Determination of Micronutrient Accumulation in Greenhouse Cucumber Crop Using a Modeling Approach
Abstract
:1. Introduction
2. Results and Discussion
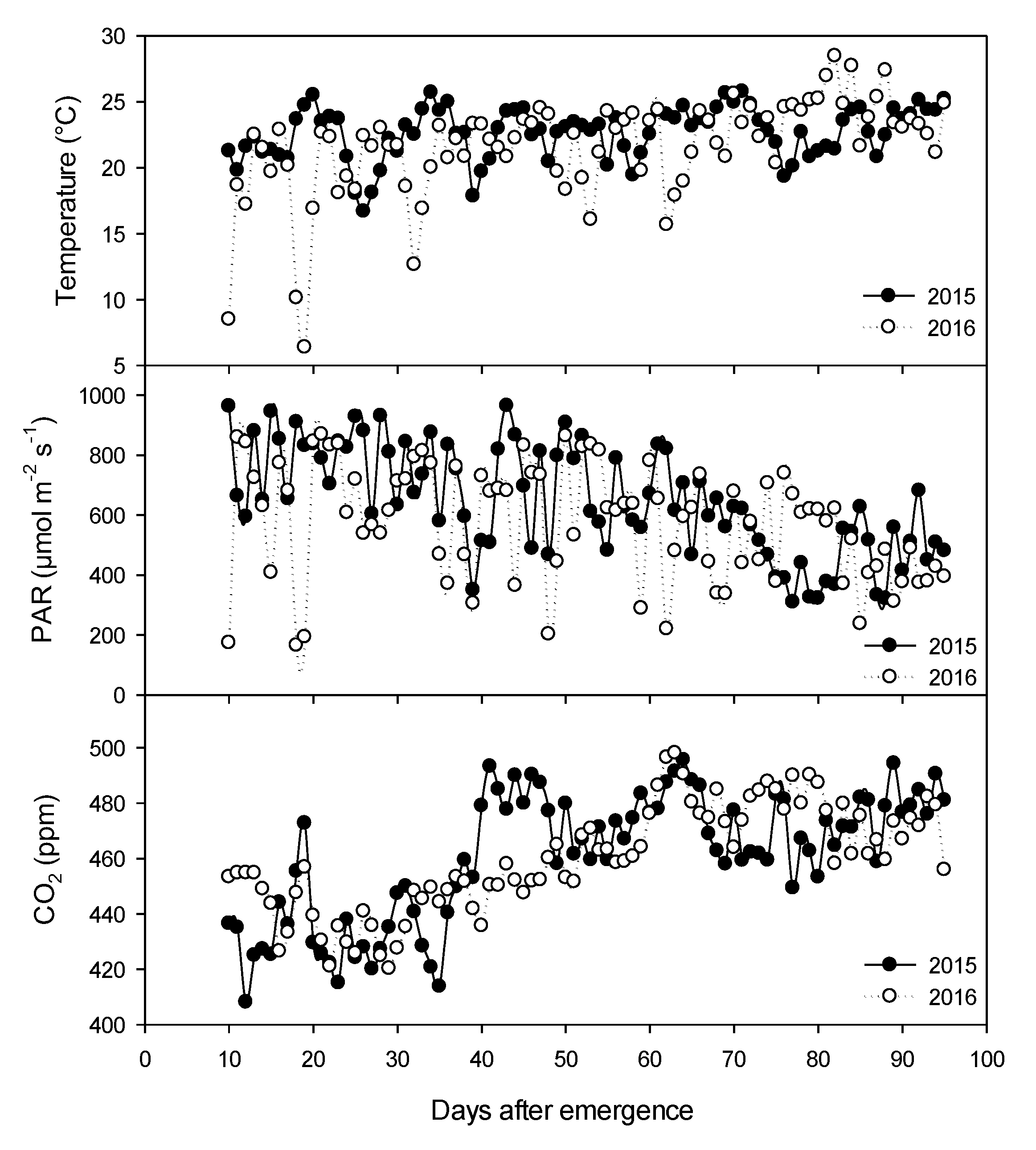
2.1. Climatic Variables
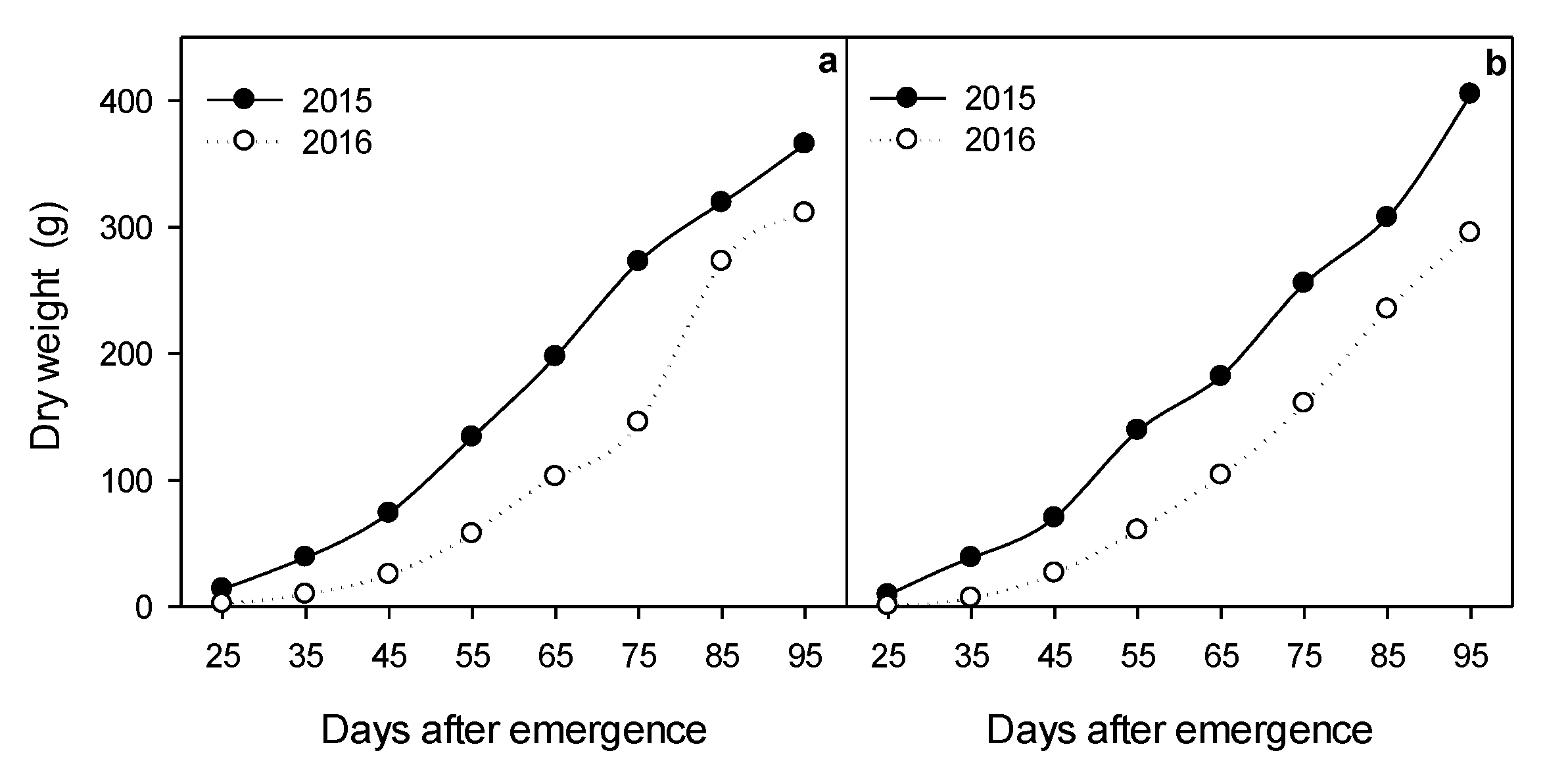
2.2. Biomass Accumulation
2.3. Micronutrient Accumulation
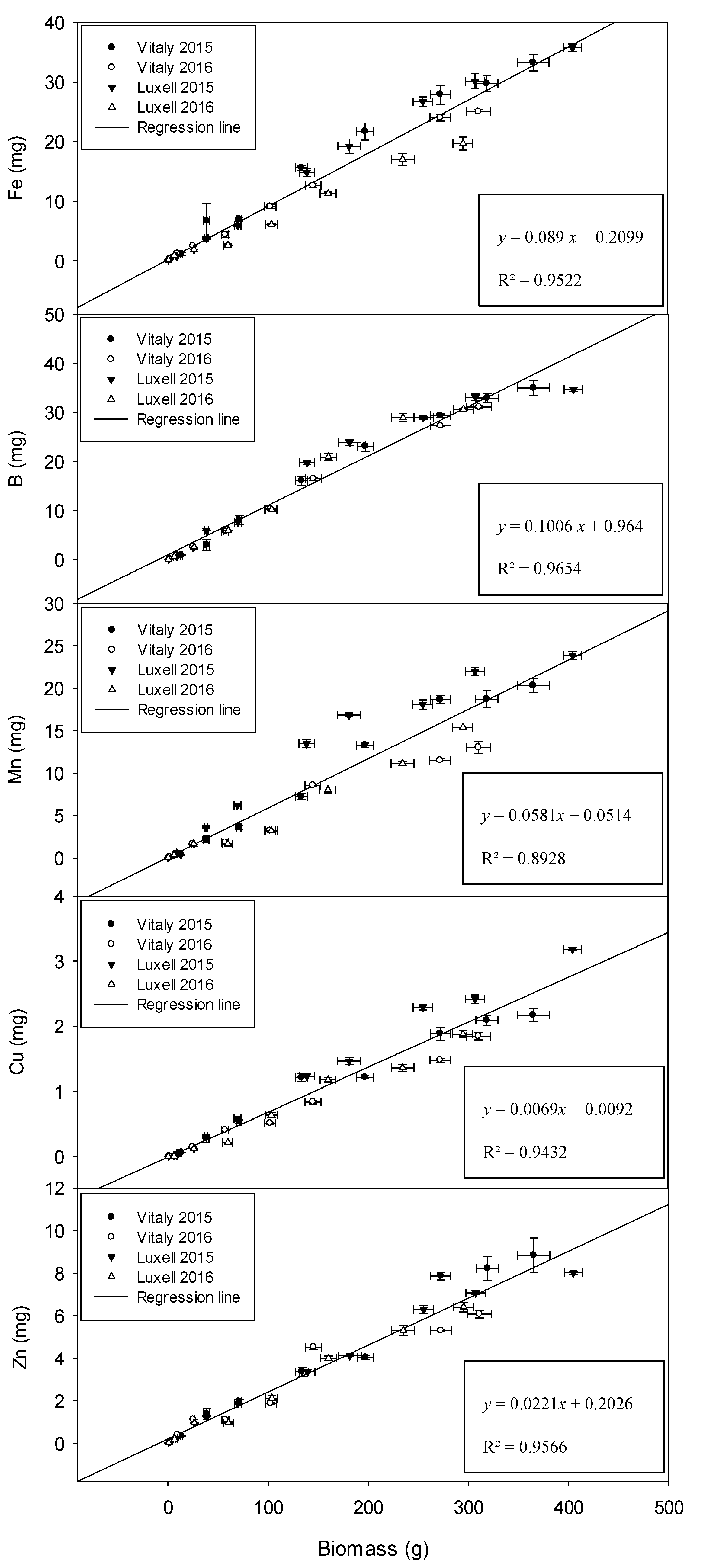
2.4. Relation between Biomass and Micronutrients
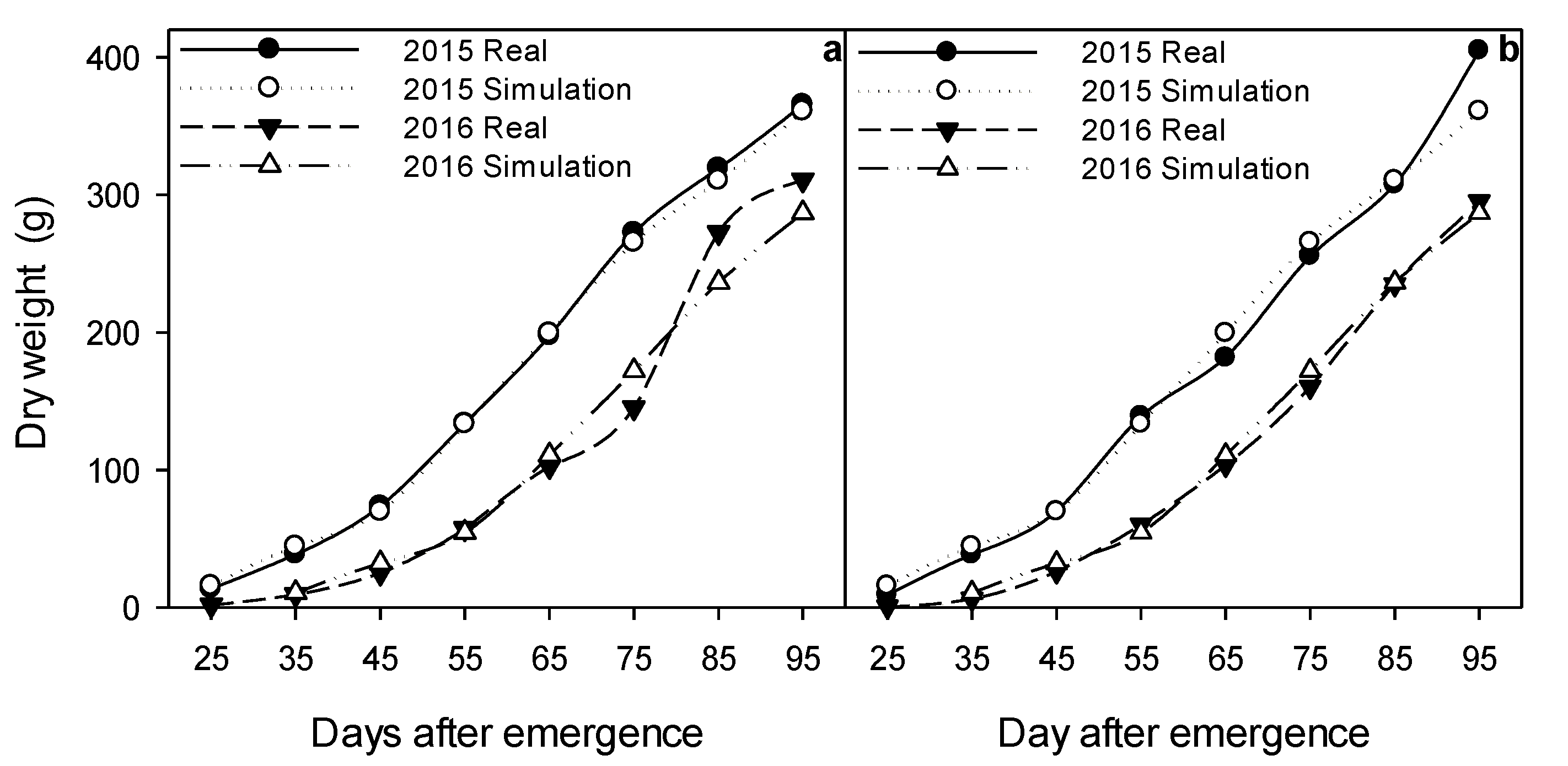
2.5. Dynamic Modeling of Growth and Micronutrient Accumulation
3. Materials and Methods
3.1. Greenhouse Description
3.2. Development of Cucumber Crop
3.3. Recording Climate Variables
3.4. Accumulated Biomass
3.5. Determination of Micronutrient Accumulation
3.6. Description of the Dynamic Model
3.7. Calibration and Validation of the Dynamic Model
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Parameters | Nominal Value | Calibrated Value | Units | Description |
|---|---|---|---|---|
| b1 | 2.7 | m2 g−1 | Coefficient of the change buffer function | |
| d1 | 2.13 × 10−7 | 5.9332 × 10−7 | s−1 | Growth Rate Parameter |
| d2 | 2.47 × 10−7 | 5.4664 × 10−7 | s−1 | Growth Rate Parameter |
| d3 | 20 | °C | Growth Rate Parameter | |
