Phytol. 2009, 182, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Smith, F.A.; Dickson, S.; Holloway, R.E.; Smith, S.E. Plant growth depressions in arbuscular mycorrhizal symbioses: Not just caused by carbon drain? New Phytol. 2008, 178, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Barea, J.M.; Azcon-Aguilar, C. Mycorrhizas and their significance in nodulating nitrogen-fixing plants. Adv. Agron. 1983, 36, 1–54. [Google Scholar] [CrossRef]
- Azcon, R.; Rubio, R.; Barea, J.M. Selective interactions between different species of mycorrhizal fungi and rhizobium-meliloti strains, and their effects on growth, N2-fixation (N-15) and nutrition of Medicago-sativa-L. New Phytol. 1991, 117, 399–404. [Google Scholar] [CrossRef]
- Ames, R.N.; Reid, C.P.P.; Porter, L.K.; Cambardella, C. Hyphal uptake and transport of nitrogen from two 15N-labelled sources by glomus mosseae, a vesicular-arbuscular mycorrhizal fungus. New Phytol. 1983, 95, 381–396. [Google Scholar] [CrossRef]
- Frey, B.; Schüepp, H. Acquisition of nitrogen by external hyphae of arbuscular mycorrhizal fungi associated with Zea mays L. New Phytol. 1993, 124, 221–230. [Google Scholar] [CrossRef]
- George, E.; Haussler, K.-W.; Vetterlein, D.; Gorgus, E.; Marschner, H. Water and nutrient translocation by hyphae of Glomus mosseae compartment. Can. J. Bot. 1992, 70, 2130–2137. [Google Scholar] [CrossRef]
- Hawkins, H. Reduced15N-nitrogen transport through arbuscular mycorrhizal hyphae to Triticum aestivum L. supplied with ammonium vs. nitrate nutrition. Ann. Bot. 2001, 87, 303–311. [Google Scholar] [CrossRef]
- Guether, M.; Neuhauser, B.; Balestrini, R.; Dynowski, M.; Ludewig, U.; Bonfante, P. A mycorrhizal-specific ammonium transporter from Lotus japonicus acquires nitrogen released by arbuscular mycorrhizal fungi. Plant Physiol. 2009, 150, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Augé, R.M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Azcon, R.; Gomez, M. Effects of arbuscular-mycorrhizal glomus species on drought tolerance: Physiological and nutritional plant responses. Appl. Environ. Microbiol. 1995, 61, 456–460. [Google Scholar] [PubMed]
- Giri, B.; Kapoor, R.; Mukerji, K.G. Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues. Microb. Ecol. 2007, 54, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Al-Karaki, G.; Al-Raddad, A. Effects of arbuscular mycorrhizal fungi and drought stress on growth and nutrient uptake of two wheat genotypes differing in drought resistance. Mycorrhiza 1997, 7, 83–88. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Ultra, V.U.; Tanaka, S.; Sakurai, K.; Iwasaki, K. Effects of arbuscular mycorrhiza and phosphorus application on arsenic toxicity in sunflower (Helianthus annuus L.) and on the transformation of arsenic in the rhizosphere. Plant Soil 2007, 290, 29–41. [Google Scholar] [CrossRef]
- Sharma, S.; Anand, G.; Singh, N.; Kapoor, R. Arbuscular mycorrhiza augments arsenic tolerance in wheat (Triticum aestivum L.) by strengthening antioxidant defense sstem and thiol metabolism. Front. Plant Sci. 2017, 8, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.W.; Shachar-Hill, Y. Sulfur transfer through an arbuscular mycorrhiza. Plant Physiol. 2009, 149, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Sieh, D.; Watanabe, M.; Devers, E.A.; Brueckner, F.; Hoefgen, R.; Krajinski, F. The arbuscular mycorrhizal symbiosis influences sulfur starvation responses of Medicago truncatula. New Phytol. 