Climate Change and Pest Management: Unanticipated Consequences of Trophic Dislocation
Abstract
:1. Introduction
1.1. Trophic Dislocation
1.2. Implications for Agriculture
2. Methods
2.1. Simulation Models
2.2. The Pests
3. Results
3.1. Species Responses
3.1.1. Stalk Borer Damage
3.1.2. Armyworm Summer Population
3.1.3. Mexican Bean Beetle Survival
3.1.4. Potato Leafhopper Generations
3.2. Thermoperiodic Response to Climate Change
4. Discussion and Conclusions
4.1. Ecological Implications
4.2. Agricultural Implications
4.3. Broader Implications
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schiermeier, Q. The costs of global warming. Nature 2006, 439, 374–375. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.J.; Edmonds, J.; Hartin, C.A.; Mundra, A.; Calvin, K. Near-term acceleration in the rate of temperature change. Nat. Clim. Chang. 2015, 5, 333–336. [Google Scholar] [CrossRef]
- Seager, R.; Ting, M.; Held, I.; Kushnir, Y.; Lu, J.; Vecchi, G.; Huang, H.-P.; Harnik, N.; Leetmaa, A.; Lau, N.-C.; et al. Model projections of an imminent transition to a more arid climate in southwestern North America. Science 2007, 316, 1181–1184. [Google Scholar] [CrossRef] [PubMed]
- Allan, R.P.; Soden, B.J. Atmospheric warming and the amplification of precipitation extremes. Science 2008, 321, 1481–1484. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.G.; Mosley-Thompson, E.; Brecher, H.; Davis, M.; Leon, B.; Les, D.; Lin, P.N.; Mashiotta, T.; Mountain, K. Abrupt tropical climate change: Past and present. Proc. Natl. Acad. Sci. USA 2006, 103, 10536–10543. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, R.J.; Cazenave, A. Sea-level rise and its impact on coastal zones. Science 2010, 328, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Dukes, J.S.; Pontius, J.; Orwig, D.; Garnas, J.R.; Rodgers, V.L.; Brazee, N.; Cooke, B.; Theoharides, K.A.; Stange, E.E.; Harrington, R.; et al. Responses of insect pests, pathogens and invasive plant species to climate change in the forests of northeastern North America: What can we predict? Can. J. For. Res. 2009, 39, 231–248. [Google Scholar] [CrossRef]
- Maclean, I.M.D.; Wilson, R.J. Recent ecological responses to climate change support predictions of high extinction risk. Proc. Natl. Acad. Sci. USA 2011, 108, 12337–12342. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.M.; Parker, G.G.; Miller, D.R. Evidence for a recent increase in forest growth. Proc. Natl. Acad. Sci. USA 2010, 107, 3611–3615. [Google Scholar] [CrossRef] [PubMed]
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate trends and global crop production since 1980. Science 2011, 29, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Schlenker, W.; Roberts, M.J. Nonlinear temperature effects indicate severe damages to U.S. crop yields under climate change. BioScience 2008, 58, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- Zvereva, E.L.; Kozlov, M.V. Consequences of simultaneous elevation of carbon dioxide and temperature for plant-herbivore interactions: A meta-analysis. Glob. Chang. Biol. 2006, 72, 27–41. [Google Scholar] [CrossRef]
- Hoekman, D. Turning up the heat: Temperature influences the relative importance of top-down and bottom-up effects. Ecology 2010, 91, 2819–2825. [Google Scholar] [CrossRef] [PubMed]
- Harrington, R.; Woiwod, I.; Sparks, T.H. Climate change and trophic interactions. Trend. Ecol. Evol. 1999, 14, 146–150. [Google Scholar] [CrossRef]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasas, A.; Ahass, R.; Alm-Tüsler, K.; Bissolli, P.; Braslavská, O.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Glob. Chang. Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Schwartz, M.D.; Reiter, B.E. Changes in North American spring. Int. J. Clim. 2000, 20, 929–932. [Google Scholar] [CrossRef]
- Gibbs, J.P.; Breisch, A.R. Climate warming and calling phenology of frogs near Ithaca, New York, 1900–1999. Conserv. Biol. 2001, 15, 1175–1178. [Google Scholar] [CrossRef]
- Dunn, P.O.; Winkler, D.W. Climate change has affected the breeding date of tree swallows throughout North America. Proc. R. Soc. Lond. B 1999, 266, 2487–2490. [Google Scholar] [CrossRef]
- Jonzén, N.; Lindén, A.; Ergon, T.; Knudsen, E.; Vik, J.O.; Rubolini, D.; Piacentini, D.; Brinch, C.; Spina, F.; Karlsson, L.; et al. Rapid advance of spring arrival dates in long-distance migratory birds. Science 2006, 312, 1959–1961. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.B.; Sparks, T.H. Phenology of British butterflies and climate change. Glob. Chang. Biol. 2000, 6, 407–416. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Fleming, R.A.; Tatchell, G.M. Shifts in the flight responses of British aphids: A response to climate warming? In Insects in a Changing Environment; Harrington, R., Stork, N.E., Eds.; Academic Press: London, UK, 1995; pp. 505–508. [Google Scholar]