| d4 | 7.50 × 10−11 | 3.46 × 10−13 | - | Growth Rate Parameter |
| F | 1.2 | 1.5 | - | Ratio of assimilated fruit requirements |
| f1 | 8.10 × 10−7 | 6.1 × 10−6 | s−1 | Fruit growth rate coefficient |
| f2 | 4.63 × 10−6 | s−1 | Fruit growth rate coefficient | |
| M | 2.511 | - | Correction-LAI function parameter | |
| mF | 1.157 × 10−7 | 1.5 × 10−6 | s−1 | Breathing coefficient of maintenance of the fruit |
| mL | 2.894 × 10−7 | 2.89 × 10−9 | s−1 | Breathing coefficient of vegetative maintenance |
| p3 | 577 | W m2 | Net photosynthesis parameter | |
| p4 | 221 | g s−1 m−2 | Net photosynthesis parameter | |
| Pm | 2.25 × 10−3 | 2.6 × 10−3 | g s−1 m−2 | Maximum photosynthesis |
| QG | 1 | - | Temperature of the rate of growth of the fruit | |
| QR | 2 | - | Respiration maintenance | |
| T | 86,400 | S | Weather | |
| TG | 20 | °C | Reference temperature of the growth rate | |
| TR | 25 | °C | Reference temperature for maintenance breath | |
| V | 1.23 | 2.23 | - | Ratio of requirements of vegetative assimilates |
| v1 | 1.3774 | 0.45 | - | Relationship of growth vegetative fruit |
| v2 | −0.168 | °C−1 | Relationship of growth vegetative fruit | |