2013, 197, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Kariman, K.; Barker, S.J.; Finnegan, P.M.; Tibbett, M. Ecto- and arbuscular mycorrhizal symbiosis can induce tolerance to toxic pulses of phosphorus in jarrah (Eucalyptus marginata) seedlings. Mycorrhiza 2014, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezvani, M.; Ardakani, M.R.; Rejali, F.; Zaefarian, F.; Teimouri, S.; Noormohammadi, G.; Miransari, M. Uptake of heavy metals by mycorrhizal barley (Hordeum vulgare L.). J. Plant Nutr. 2014, 38, 904–919. [Google Scholar] [CrossRef]
- Slezack, S.; Dumas-Gaudot, E.; Rosendahl, S.; Kjøller, R.; Paynot, M.; Negrel, J.; Gianinazzi, S. Endoproteolytic activities in pea roots inoculated with the arbuscular mycorrhizal fungus Glomus mosseae and/or Aphanomyces euteiches in relation to bioprotection. New Phytol. 1999, 142, 517–529. [Google Scholar] [CrossRef]
- Bødker, L.; Kjøller, R.; Kristensen, K.; Rosendahl, S. Interactions between indigenous arbuscular mycorrhizal fungi and Aphanomyces euteiches in field-grown pea. Mycorrhiza 2002, 12, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Cordier, C.; Pozo, M.J.; Barea, J.M.; Gianinazzi, S.; Gianinazzi-Pearson, V. Cell defense responses associated with localized and systemic resistance to Phytophthora parasitica induced in tomato by an arbuscular mycorrhizal fungus. Mol. Plant-Microbe Interact. 1998, 11, 1017–1028. [Google Scholar] [CrossRef]
- Rosendahl, C.N.; Rosendahl, S. The role of vesicular-arbuscular mycorrhiza in controlling damping-off and growth reduction in cucumber caused by pythium-ultimum. Symbiosis 1990, 9, 363–366. [Google Scholar]
- Campos-Soriano, L.; García-Martínez, J.; Segundo, B.S. The arbuscular mycorrhizal symbiosis promotes the systemic induction of regulatory defence-related genes in rice leaves and confers resistance to pathogen infection. Mol. Plant Pathol. 2012, 13, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Fritz, M.; Jakobsen, I.; Lyngkjær, M.F.; Thordal-Christensen, H.; Pons-Kühnemann, J. Arbuscular mycorrhiza reduces susceptibility of tomato to Alternaria solani. Mycorrhiza 2006, 16, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Møller, K.; Kristensen, K.; Yohalem, D.; Larsen, J. Biological management of gray mold in pot roses by co-inoculation of the biocontrol agent Ulocladium atrum and the mycorrhizal fungus Glomus mosseae. Biol. Control 2009, 49, 120–125. [Google Scholar] [CrossRef]
- Pozo, M.J.; Jung, S.C.; Lopez-Raez, J.A.; Azcón-Aguilar, C. Impact of arbuscular mycorrhizal symbiosis on plant response to biotic stress: The role of plant defence mechanisms. In Arbuscular Mycorrhizas: Physiology and Function; Springer: Dordrecht, The Netherlands, 2010; pp. 193–2007. ISBN 978-90-481-9488-9. [Google Scholar]
- Ruiz-Lozano, J.M.; Gianinazzi, S.; Gianinazzi-Pearson, V. Genes involved in resistance to powdery mildew in barley differentially modulate root colonization by the mycorrhizal fungus Glomus mosseae. Mycorrhiza 1999, 9, 237–240. [Google Scholar] [CrossRef]
- Pozo, M.J.; Azcón-Aguilar, C. Unraveling mycorrhiza-induced resistance. Curr. Opin. Plant Biol. 2007, 10, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Van der Ent, S.; Van Wees, S.C.M.; Pieterse, C.M.J. Jasmonate signaling in plant interactions with resistance-inducing beneficial microbes. Phytochemistry 2009, 70, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Pozo, M.J.; Van Loon, L.C.; Pieterse, C.M.J. Jasmonates—Signals in plant-microbe interactions. J. Plant Growth Regul. 2004, 23, 211–222. [Google Scholar]