- Buse, A.; Dury, S.J.; Woodburn, R.J.W.; Perrins, C.M.; Good, J.E.G. Effects of elevated temperature on multi-species interactions: The case of pedunculate oak, winter moth and tits. Funct. Ecol. 1999, 13, 74–82. [Google Scholar] [CrossRef]
- Sanz, J.J.; Potti, J.; Moreno, J.; Merino, S.; Frias, O. Climate change and fitness components of a migratory bird breeding in the Mediterranean region. Glob. Chang. Biol. 2003, 9, 461–472. [Google Scholar] [CrossRef]
- Winkler, D.W.; Dunn, P.O.; McCulloch, C.E. Predicting the effects of climate change on avian life-history traits. Proc. Natl. Acad. Sci. USA 2002, 99, 13595–13599. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.E.; Adriaensen, F.; van Balen, J.H.; Blondel, J.; Dhondt, A.A.; van Dongen, S.; du Feu, C.; Ivankina, E.V.; Kerimov, A.B.; de Laet, J.; et al. Variable responses to large-scale climate change in European Parus populations. Proc. R. Soc. Lond. B 2003, 270, 367–372. [Google Scholar]
- Pounds, J.A.; Bustamante, M.R.; Coloma, L.A.; Consuegra, J.A.; Fogden, M.P.L.; Foster, P.N.; la Marca, E.; Masters, K.L.; Merino-Viteri, A.; Puschendorf, R.; et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 2006, 439, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Coates, K.D.; Hamann, A. Is an unprecedented Dothistroma needle blight epidemic related to climate change? BioScience 2005, 55, 761–769. [Google Scholar] [CrossRef]
- Ghandi, K.J.K.; Herms, D.A. Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forest of eastern North America. Biol. Invasions 2010, 12, 389–405. [Google Scholar]
- Bentz, B.J.; Régnière, J.; Fettig, C.J.; Hansen, E.M.; Hayes, J.L.; Hicke, J.A.; Kelsey, R.G.; Negrón, J.F.; Seybold, S.J. Climate change and bark beetles of the western United States and Canada: Direct and indirect effects. BioScience 2010, 60, 602–613. [Google Scholar] [CrossRef]
- Cudmore, T.J.; Bjorklund, N.; Carroll, A.L.; Lindgren, S. Climate change and range expansion of an aggressive bark beetle: Evidence of higher beetle reproduction in naïve host tree populations. J. Appl. Ecol. 2010, 47, 1036–1043. [Google Scholar] [CrossRef]
- Volney, W.J.A.; Fleming, R.A. Climate change and impacts of boreal forest insects. Agric. Ecosyst. Environ. 2000, 82, 283–294. [Google Scholar] [CrossRef]
- Stinner, B.R.; Taylor, R.A.J.; Hammond, R.B.; Purrington, F.F.; McCartney, D.A.; Rodenhouse, N.; Barrett, G.W. Potential effects of climate change on plant-pest interactions. In The Potential Effects of Global Climate Change on the United States. Appendix C—Agriculture; Smith, J.B., Tirpak, D.A., Eds.; U.S. Environmental Protection Agency: Washington, DC, USA, 1989; p. 35. [Google Scholar]
- DeLucia, E.H.; Casteel, C.L.; Nabity, P.D.; O’Neill, B.F. Insects take a bigger bite out of plants in a warmer, higher carbon dioxide world. Proc. Natl. Acad. Sci. USA 2009, 105, 1781–1782. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fleischer, S.J.; Tobin, P.C.; Saunders, M.C. Projecting insect voltinism under high and low greenhouse gas emission conditions. Environ. Entomol. 2011, 40, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Trumble, J.T.; Butler, C.D. Change will exacerbate California’s insect pest problems. Calif. Agric. 2009, 63, 73–78. [Google Scholar] [CrossRef]
- North American Plant Protection Organization (NAPPO). Climate Change and Pest Risk Analysis; Discussion Paper. Available online: https://www.nappo.org/files/4814/3781/8174/Climate_Change_Discussion_DocumentRev-07-08-12-e.pdf (accessed on 10 December 2017).
- Lindroth, R.L. Impacts of elevated atmospheric CO2 and O3 on forests: Phytochemistry, trophic interactions and ecosystem dynamics. J. Chem. Ecol. 2010, 36, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Sharpley, A.N.; Williams, J.R. (Eds.) EPIC—Erosion/Productivity Impact Calculator: 1. Model Documentation; United States Department of Agriculture: Washington, DC, USA, 1990.
- Gassman, P.W.; Williams, J.R.; Benson, V.W.; Izaurralde, R.C.; Hauck, L.; Jones, C.A.; Atwood, J.D.; Kiniry, J.; Flowers, J.D. Historical Development and Applications of the EPIC and APEX Models; Center for Agricultural and Rural Development, Iowa State University: Ames, IA, USA, 2005. [Google Scholar]
- Rasche, L.; Taylor, R.A.J. A generic pest submodel for use in integrated assessment models. Trans. ASABE 2017, 60, 147–158. [Google Scholar]
- Source of County Soils Data. Available online: https://datagateway.nrcs.usda.gov (accessed on 10 December 2017).
- Source of Historical Weather Data. 1901–2000. Available online: https://www.ncdc.noaa.gov (accessed on 10 December 2017).
- Source of Predicted Weather Data. 2001–2100. Available online: http://nomads.gfdl.noaa.gov (accessed on 10 December 2017).