| v3 | 19 | 20 | °C | Relationship of growth vegetative fruit |
| WR | 32.23 | g m−2 | Parameter of the LAI correction function | |
| yF | 0.5983 | 1.05 | - | Parameter of the fruit harvest coefficient |
| yFc | 5.3 | g m−2 | Parameter of cucumber fruit harvest | |
| yL | 0.5983 | 0.35 | - | Leaf Harvest Coefficient Parameter |
| z | 0.6081 | - | Fraction of vegetative dry weight leaf | |
| Fe | 108 | mg kg−1 | Average content of Fe on dry matter base | |
| B | 104 | mg kg−1 | Average content of B on dry matter base | |
| Mn | 54 | mg kg−1 | Average content of Mn on dry matter base | |
| Cu | 6.8 | mg kg−1 | Average content of Cu on dry matter base | |
| Zn | 26 | mg kg−1 | Average content of Zn on dry matter base |
References
- Patidar, D.K.; Maurya, I.B.; Singh, P. Effect of micronutrients on yield and economics of gynoecious cucumber (Cucumis sativus L.) var Kian under naturally-ventilated polyhouse. Int. J. Farm Sci. 2017, 7, 29–32. [Google Scholar]
- USDA, United States Deparment of Agriculture. Vegetables and Outlook. Available online: http://usda.mannlib.cornell.edu/MannUsda/viewDocumentInfo.do?documentID=1401 (accessed on 11 August 2017).