- Hao, Z.; Fayolle, L.; Van Tuinen, D.; Chatagnier, O.; Li, X.; Gianinazzi, S.; Gianinazzi-Pearson, V. Local and systemic mycorrhiza-induced protection against the ectoparasitic nematode Xiphinema index involves priming of defence gene responses in grapevine. J. Exp. Bot. 2012, 63, 3657–3672. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, W.; Xie, Q.; Liu, N.; Liu, L.; Wang, D.; Zhang, X.; Yang, C.; Chen, X.; Tang, D.; et al. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 2017, 356, 1172–1175. [Google Scholar] [CrossRef] [PubMed]
- Gobbato, E.; Wang, E.; Higgins, G.; Bano, S.A.; Henry, C.; Schultze, M.; Oldroyd, G.E.D. RAM1 and RAM2 function and expression during arbuscular mycorrhizal symbiosis and Aphanomyces euteiches colonization. Plant Signal. Behav. 2013, 8, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Glazebrook, J. Contrasting Mechanisms of Defense Against Biotrophic and Necrotrophic Pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Y.; Cao, M.; Xie, L.J.; Liang, X.T.; Zeng, R.S.; Su, Y.J.; Huang, J.H.; Wang, R.L.; Luo, S.M. Induction of DIMBOA accumulation and systemic defense responses as a mechanism of enhanced resistance of mycorrhizal corn (Zea mays L.) to sheath blight. Mycorrhiza 2011, 21, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Pozo, M.J.; Azcón-Aguilar, C.; Dumas-Gaudot, E.; Barea, J.M. B-1,3-glucanase activities in tomato roots inoculated with arbuscular mycorrhizal fungi and/or Phytophthora parasitica and their possible involvement in bioprotection. Plant Sci. 1999, 141, 149–157. [Google Scholar] [CrossRef]
- Pozo, M.J.; Cordier, C.; Dumas-Gaudot, E.; Gianinazzi, S.; Barea, J.M.; Azcón-Aguilar, C. Localized versus systemic effect of arbuscular mycorrhizal fungi on defence responses to Phytophthora infection in tomato plants. J. Exp. Bot. 2002, 53, 525–534. [Google Scholar] [CrossRef] [PubMed]
- López-Ráez, J.A.; Flors, V.; García, J.M.; Pozo, M.J. AM symbiosis alters phenolic acid content in tomato roots. Plant Signal. Behav. 2010, 5, 1138–1140. [Google Scholar] [CrossRef]
- Benhamou, N.; Fortin, J.A.; Hamel, C.; St-Arnaud, M.; Shatilla, A. Resistance responses of mycorrhizal Ri T-DNA-transformed carrot roots to infection by Fusarium oxysporum f.sp. chrysanthemi. Phytopathology 1994, 85, 958–968. [Google Scholar] [CrossRef]
- Haynes, R.J. Effects of liming on phosphate availability in acid soils—A critical review. Plant Soil 1982, 68, 289–308. [Google Scholar] [CrossRef]
- Kumar, M.; Yadav, V.; Kumar, H.; Sharma, R.; Singh, A.; Tuteja, N.; Johri, A.K. Piriformospora indica enhances plant growth by transferring phosphate. Plant Signal. Behav. 2011, 6, 723–725. [Google Scholar] [CrossRef] [PubMed]
- Bethlenfalvay, G.J. Parasitic and mutualistic associations between a mycorrhizal fungus and soybean: Development of the host plant. Phytopathology 1982, 72, 889. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhu, Y.G.; Marschner, P.; Smith, F.A.; Smith, S.E. Wheat responses to arbuscular mycorrhizal fungi in a highly calcareous soil differ from those of clover, and change with plant development and P supply. Plant Soil 2005, 277, 221–232. [Google Scholar] [CrossRef]