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2007: The Physical Science Basis: Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Stouffer, R.J.; Broccoli, A.J.; Delworth, T.L.; Dixon, K.W.; Gudgel, R.; Held, I.; Hemler, R.; Knutseon, T.; Lee, H.-C.; Schwartzkopf, M.D.; et al. GFDL’s CM2 Global coupled climate models. Part IV: Idealized climate response. J. Clim. 2006, 19, 723–740. [Google Scholar] [CrossRef]
- Taylor, R.A.J.; Shields, E.J. Revisiting potato leafhopper, Empoasca fabae, migration: Implications in a world where invasive insects are all too common. Am. Entomol. 2018, in press. [Google Scholar]
- Mann, M.E.; Bradley, R.S.; Hughes, M.K. Global-scale temperature patterns and climate forcing over the past six centuries. Nature 1998, 392, 779–787. [Google Scholar] [CrossRef]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Ainsworth, E.A.; Leakey, A.D.B.; Nösberger, J.; Ort, D.R. Food for thought: Lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 2006, 312, 1918–1921. [Google Scholar] [CrossRef] [PubMed]
- Clements, D.R.; DiTommaso, A. Predicting weed invasion in Canada under climate change: Evaluating evolutionary potential. Can. J. Plant Sci. 2012, 92, 1013–1020. [Google Scholar] [CrossRef]
- Ziska, L.H. Climate change and plant protection: Emerging viral and weed threats. Abstracts of Special Session at the 2011 APS-IPPC Joint Meeting in Honolulu, Hawaii. Phytopathology 2011, 101, S240. [Google Scholar]
- Bradley, B.A.; Estes, L.D.; Hole, D.G.; Holness, S.; Oppenheimer, M.; Turner, W.R.; Beukes, H.; Schulze, R.E.; Tadross, M.A. Predicting how adaptation to climate change could affect ecological conservation: Secondary impacts of shifting agricultural suitability. Divers. Distrib. 2012, 18, 425–437. [Google Scholar] [CrossRef]
- Ziska, L.H. The role of climate change and increasing atmospheric carbon dioxide on weed management: Herbicide efficacy. Agric. Ecosyst. Environ. 2016, 231, 304–309. [Google Scholar] [CrossRef]
- Schmitz, O.J.; Post, E.; Burns, C.E.; Johnston, K.M. Ecosystem responses to global climate change: Moving beyond color mapping. BioScience 2003, 53, 1199–1205. [Google Scholar] [CrossRef]