- Hao, H.; Wei, Y.; Yang, X.; Feng, Y.; Wu, C. Effects of different nitrogen fertilizer levels on Fe, Mn, Cu and Zn concentrations in shoot and grain quality in rice (Oryza sativa). Rice Sci. 2007, 14, 289–294. [Google Scholar] [CrossRef]
- Hanjagi, P.S.; Singh, B. Interactive regulation of iron and zinc nutrition in wheat (Triticum aestivum L.). Indian J. Plant Physiol. 2017, 22, 70–78. [Google Scholar] [CrossRef]
- Fan, Z.; Lin, S.; Zhang, X.; Jiang, Z.; Yang, K.; Jian, D.; Chen, Y.; Li, J.; Chen, Q.; Wang, J. Conventional flooding irrigation causes an overuse of nitrogen fertilizer and low nitrogen use efficiency in intensively used solar greenhouse vegetable production. Agric. Water Manag. 2014, 144, 11–19. [Google Scholar] [CrossRef]
- Wang, C.; Gu, F.; Chen, J.; Yang, H.; Jiang, J.; Du, T.; Zhang, J. Assessing the response of yield and comprehensive fruit quality of tomato grown in greenhouse to deficit irrigation and nitrogen application strategies. Agric. Water Manag. 2015. [Google Scholar] [CrossRef]
- Marcelis, L.F.M.; Brajeul, E.; Elings, A.; Garate, A.; Heuvelink, E.; de Visser, P.H.B. Modelling nutrient uptake of sweet pepper. Acta Hortic. 2005, 691, 285–292. [Google Scholar] [CrossRef]
- Du, T.; Kang, S.; Zhang, X.; Zhang, J. China’s food security is threatened by the unsustainable use of water resources in North and Northwest China. Food Energy Secur. 2013, 3, 7–18. [Google Scholar] [CrossRef]
- Cao, J.; Lee, J.; Six, J.; Yan, Y.; Zhang, F.; Fan, M. Changes in potential denitrification-derived N2O emissions following conversion of grain to greenhouse vegetable cropping systems. Eur. J. Soil Biol. 2015, 68, 94–100. [Google Scholar] [CrossRef]
- Alaoui-Sossé, B.; Genet, P.; Vinit-Dunand, F.; Toussaint, M.L.; Epron, D.; Badot, P.M. Effect of copper on growth in cucumber plants (Cucumis sativus) and its relationships with carbohydrate accumulation and changes in ion contents. Plant Sci. 2004, 166, 1213–1218. [Google Scholar] [CrossRef]
- Briat, J.F.; Ravet, K.; Arnaud, N.; Duc, C.; Boucherez, J.; Touraine, B.; Cellier, F.; Gaymard, F. New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann. Bot. 2010, 105, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Aravind, P.; Prasad, M.N.V. Zinc protects chloroplasts and associated photochemical functions in cadmium exposed Ceratophyllum demersum L., a freshwater macrophyte. Plant Sci. 2004, 166, 1321–1327. [Google Scholar] [CrossRef]
- Goussias, C.; Boussac, A.; Rutherford, A.W. Photosystem II and photosynthetic oxidation of water: An overview. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002, 357, 1369–1420. [Google Scholar] [CrossRef] [PubMed]
- Millaleo, R.; Reyes-Diaz, M.; Ivanov, A.G.; Mora, M.L.; Alberdi, M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 470–481. [Google Scholar] [CrossRef]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of Nutrients: Micronutrients. In Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Academic Press: Amsterdam, The Netherlands, 2012; pp. 191–248. [Google Scholar]