- Tinker, P.B. Soil chemistry of phosphorus and mycorrhizal effects on plant growth. In Endomycorrhizas; Sanders, F.E., Mosse, B., Tinker, P.B., Eds.; Academic Press: London, UK, 1975; pp. 353–371. [Google Scholar]
- Paszkowski, U.; Kroken, S.; Roux, C.; Briggs, S.P. Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2002, 99, 13324–13329. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Trieu, A.T.; Blaylock, L.A.; Harrison, M.J. Cloning and characterization of two phosphate transporters from Medicago truncatula roots: Regulation in response to phosphate and to colonization by arbuscular mycorrhizal (AM) fungi. Mol. Plant Microbe Interact. 1998, 11, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Karandashov, V.; Nagy, R.; Wegmüller, S.; Amrhein, N.; Bucher, M. Evolutionary conservation of a phosphate transporter in the arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2004, 101, 6285–6290. [Google Scholar] [CrossRef] [PubMed]
- Fitter, A.H. What is the link between carbon and phosphorus fluxes in arbuscular mycorrhizas? A null hypothesis for symbiotic function. New Phytol. 2006, 172, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Klironomos, J.N. Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 2003, 84, 2292–2301. [Google Scholar] [CrossRef]
- Azcón-Aguilar, C.; Barea, J. Arbuscular mycorrhizas and biological control of soil-borne plant pathogens—An overview of the mechanisms involved. Mycorrhiza 1996, 457–464. [Google Scholar] [CrossRef]
- Whipps, J.M. Prospects and limitations for mycorrhizas in biocontrol of root pathogens. Can. J. Bot. 2004, 82, 1198–1227. [Google Scholar] [CrossRef]
- Borowicz, V.A. Do arbuscular mycorrhizal fungi alter plant-pathogen interactions. Ecology 2001, 82, 3057–3068. [Google Scholar] [CrossRef]
- Perfect, S.E.; Green, J.R. Infection structures of biotrophic and hemibiotrophic fungal plant pathogens. Mol. Plant Pathol. 2001, 2, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C. Pattern-recognition receptors in plant innate immunity. Curr. Opin. Immunol. 2008, 20, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Boyd, L.A.; Ridout, C.; O’Sullivan, D.M.; Leach, J.E.; Leung, H. Plant-pathogen interactions: Disease resistance in modern agriculture. Trends Genet. 2013, 29, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Tisserant, E.; Kohler, A.; Dozolme-Seddas, P.; Balestrini, R.; Benabdellah, K.; Colard, A.; Croll, D.; da Silva, C.; Gomez, S.K.; Koul, R.; et al. The transcriptome of the arbuscular mycorrhizal fungus Glomus intraradices (DAOM 197198) reveals functional tradeoffs in an obligate symbiont. New Phytol. 2012, 193, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Kaku, H.; Nishizawa, Y.; Ishii-Minami, N.; Akimoto-Tomiyama, C.; Dohmae, N.; Takio, K.; Minami, E.; Shibuya, N. Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 11086–11091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, W.; Sun, J.; Feng, F.; Deng, Y.; He, Z.; Oldroyd, G.E.D.; Wang, E. The receptor kinase CERK1 has dual functions in symbiosis and immunity signalling. Plant J. 2015, 81, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Miya, A.; Albert, P.; Shinya, T.; Desaki, Y.; Ichimura, K.; Shirasu, K.; Narusaka, Y.; Kawakami, N.; Kaku, H.; Shibuya, N. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 19613–19618. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Nakano, T.; Takamizawa, D.; Desaki, Y.; Ishii-Minami, N.; Nishizawa, Y.; Minami, E.; Okada, K.; Yamane, H.; Kaku, H.; et al. Two LysM receptor molecules, CEBiP and OsCERK1, cooperatively regulate chitin elicitor signaling in rice. Plant J. 2010, 64, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, G.; Chabaud, M.; Miyata, K.; Capozzi, M.; Takeda, N.; Kaku, H.; Shibuya, N.; Nakagawa, T.; Barker, D.G.; Genre, A. The rice LysM receptor-like kinase OsCERK1 is required for the perception of short-chain chitin oligomers in arbuscular mycorrhizal signaling. New Phytol. 2017, 214, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Kloppholz, S.; Kuhn, H.; Requena, N. A secreted fungal effector of glomus intraradices promotes symbiotic biotrophy. Curr. Biol. 2011, 21, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Koeck, M.; Hardham, A.R.; Dodds, P.N. The role of effectors of biotrophic and hemibiotrophic fungi in infection. Cell. Microbiol. 2011, 13, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Sędzielewska Toro, K.; Brachmann, A. The effector candidate repertoire of the arbuscular mycorrhizal fungus Rhizophagus clarus (supp. data). BMC Genom. 2016, 53, 1689–1699. [Google Scholar] [CrossRef]
- Tisserant, E.; Malbreil, M.; Kuo, A.; Kohler, A.; Symeonidi, A.; Balestrini, R.; Charron, P.; Duensing, N.; Frei dit Frey, N.; Gianinazzi-Pearson, V.; et al. Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proc. Natl. Acad. Sci. USA 2013, 110, 20117–20122. [Google Scholar] [CrossRef] [PubMed]
- Wewer, V.; Brands, M.; Dörmann, P. Fatty acid synthesis and lipid metabolism in the obligate biotrophic fungus Rhizophagus irregularis during mycorrhization of Lotus japonicus. Plant J. 2014, 79, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; San Clemente, H.; Roy, S.; Bécard, G.; Zhao, B.; Roux, C. A survey of the gene repertoire of Gigaspora rosea unravels conserved features among Glomeromycota for obligate biotrophy. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Luginbuehl, L.H.; Menard, G.N.; Kurup, S.; Van Erp, H.; Radhakrishnan, G.V.; Breakspear, A.; Oldroyd, G.E.D.; Eastmond, P.J. Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 2017, 356, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Gobbato, E.; Marsh, J.F.; Vernié, T.; Wang, E.; Maillet, F.; Kim, J.; Miller, J.B.; Sun, J.; Bano, S.A.; Ratet, P.; et al. A GRAS-type transcription factor with a specific function in mycorrhizal signaling. Curr. Biol. 2012, 22, 2236–2241. [Google Scholar] [CrossRef] [PubMed]
- Huisman, R.; Bouwmeester, K.; Brattinga, M.; Govers, F.; Bisseling, T.; Limpens, E. Haustorium formation in Medicago truncatula roots infected by Phytophthora palmivora does not involve the common endosymbiotic program shared by arbuscular mycorrhizal fungi and rhizobia. Mol. Plant-Microbe Interact. 2015, 28, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Dreher, D.; Yadav, H.; Zander, S.; Hause, B. Is there genetic variation in mycorrhization of Medicago truncatula? PeerJ 2017, 5, e3713. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.H. Discovery, characterization and exploitation of Mlo powdery mildew resistance in barley. Euphytica 1992, 66, 141–152. [Google Scholar] [CrossRef]
- Piffanelli, P. The barley MLO modulator of defense and cell death is responsive to biotic and abiotic stress stimuli. Plant Physiol. 2002, 129, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.K.M.; Andrivon, D.; Collinge, D.B.; Nicholson, P. Fitness costs and trade-offs in plant disease. Plant Pathol. 2013, 62, 1. [Google Scholar] [CrossRef]