- Watt, A.D.; Whittaker, J.B.; Docherty, M.; Brooks, G.; Lindsay, E.; Salt, D. The impact of elevated atmospheric CO2 on insect herbivores. In Insects in a Changing Environment; Harrington, R., Stork, N.E., Eds.; Academic Press: London, UK, 1995; pp. 198–217. [Google Scholar]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Parker, J.S.; Moore, R.; Weaver, M. Land tenure as a variable in community based watershed projects: Some lessons from the Sugar Creek watershed, Wayne and Holmes County, Ohio. Soc. Nat. Res. 2007, 20, 815–833. [Google Scholar] [CrossRef]
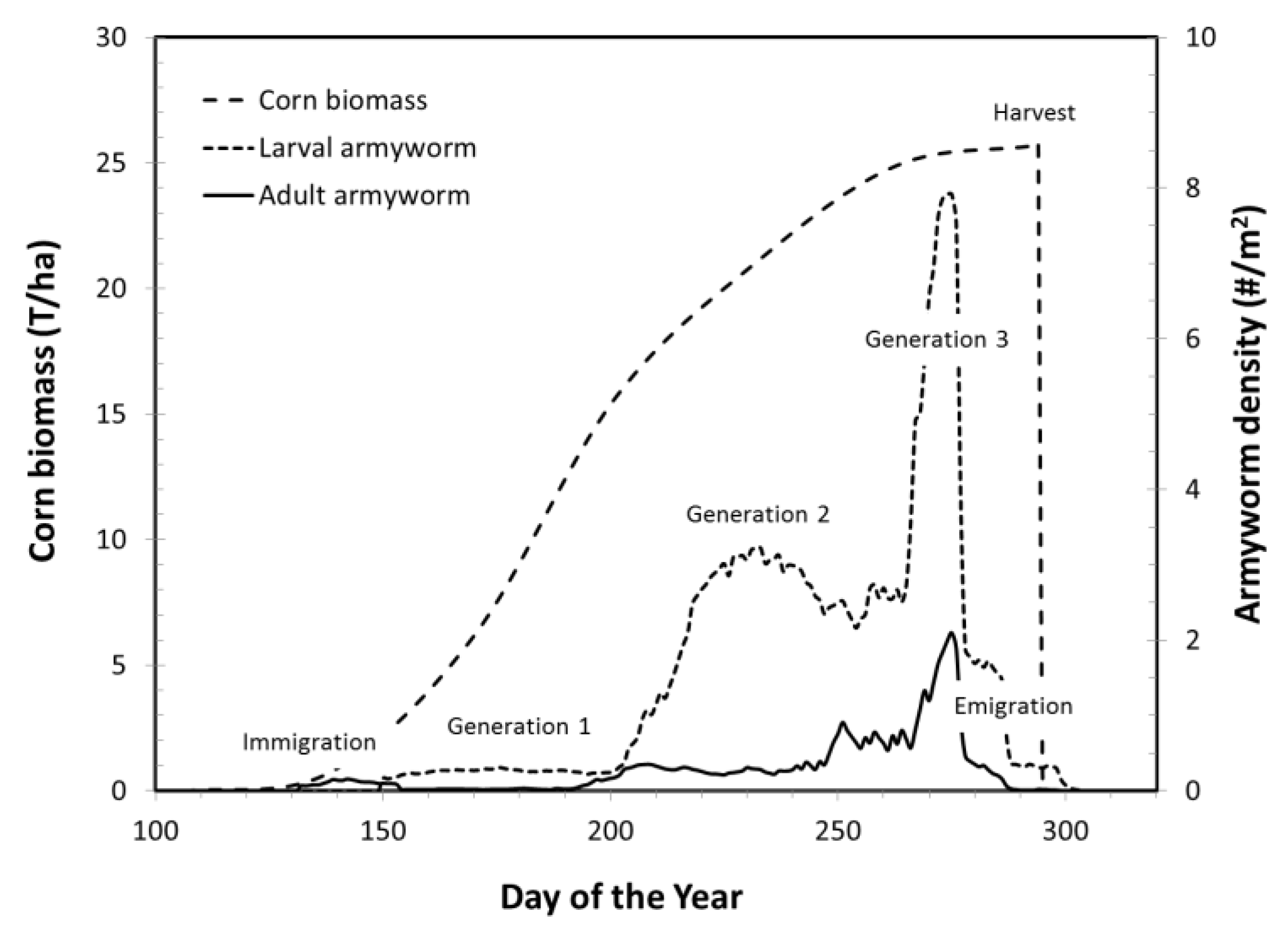

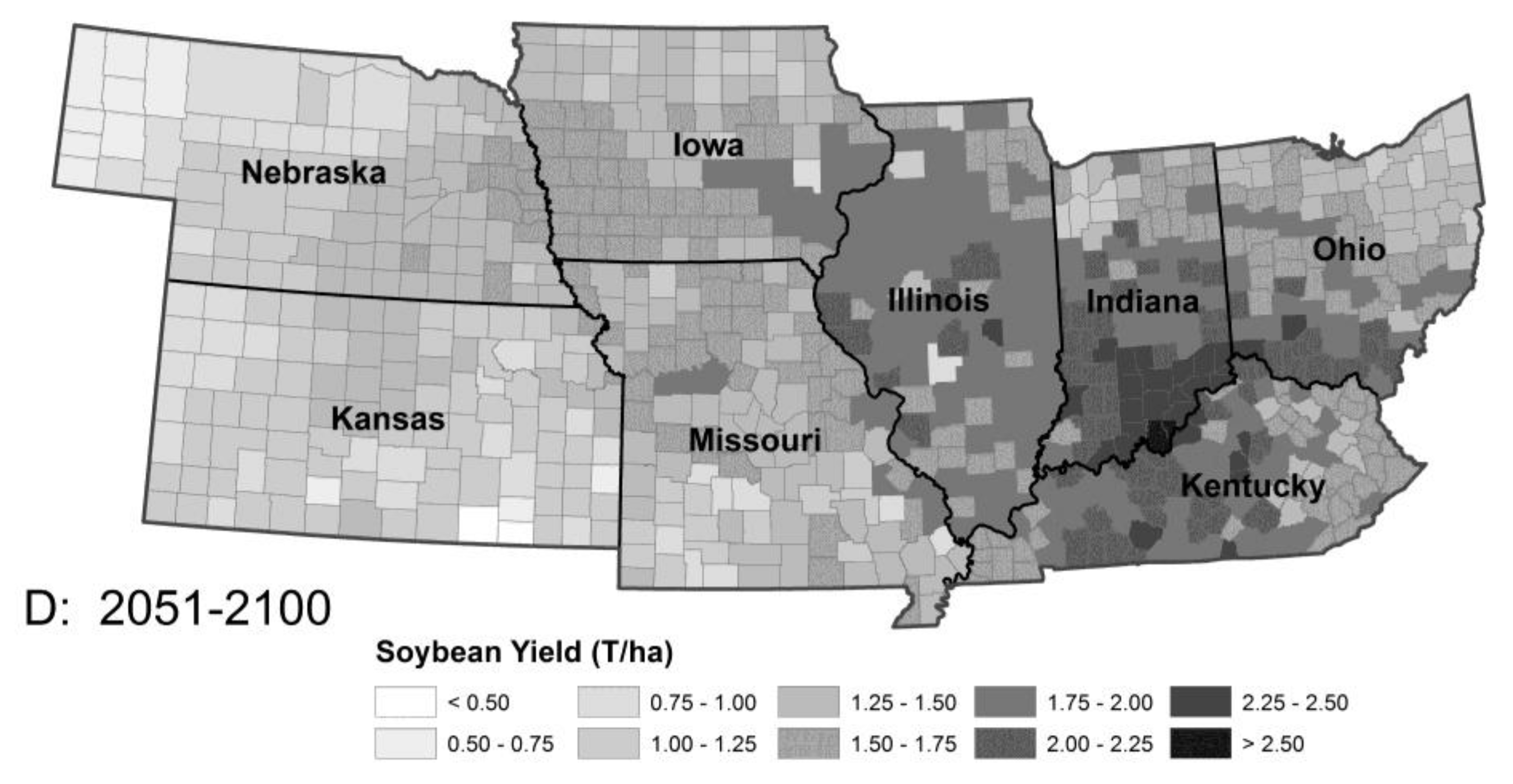
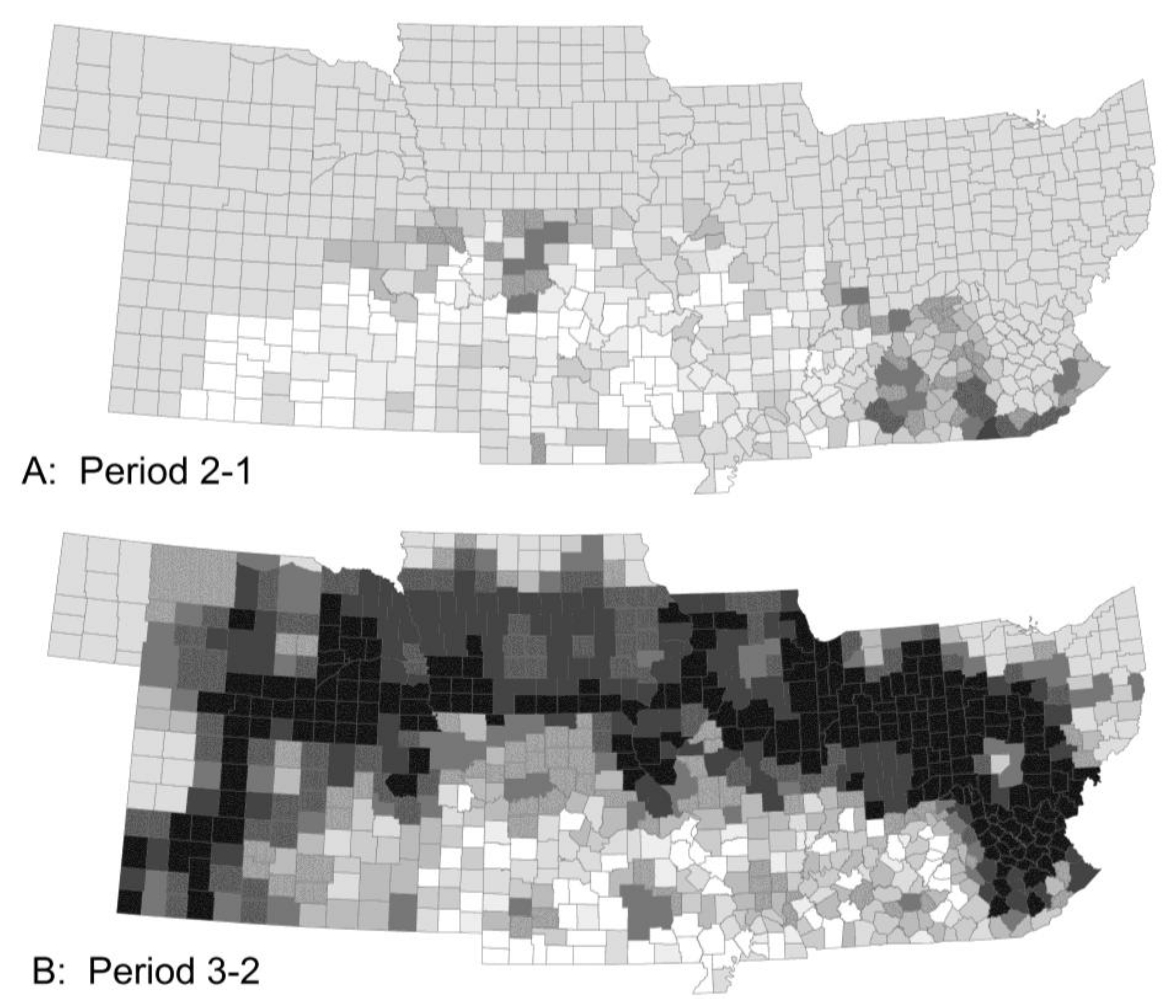
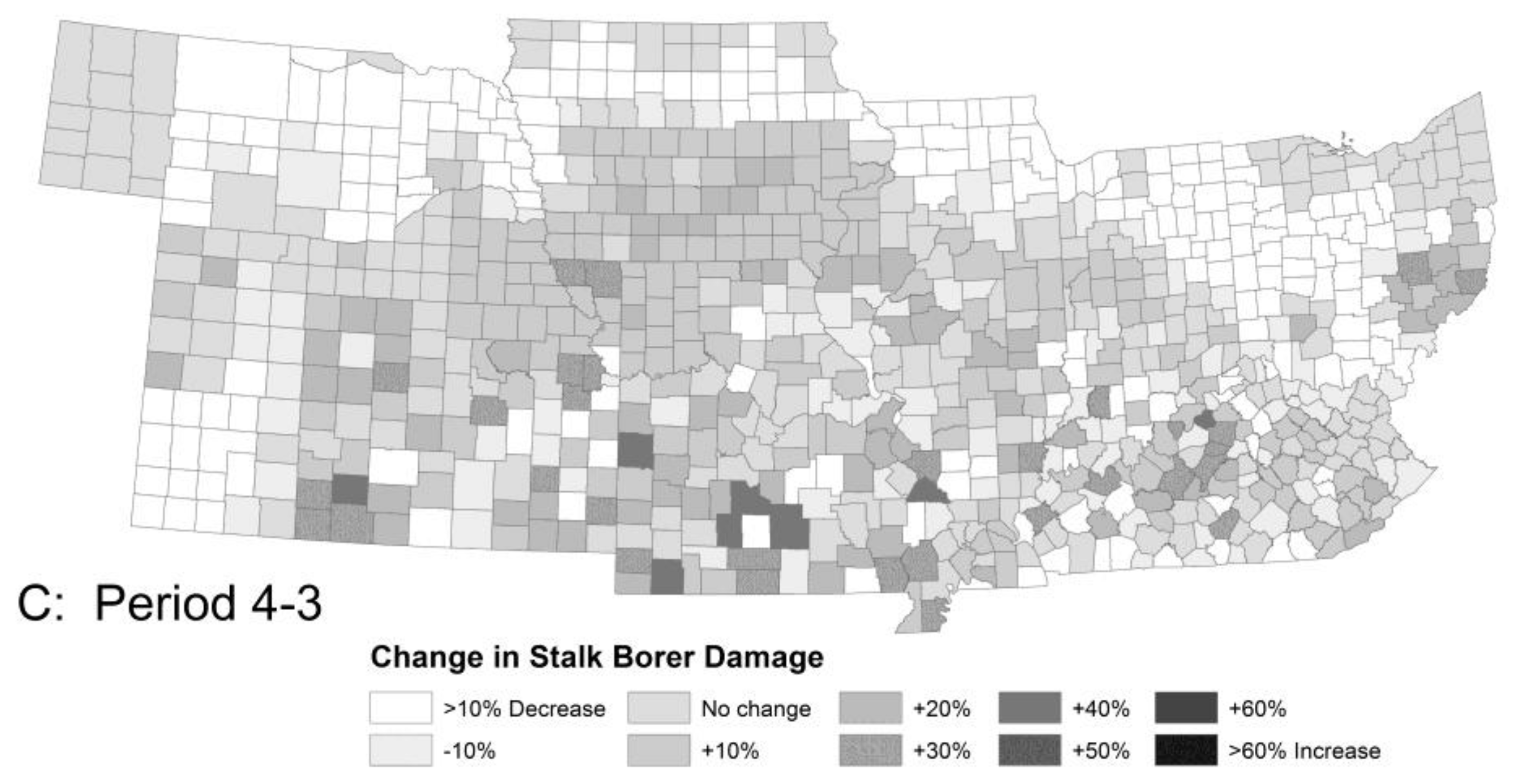

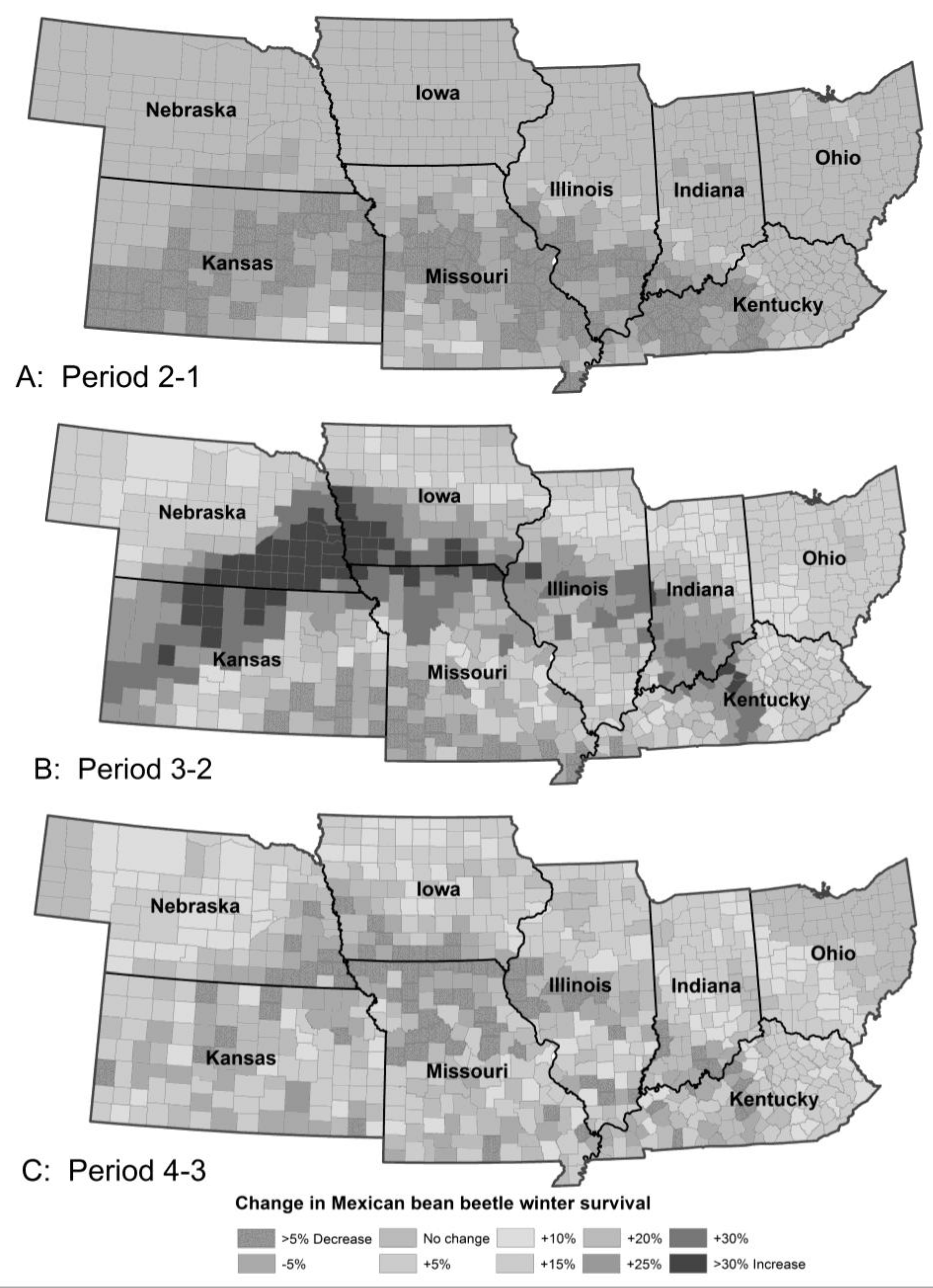
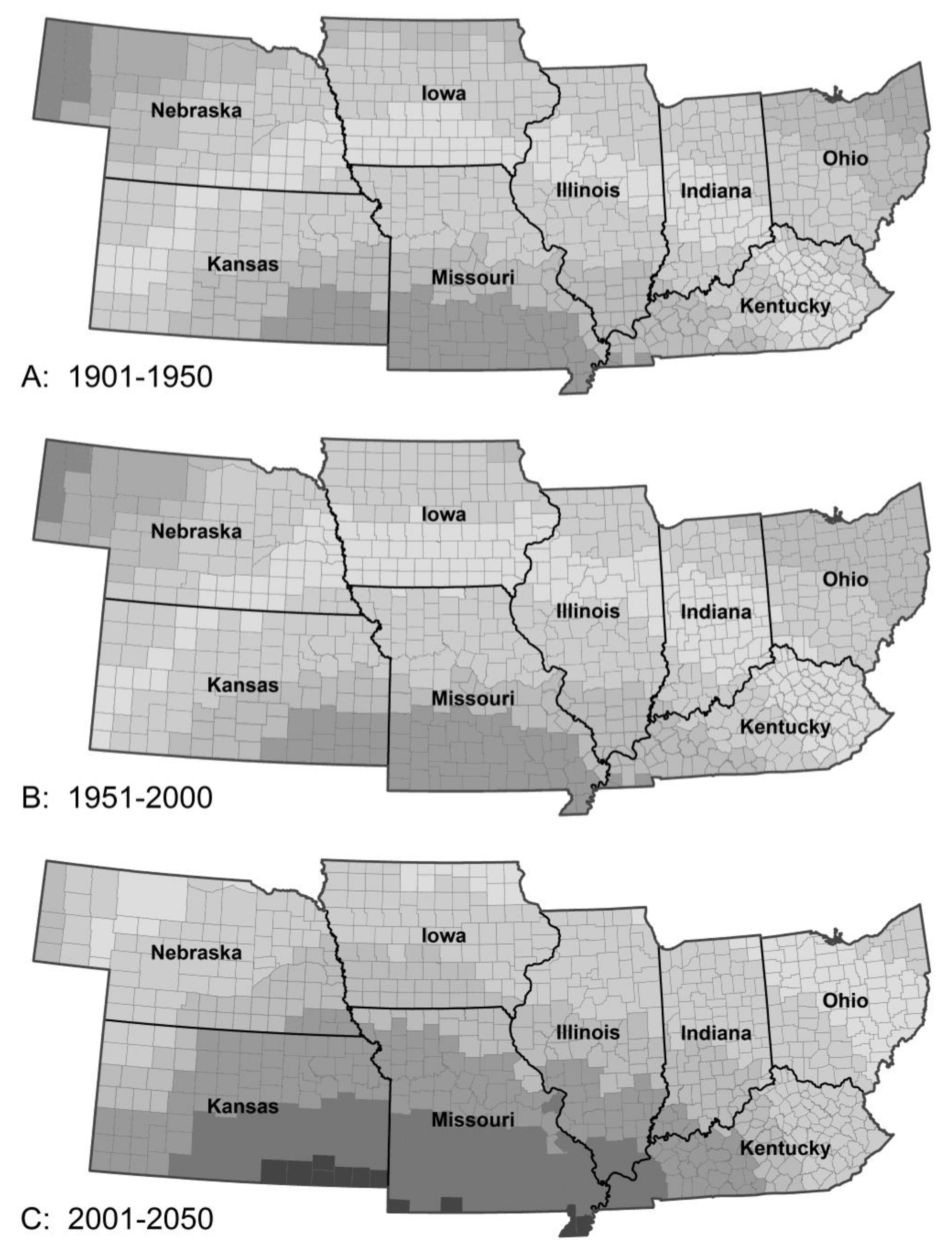

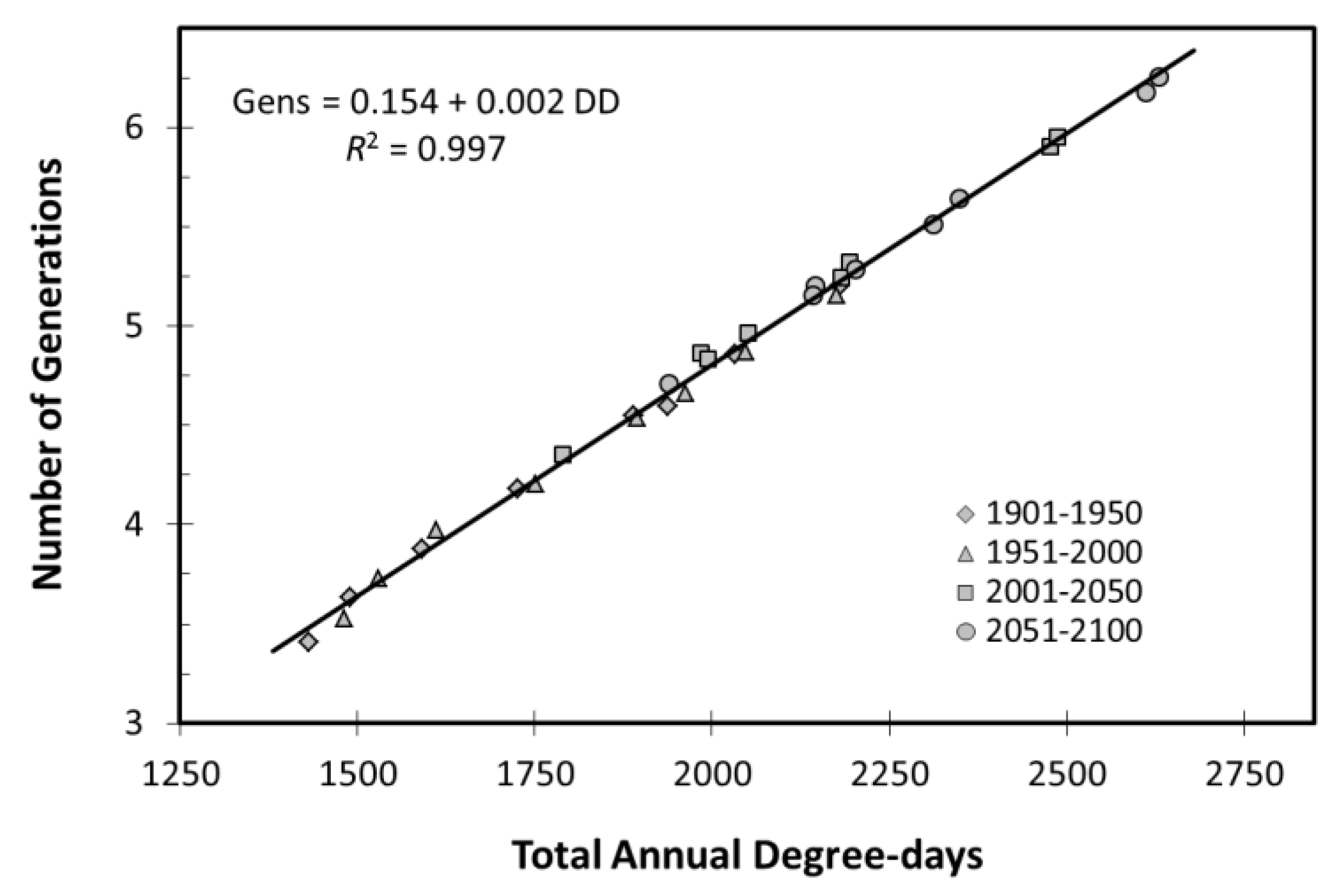

| Pest Species | Status | Crops | Fecundity: Total Eggs; Eggs/Day | Feeding Rate (mg/Day) | Damage Potential | Thresholds (°C) | Degree-Days to Adult | Adult Lifespan (Days) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Beans | Corn | Upper | Lower | |||||||