- Enriquez-Reyes, S.A.; Alcántar-González, G.; Castellanos-Ramos, J.Z.; Suárez, E.A.; González-Eguiarte, D.; Lazcano-Ferrat, I. Mineral Nutrición mineral acoplada al crecimiento (NUMAC): Nutrición con n para tomate en invernadero 3. Evaluación del Modelo. Terra Latinoam. 2003, 21, 167–175. [Google Scholar]
- Bugarín, M.; Galvis, S.; Sánchez, G.; García, P. Acumulación diaria de materia seca y de potasio en la biomasa aerea total de tomate. Terra Latinoam. 2002, 20, 401–409. [Google Scholar]
- Juárez-Maldonado, A.; Benavides-Mendoza, A.; de-Alba-Romenus, K.; Morales-Díaz, A.B. Dynamic modeling of mineral contents in greenhouse tomato crop. Agric. Sci. 2014, 5, 114–123. [Google Scholar] [CrossRef]
- Bar-Yosef, B.; Fishman, S.; Kläring, H.P. A model-based decision support system for closed irrigation loop greenhouses. Acta Hortic. 2004, 654, 107–122. [Google Scholar] [CrossRef]
- López-Cruz, I.L.; Ramírez-Arias, A.; Rojano-Aguilar, A. Modelos matemáticos de hortalizas en invernadero: Trascendiendo la contemplación de la dinámica de cultivos. Rev. Chapingo Ser. Hortic. 2005, 11, 257–267. [Google Scholar] [CrossRef]
- Kiba, T.; Krapp, A. Plant nitrogen acquisition under low availability: Regulation of uptake and root architecture. Plant Cell Physiol. 2016, 57, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Osvalde, A. Optimization of plant mineral nutrition revisited: The roles of plant requirements, nutrient interactions, and soil properties in fertilization management. Environ. Exp. Biol. 2011, 9, 1–8. [Google Scholar]
- Zhu, L.; Li, Z.; Ketola, T. Biomass accumulations and nutrient uptake of plants cultivated on artificial floating beds in China’s rural area. Ecol. Eng. 2011, 37, 1460–1466. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, C.; Xiao, B.; Cheng, Z. The role of plants in channel-dyke and field irrigation systems for domestic wastewater treatment in an integrated eco-engineering system. Ecol. Eng. 2000, 16, 235–241. [Google Scholar] [CrossRef]
- Marcelis, L.F.M.; Heuvelink, E.; Goudriaan, J. Modelling biomass production and yield of horticultural crops: A review. Sci. Hortic. 1998, 74, 83–111. [Google Scholar] [CrossRef]
- Sakurai, G.; Yamaji, N.; Mitani-Ueno, N.; Yokozawa, M.; Ono, K.; Ma, J.F. A model of silicon dynamics in rice: An analysis of the investment efficiency of Si transporters. Front. Plant Sci. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.J.; Mittra, B.N. Growth and yield of peanut as influenced by degree and duration of shading. J. Agron. Crop Sci. 1988, 160, 260–265. [Google Scholar] [CrossRef]
- Alsadon, A.; Al-Helal, I.; Ibrahim, A.; Abdel-Ghany, A.; Al-Zaharani, S.; Ashour, T. The effects of plastic greenhouse covering on cucumber (Cucumis sativus L.) growth. Ecol. Eng. 2016, 87, 305–312. [Google Scholar] [CrossRef]
- Krizek, D.T. Influence of PAR and UV-A in determining plant sensitivity and photomorphogenic responses to UV-B radiation. Photochem. Photobiol. 2004, 79, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.M.; Hasanuzzaman, M.; Rahman, M.L. Morpho-physiology and yield of cucumber (Cucumis sativa) under varying light intensity. Acad. J. Plant Sci. 2009, 2, 154–157. [Google Scholar]