- Bravo, A.; York, T.; Pumplin, N.; Mueller, L.A.; Harrison, M.J. Genes conserved for arbuscular mycorrhizal symbiosis identified through phylogenomics. Nat. Plants 2015, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kemen, E.; Gardiner, A.; Schultz-Larsen, T.; Kemen, A.C.; Balmuth, A.L.; Robert-Seilaniantz, A.; Bailey, K.; Holub, E.; Studholme, D.J.; MacLean, D.; et al. Gene gain and loss during evolution of obligate parasitism in the white rust pathogen of Arabidopsis thaliana. PLoS Biol. 2011, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, F.; Aerts, A.; Ahrén, D.; Brun, A.; Danchin, E.G.J.; Duchaussoy, F.; Gibon, J.; Kohler, A.; Lindquist, E.; Pereda, V.; et al. The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature 2008, 452, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Baxter, L.; Tripathy, S.; Ishaque, N.; Boot, N.; Cabral, A.; Kemen, E.; Thines, M.; Ah-Fong, A.; Anderson, R.; Badejoko, W.; et al. Signatures of adaptation to obligate biotrophy in the Hyaloperonospora arabidopsidis genome. Science 2010, 330, 1549–1551. [Google Scholar] [CrossRef] [PubMed]
- Sawers, R.J.H.; Gutjahr, C.; Paszkowski, U. Cereal mycorrhiza: An ancient symbiosis in modern agriculture. Trends Plant Sci. 2008, 13, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.C. Can fertilization of soil select less mutualistic mycorrhizae? Ecol. Appl. 1993, 3, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Kaeppler, S.M.; Parke, J.L.; Mueller, S.M.; Senior, L.; Stuber, C.; Tracy, W.F. Variation among maize inbred lines and detection of quantitative trait loci for growth at low phosphorus and responsiveness to arbuscular mycorrhizal fungi. Crop Sci. 2000, 40, 358–364. [Google Scholar] [CrossRef]
- Mark, G.L.; Cassells, A.C. Genotype-dependence in the interaction between Glomus fistulosum, Phytophthora fragariae and the wild strawberry (Fragaria vesca). Plant Soil 1996, 185, 233–239. [Google Scholar] [CrossRef]
- Steinkellner, S.; Hage-Ahmed, K.; García-Garrido, J.M.; Illana, A.; Ocampo, J.A.; Vierheilig, H. A comparison of wild-type, old and modern tomato cultivars in the interaction with the arbuscular mycorrhizal fungus Glomus mosseae and the tomato pathogen Fusarium oxysporum f. sp. lycopersici. Mycorrhiza 2012, 22, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Miyata, K.; Kozaki, T.; Kouzai, Y.; Ozawa, K.; Ishii, K.; Asamizu, E.; Okabe, Y.; Umehara, Y.; Miyamoto, A.; Kobae, Y.; et al. The bifunctional plant receptor, OsCERK1, regulates both chitin-triggered immunity and arbuscular mycorrhizal symbiosis in rice. Plant Cell Physiol. 2014, 55, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, H.; Serfling, A.; Enders, M.; Friedt, W.; Ordon, F. Genetics of mycorrhizal symbiosis in winter wheat (Triticum aestivum). New Phytol. 2017, 215, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Küster, H.; Becker, A.; Samac, D.; Tesfaye, M. The Medicago Truncatula Handbook; Samuel Roberts Noble Foundation: Ardmore, TN, USA, 2006. [Google Scholar]