| Coleoptera | ||||||||||
| Bean leaf beetle | R | B | 350; 35 | 3.5 | 6.5 | - | 30 | 10 | 490 | 30 |
| (Ceratoma trifurcata) | ||||||||||
| Mexican bean beetle | R | B | 300; 30 | 3.5 | 12.5 | - | 30 | 11.5 | 385 | 20 |
| (Epilachna varivestis) | ||||||||||
| Lepidoptera | ||||||||||
| Armyworm | M | C | 200; 20 | 25 | - | 25 | 30 | 9 | 610 | 15 |
| (Pseudaletia unipuncta) | ||||||||||
| Black cutworm | M | C | 100; 10 | 8 | - | 10 | 35 | 8 | 350 | 30 |
| (Agrotis ipsilon) | ||||||||||
| Corn earworm | RM | BC | 200; 40 | 6 | 8 | 50 | 35 | 12.5 | 480 | 20 |
| (Heliothis zea) | ||||||||||
| European corn borer | R | BC | 300; 30 | 8 | 8 | 3 | 30 | 9.5 | 660 | 15 |
| (Ostrinia nubilalis) | ||||||||||
| Stalk borer | R | BC | 750; 75 | 20 | 5 | 5 | 30 | 9 | 1250 | 10 |
| (Papaipama nebris) | ||||||||||
| Velvetbean caterpillar | M | B | 300; 30 | 25 | 25 | - | 35 | 11 | 485 | 20 |
| (Anticarsia gemmatalis) | ||||||||||
| Homoptera | ||||||||||
| Potato leafhopper | M | B | 600; 20 | 1.5 | 6.5 | - | 30 | 8.5 | 415 | 35 |
| (Empoasca fabae) | ||||||||||