- Falk, S.; Maxwell, D.P.; Laudenbach, D.E.; Huner, N.P. Photosynthetic adjustment to temperature. In Photosynthesis and the Environment; Baker, N.R., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; pp. 36–385. [Google Scholar]
- Li, H.; Min, X.; Chen, L.; Jalal, G.; Jian, X.; Shi, K.; Considine, M.J.; Quan, J.; Hong, Y. Plant Physiology and Biochemistry Growth temperature-induced changes in biomass accumulation, photosynthesis and glutathione redox homeostasis as in fluenced by hydrogen peroxide in cucumber. Plant Physiol. Biochem. 2013, 71, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Parthasaranthi, T.; Velu, G.; Jeyakumar, P. Impact of crop heat units on growth and developmental physiology of future crop production: A Review. Res. Rev. J. Crop Sci. Technol. 2013, 2, 11–18. [Google Scholar]
- Ghehsareh, A.M.; Samadi, N. Effect of soil acidification on growth indices and microelements uptake by greenhouse cucumber. Afr. J. Agric. Res. 2012, 7, 1659–1665. [Google Scholar]
- Kreij De, C.; Sonneveld, C.; Warmenhoven, M.G.; Straver, N.A. Guide Values for Nutrient Element Contents of Vegetables and Flowers under Glass; No. 15; Research Station for Floriculture and Greenhouse Vegetables Report; Proefstation voor Tuinbouw onder Glas te Naaldwijk: Naaldwijk, The Netherlands, 1992. [Google Scholar]
- Bacaicoa, E.; Garcia-Mina, J.M. Iron Efficiency in Different Cucumber Cultivars: The Importance of Optimizing the Use of Foliar Iron. J. Am. Soc. Hortic. Sci. 2009, 134, 405–416. [Google Scholar]
- Zanin, L.; Tomasi, N.; Rizzardo, C.; Gottardi, S.; Terzano, R.; Alfeld, M.; Janssens, K.; De Nobili, M.; Mimmo, T.; Cesco, S. Iron allocation in leaves of Fe-deficient cucumber plants fed with natural Fe complexes. Physiol. Plant. 2015, 154, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Gopal, R. Manganese and Oxidative Damage in Cucumber. Int. J. Veg. Sci. 2008, 14, 55–66. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, L.; Dixon, M. Greenhouse pepper growth and yield response to copper application. HortScience 2005, 40, 2132–2134. [Google Scholar]
- Soydam, A.S.; Gökçe, E.; Büyük, İ.; Aras, S. Characterization of stress induced by copper and zinc on cucumber (Cucumis sativus L.) seedlings by means of molecular and population parameters. Mutat. Res. Toxicol. Environ. Mutagen. 2012, 746, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Tzerakis, C.; Savvas, D.; Sigrimis, N.; Mavrogiannopoulos, G. Uptake of Mn and Zn by cucumber grown in closed hydroponic systems as influenced by the Mn and Zn concentrations in the supplied nutrient solution. HortScience 2013, 48, 373–379. [Google Scholar]
- Engels, C.; Kirkby, E.; White, P. Mineral Nutrition, Yield and Source–Sink Relationships. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Academic Press: Amsterdam, The Netherlands, 2012; pp. 85–133. [Google Scholar]
- Tap, R.F. Economics-Based Optimal Control of Greenhouse Tomato Crop Production; Wageningen Agricultural University: Wageningen, The Netherlands, 2000. [Google Scholar]