- Gutjahr, C.; Banba, M.; Croset, V.; An, K.; Miyao, A.; An, G.; Hirochika, H.; Imaizumi-Anraku, H.; Paszkowski, U. Arbuscular mycorrhiza-specific signaling in rice transcends the common symbiosis signaling pathway. Plant Cell 2008, 20, 2989–3005. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Ané, J.-M. Germinating spore exudates from arbuscular mycorrhizal fungi: Molecular and developmental responses in plants and their regulation by ethylene. Mol. Plant-Microbe Interact. 2011, 24, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.D.; Neal, A.L.; van Wees, S.C.M.; Ton, J. Mycorrhiza-induced resistance: More than the sum of its parts? Trends Plant Sci. 2013, 18, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Öpik, M.; Vanatoa, A.; Vanatoa, E.; Moora, M.; Davison, J.; Kalwij, J.M.; Reier, Ü.; Zobel, M. The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol. 2010, 188, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Daniell, T.J.; Husband, R.; Fitter, A.H.; Young, J.P. Molecular diversity of arbuscular mycorrhizal fungi colonising arable crops. FEMS Microbiol. Ecol. 2001, 36, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Abbott, L.K.; Lumley, S. Mycorrhizal Fungi: Use in Sustainable Agriculture and Land Restoration; Springer: Berlin, Germany, 2014; Volume 41, ISBN 978-3-662-45369-8. [Google Scholar]
- Verzeaux, J.; Hirel, B.; Dubois, F.; Lea, P.J.; Tétu, T. Agricultural practices to improve nitrogen use efficiency through the use of arbuscular mycorrhizae: Basic and agronomic aspects. Plant Sci. 2017, 264, 48–56. [Google Scholar] [CrossRef] [PubMed]
- An, Z.Q.; Hendrix, J.W.; Hershman, D.E.; Ferriss, R.S.; Henson, G.T. The influence of crop rotation and soil fumigation on a mycorrhizal fungal community associated with soybean. Mycorrhiza 1993, 3, 171–182. [Google Scholar] [CrossRef]
| Responses | Positive | Negative |
|---|---|---|
| Plant growth and abiotic stresses | Increased biomass [2,39,40,41,42,43,44,45] | Reduced biomass [46,47,48,49,50,51,52] |
| Phosphate acquisition [45,46,53,54,55,56,57] | Reduced phosphate [2,27,47,58,59] | |
| Nitrogen (ammonium) acquisition [4,60,61,62,63,64 ,65,66] | ||
| Water acquisition [67,68] | ||
| Zinc acquisition [5,6] | ||
| Drought tolerance [67,68] | ||
| Salinity tolerance [29,69,70,71] | ||
| Reduced arsenic toxicity [72,73] Reduced sulphur starvation [74,75] | ||
| Reduced phosphate toxicity [76] | ||
| Reduced assimilation of adverse heavy metals [19,36,77] | ||
| Disease resistance | Increased resistance to root [31,78,79,80] and foliar [81,82,83,84,85] pathogens | Reduced arbuscular mycorrhizal colonization in mlo mutants [86] |
| Jasmonic acid production [87,88,89,90] | Components required for AM colonization used by pathogens, Golovinomyces cichoracerum (DC.) V.P. Heluta [91], Phytophthora palmivora E.J. Butler [35] and Aphanomyces euteiches Drechsler [92] | |
| Salicylic acid production [87,93] | ||
| Systemic acquired resistance [80,90,94,95,96,97] | ||
| Production of other defence compounds, e.g., phenolics [98], β-1,3-glucanase [96,97] and chitinolytic enzymes [99] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacott, C.N.; Murray, J.D.; Ridout, C.J. Trade-Offs in Arbuscular Mycorrhizal Symbiosis: Disease Resistance, Growth Responses and Perspectives for Crop Breeding. Agronomy 2017, 7, 75. https://doi.org/10.3390/agronomy7040075
Jacott CN, Murray JD, Ridout CJ. Trade-Offs in Arbuscular Mycorrhizal Symbiosis: Disease Resistance, Growth Responses and Perspectives for Crop Breeding. Agronomy. 2017; 7(4):75. https://doi.org/10.3390/agronomy7040075
Chicago/Turabian StyleJacott, Catherine N., Jeremy D. Murray, and Christopher J. Ridout. 2017. "Trade-Offs in Arbuscular Mycorrhizal Symbiosis: Disease Resistance, Growth Responses and Perspectives for Crop Breeding" Agronomy 7, no. 4: 75. https://doi.org/10.3390/agronomy7040075