| Pest Species & Crop | Change from 1901 to 2000 | Change from 1901 to 2100 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield | Damage | Expose | Peak | Initial | Gens | Yield | Damage | Expose | Peak | Initial | Gens | ||
| (%) | (%) | (days) | (days) | (% or d) | (%) | (%) | (%) | (days) | (days) | (% or d) | (%) | ||
| Bean leaf beetle | B | 3.1 | −8.4 | −1.4 | −3.4 | −0.9% | −8.4 | 37 | 430 | 15.3 | 18.5 | 13.3% | 73.4 |
| Mexican bean beetle | B | 3.1 | −2.1 | −1.7 | −1.6 | −2.5% | 0.8 | 37 | 42 | 2.6 | 16.6 | 4.4% | 7.8 |
| Armyworm | C | 2.9 | 30.1 | 2.6 | 5.0 | −2.5 d | 7.1 | 21 | 100 | 14.4 | 19.2 | −7.0d | 26.0 |
| Black cutworm | C | 2.9 | 4.7 | 4.5 | 5.5 | −3.6 d | 5.6 | 21 | 16 | 18.9 | 18.8 | −9.1d | 23.8 |
| Corn earworm | C | 3.1 | 0 | 1.3 | 1.1 | 0.9 d | 1.3 | 37 | 480 | 14.2 | 32.8 | −7.1d | 31.6 |
| B | 2.9 | 42.9 | 4.5 | 7.5 | −3.4 d | 8.6 | 21 | 136 | 14.2 | 30.7 | −8.2d | 29.0 | |
| European corn borer | C | 3.1 | 5.0 | 2.7 | −0.1 | 2.6% | 1.2 | 37 | 1000 | 35.4 | 39.2 | 17.6% | 28.4 |
| B | 2.9 | 38.6 | 11.7 | 4.7 | 1.9% | 7.7 | 21 | 800 | 36.7 | 37.8 | 18.7% | 27.5 | |