- Wallach, D.; Makowski, D.; Jones, J.W.; Brun, F. Working with Dynamic Crop Models; Academic Press: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Abdel-Rahman, E.M.; Mutanga, O.; Odindi, J.; Adam, E.; Odindo, A.; Ismail, R. Estimating Swiss chard foliar macro- and micronutrient concentrations under different irrigation water sources using ground-based hyperspectral data and four partial least squares (PLS)-based (PLS1, PLS2, SPLS1 and SPLS2) regression algorithms. Comput. Electron. Agric. 2017, 132, 21–33. [Google Scholar] [CrossRef]
- Quesada-Roldán, G.; Bertsch-Hernández, F. Obtención de la curva de extracción nutrimental del híbrido de tomate FB-17. Terra Latinoam. 2013, 31, 1–7. [Google Scholar]
- Rodas-Gaitán, H.A.; Rodríguez-Fuentes, H.; Ojeda-Zacarías, M.C.; Vidales-Contreras, J.A.; Luna-Maldonado, A.I. Macronutrients absorption curves in italian squash (Cucurbita pepo L.). Rev. Fitotec. Mex. 2012, 35, 57–60. [Google Scholar]
- Lombnæs, P.; Singh, B.R. Predicting Zn and Cu status in cereals—Potential for a multiple regression model using soil parameters. J. Agric. Sci. 2003, 141, 349. [Google Scholar] [CrossRef]
- Steiner, A.A. A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil 1961, 15, 134–154. [Google Scholar] [CrossRef]
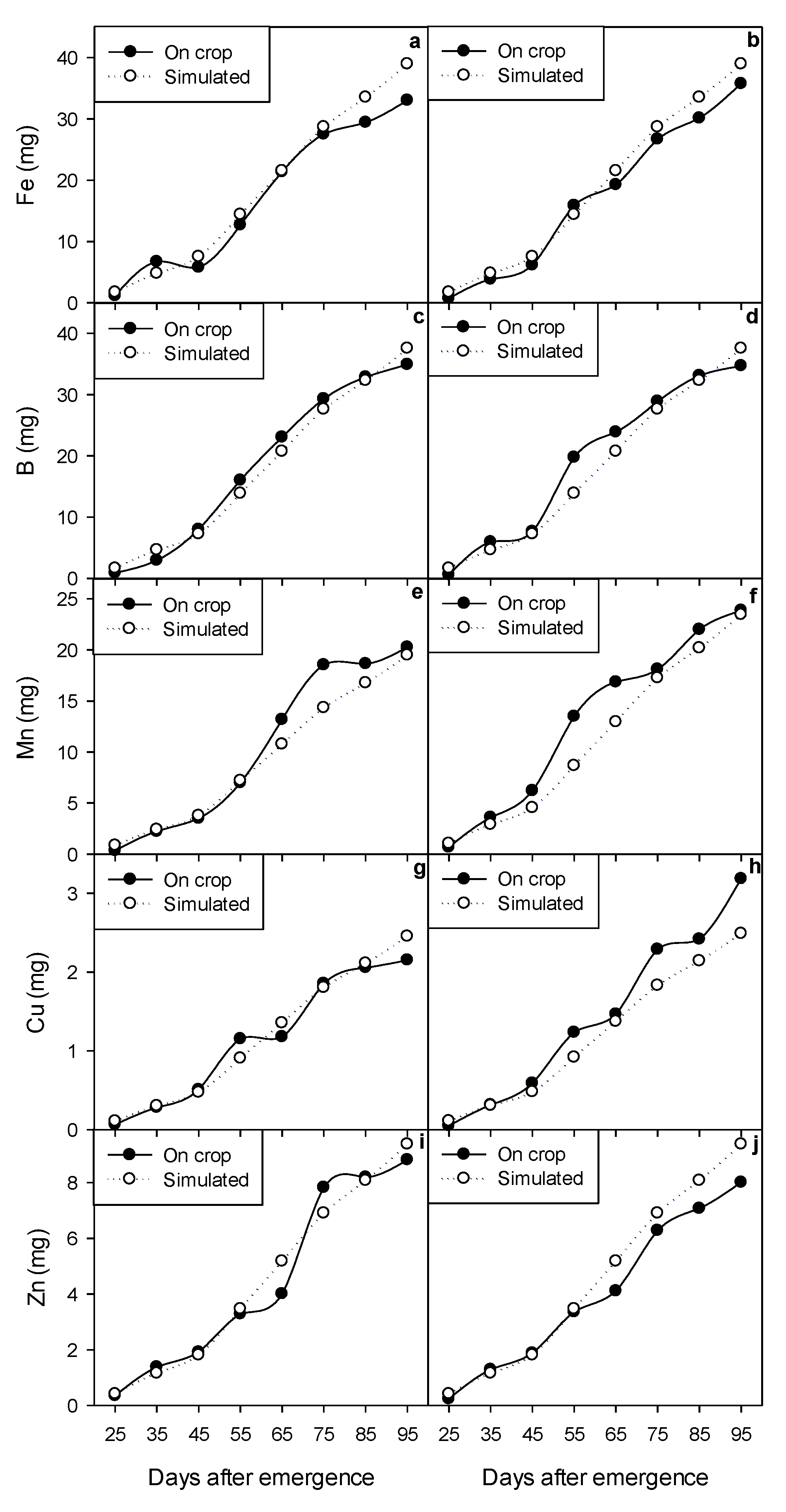
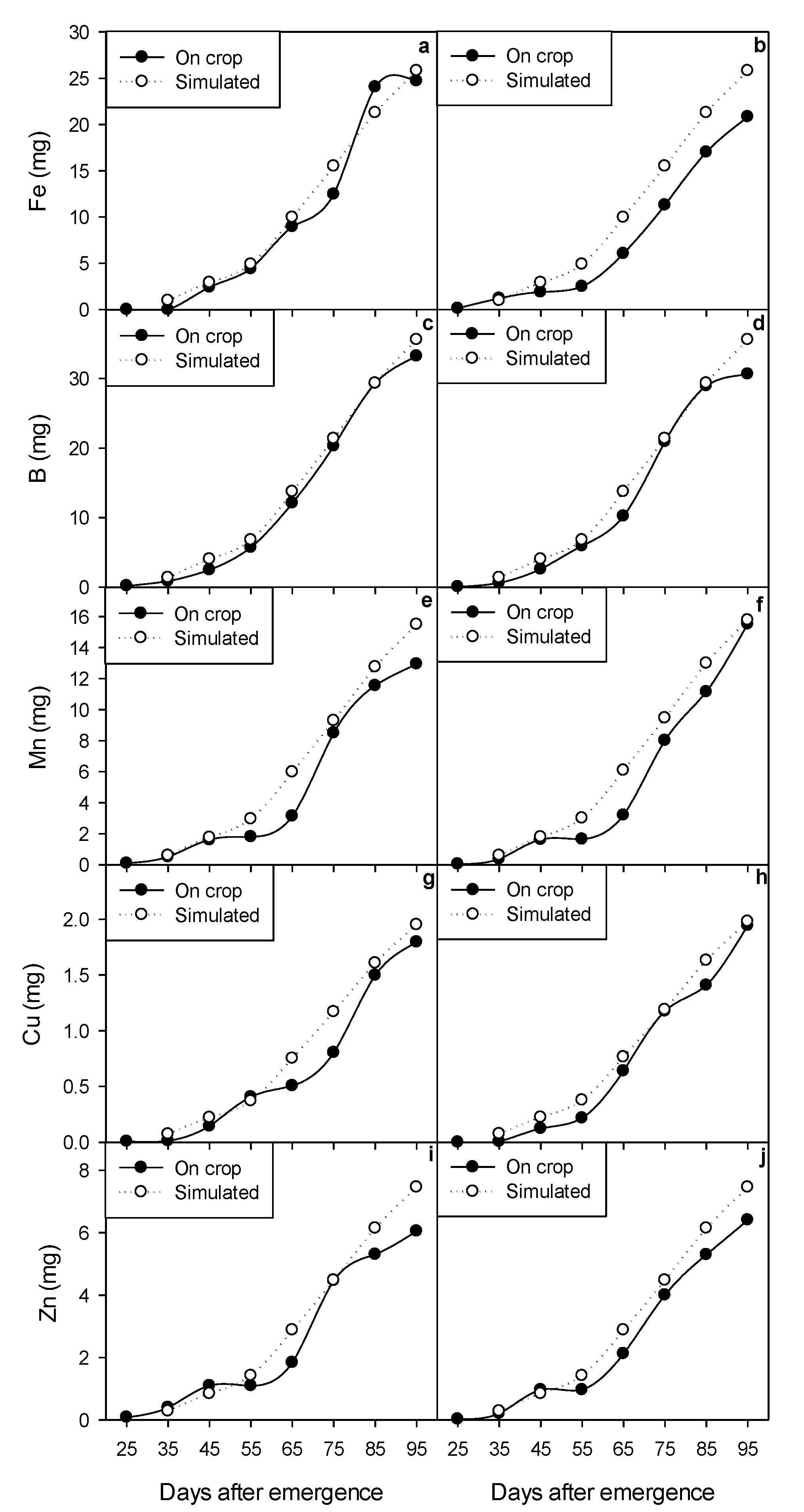
| Fe (mg kg−1 DW) | B (mg kg−1 DW) | Mn (mg kg−1 DW) | Cu (mg kg−1 DW) | Zn (mg kg−1 DW) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| cv | DAE | 2015 | 2016 | 2015 | 2016 | 2015 | 2016 | 2015 | 2016 | 2015 | 2016 |
| Vitaly | 25 | 91.5 ± 13.6 | 187.0 ± 8.0 | 71.4 ± 9.1 | 100.0 ± 0.2 | 27.8 ± 4.2 | 53.1 ± 1.6 | 5.2 ± 0.3 | 4.4 ± 0.5 | 27.7 ± 1.3 | 44.0 ± 1.6 |
| 35 | 167.0 ± 9.7 | 141.5 ± 3.8 | 82.0 ± 20.3 | 100.0 ± 0.8 | 63.3 ± 5.9 | 58.8 ± 2.3 | 7.8 ± 1.0 | 1.5 ± 0.2 | 38.3 ± 4.5 | 45.8 ± 2.1 | |
| 45 | 96.3 ± 10.7 | 99.1 ± 17.4 | 112.8 ± 22.5 | 100.0 ± 0.9 | 50.7 ± 6.5 | 63.3 ± 1.1 | 7.6 ± 0.7 | 5.8 ± 0.9 | 27.5 ± 2.1 | 43.7 ± 0.5 | |
| 55 | 95.5 ± 5.25 | 76.8 ± 29.6 | 120.2 ± 9.3 | 100.0 ± 0.9 | 53.9 ± 5.6 | 34.2 ± 1.8 | 9.1 ± 0.7 | 7.1 ± 0.5 | 25.1 ± 2.4 | 19.3 ± 2.5 | |
| 65 | 110.0 ± 12.5 | 89.2 ± 12.2 | 117.1 ± 8.7 | 118.9 ± 5.8 | 67.3 ± 2.4 | 33.8 ± 1.8 | 6.2 ± 0.3 | 5.0 ± 0.6 | 20.4 ± 0.7 | 18.3 ± 1.9 | |
| 75 | 102.4 ± 8.0 | 86.8 ± 9.0 | 107.7 ± 1.7 | 140.5 ± 15.9 | 68.5 ± 2.4 | 58.8 ± 2.9 | 6.9 ± 0.5 | 5.8 ± 0.7 | 28.8 ± 0.8 | 31.1 ± 2.8 | |
| 85 | 93.2 ± 5.9 | 88.1 ± 7.6 | 103.1 ± 4.1 | 107.7 ± 3.5 | 58.7 ± 4.6 | 42.3 ± 2.3 | 6.6 ± 0.4 | 5.3 ± 0.4 | 25.8 ± 2.5 | 19.4 ± 0.5 | |
| 95 | 91.0 ± 4.3 | 80.5 ± 3.1 | 101.3 ± 4.5 | 107.0 ± 4.8 | 55.7 ± 2.6 | 42.0 ± 5.9 | 5.9 ± 0.3 | 5.7 ± 0.5 | 24.2 ± 2.5 | 19.5 ± 1.5 | |
| Luxell | 25 | 87.3 ± 6.1 | 207.6 ± 23.4 | 72.6 ± 6.7 | 100.0 ± 2.2 | 81.3 ± 7.2 | 67.5 ± 1.3 | 6.1 ± 0.5 | 1.8 ± 0.3 | 28.7 ± 1.4 | 40.6 ± 2.1 |
| 35 | 106.2 ± 9.9 | 155.0 ± 11.0 | 164.7 ± 5.2 | 100.0 ± 1.9 | 99.0 ± 5.6 | 60.2 ± 2.5 | 8.7 ± 0.3 | 1.2 ± 0.1 | 35.7 ± 1.0 | 33.6 ± 1.8 | |
| 45 | 95.2 ± 2.6 | 74.1 ± 4.3 | 110.0 ± 11.8 | 100.0 ± 1.9 | 90.1 ± 2.8 | 62.4 ± 2.4 | 8.8 ± 0.3 | 4.7 ± 0.6 | 27.8 ± 0.9 | 37.5 ± 0.9 | |