| Stalk borer | C | 3.1 | 1.7 | 0.1 | 0.9 | 0.6% | 0 | 37 | 165 | −13.8 | 60.1 | 1.5% | 0 |
| B | 2.9 | 0.4 | −4.3 | 1.6 | 1.1% | 0 | 21 | 153 | −12.3 | 59.2 | 1.4% | 0 | |
| Velvetbean caterpillar | B | 3.1 | 2.0 | 2.7 | 1.0 | 0.5 d | 0.9 | 37 | 326 | 15.4 | 21.1 | −9.9d | 34.6 |
| Potato leafhopper | B | 3.1 | 4.1 | 1.3 | 1.1 | 1.5 d | 1.0 | 37 | 155 | 12.8 | 27.4 | −8.1d | 27.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, R.A.J.; Herms, D.A.; Cardina, J.; Moore, R.H. Climate Change and Pest Management: Unanticipated Consequences of Trophic Dislocation. Agronomy 2018, 8, 7. https://doi.org/10.3390/agronomy8010007
Taylor RAJ, Herms DA, Cardina J, Moore RH. Climate Change and Pest Management: Unanticipated Consequences of Trophic Dislocation. Agronomy. 2018; 8(1):7. https://doi.org/10.3390/agronomy8010007
Chicago/Turabian StyleTaylor, R. A. J., Daniel A. Herms, John Cardina, and Richard H. Moore. 2018. "Climate Change and Pest Management: Unanticipated Consequences of Trophic Dislocation" Agronomy 8, no. 1: 7. https://doi.org/10.3390/agronomy8010007