| 55 | 111.6 ± 9.3 | 45.4 ± 7.7 | 142.7 ± 5.6 | 100.0 ± 2.1 | 97.9 ± 6.2 | 27.8 ± 1.7 | 9.2 ± 0.6 | 3.6 ± 0.2 | 24.7 ± 1.5 | 16.8 ± 1.7 | |
| 65 | 108.0 ± 10.4 | 61.4 ± 2.6 | 131.7 ± 5.8 | 100.0 ± 2.4 | 93.5 ± 1.9 | 31.4 ± 1.5 | 8.3 ± 0.5 | 6.7 ± 0.9 | 22.8 ± 0.3 | 21.1 ± 2.3 | |
| 75 | 105.4 ± 8.2 | 71.7 ± 4.1 | 113.4 ± 2.9 | 131.1 ± 10.5 | 71.3 ± 5.7 | 50.2 ± 4.8 | 9.1 ± 0.4 | 7.4 ± 0.5 | 24.7 ± 1.9 | 25.2 ± 1.4 | |
| 85 | 99.1 ± 12.9 | 74.8 ± 9.2 | 107.9 ± 7.0 | 123.8 ± 6.9 | 72.0 ± 4.7 | 48.3 ± 2.3 | 8.0 ± 0.7 | 6.3 ± 0.5 | 23.2 ± 1.0 | 22.9 ± 2.1 | |
| 95 | 88.6 ± 6.7 | 68.5 ± 12.0 | 85.8 ± 4.0 | 104.1 ± 2.2 | 59.0 ± 5.5 | 52.5 ± 2.3 | 7.9 ± 0.3 | 6.6 ± 0.7 | 19.8 ± 0.7 | 21.9 ± 2.6 | |
| Bio | Fe | B | Mn | Cu | Zn | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DAE | 2015 | 2016 | 2015 | 2016 | 2015 | 2016 | 2015 | 2016 | 2015 | 2016 | 2015 | 2016 | |
| Bio | V | 1 | 1 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.98 | 0.98 | 0.99 | 0.99 | 0.97 |
| L | 1 | 1 | 0.99 | 0.99 | 0.99 | 0.97 | 0.99 | 0.97 | 0.99 | 0.99 | 0.99 | 0.99 | |
| Fe | V | 1 | 1 | 0.99 | 0.99 | 0.99 | 0.98 | 0.99 | 0.99 | 0.98 | 0.97 | ||
| L | 1 | 1 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | |||
| B | V | 1 | 1 | 0.99 | 0.99 | 0.99 | 0.99 | 0.97 | 0.98 | ||||
| L | 1 | 1 | 0.98 | 0.99 | 0.98 | 0.98 | 0.99 | 0.98 | |||||
| Mn | V | 1 | 1 | 0.98 | 0.98 | 0.98 | 0.99 | ||||||
| L | 1 | 1 | 0.99 | 0.98 | 0.99 | 0.98 | |||||||
| Cu | V | 1 | 1 | 0.98 | 0.97 | ||||||||
| L | 1 | 1 | 0.99 | 0.99 | |||||||||
| Zn | V | 1 | 1 | ||||||||||
| L | 1 | 1 | |||||||||||
| Biomass | Fe | B | Mn | Cu | Zn | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cv | EF | Index | EF | Index | EF | Index | EF | Index | EF | Index | EF | Index | |
| 2015 | V | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 |
| L | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.98 | 0.99 | 0.97 | 0.99 | 0.98 | 0.99 | |
| 2016 | V | 0.99 | 0.99 | 0.99 | 0.99 | 0.95 | 0.99 | 0.93 | 0.99 | 0.95 | 0.99 | 0.96 | 0.99 |
| L | 0.99 | 0.99 | 0.84 | 0.98 | 0.98 | 0.99 | 0.95 | 0.99 | 0.98 | 0.99 | 0.96 | 0.99 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Pérez, L.J.; Morales-Díaz, A.B.; De Alba-Romenus, K.; González-Morales, S.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Determination of Micronutrient Accumulation in Greenhouse Cucumber Crop Using a Modeling Approach. Agronomy 2017, 7, 79. https://doi.org/10.3390/agronomy7040079
Ramírez-Pérez LJ, Morales-Díaz AB, De Alba-Romenus K, González-Morales S, Benavides-Mendoza A, Juárez-Maldonado A. Determination of Micronutrient Accumulation in Greenhouse Cucumber Crop Using a Modeling Approach. Agronomy. 2017; 7(4):79. https://doi.org/10.3390/agronomy7040079
Chicago/Turabian StyleRamírez-Pérez, Lino J., América Berenice Morales-Díaz, Karim De Alba-Romenus, Susana González-Morales, Adalberto Benavides-Mendoza, and Antonio Juárez-Maldonado. 2017. "Determination of Micronutrient Accumulation in Greenhouse Cucumber Crop Using a Modeling Approach" Agronomy 7, no. 4: 79. https://doi.org/10.3390/agronomy7040079





