Effects of Steel Slag and Biochar Incorporation on Active Soil Organic Carbon Pools in a Subtropical Paddy Field
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Determination of Soil Properties and Calculation of CPMI
2.3. Statistical Analysis
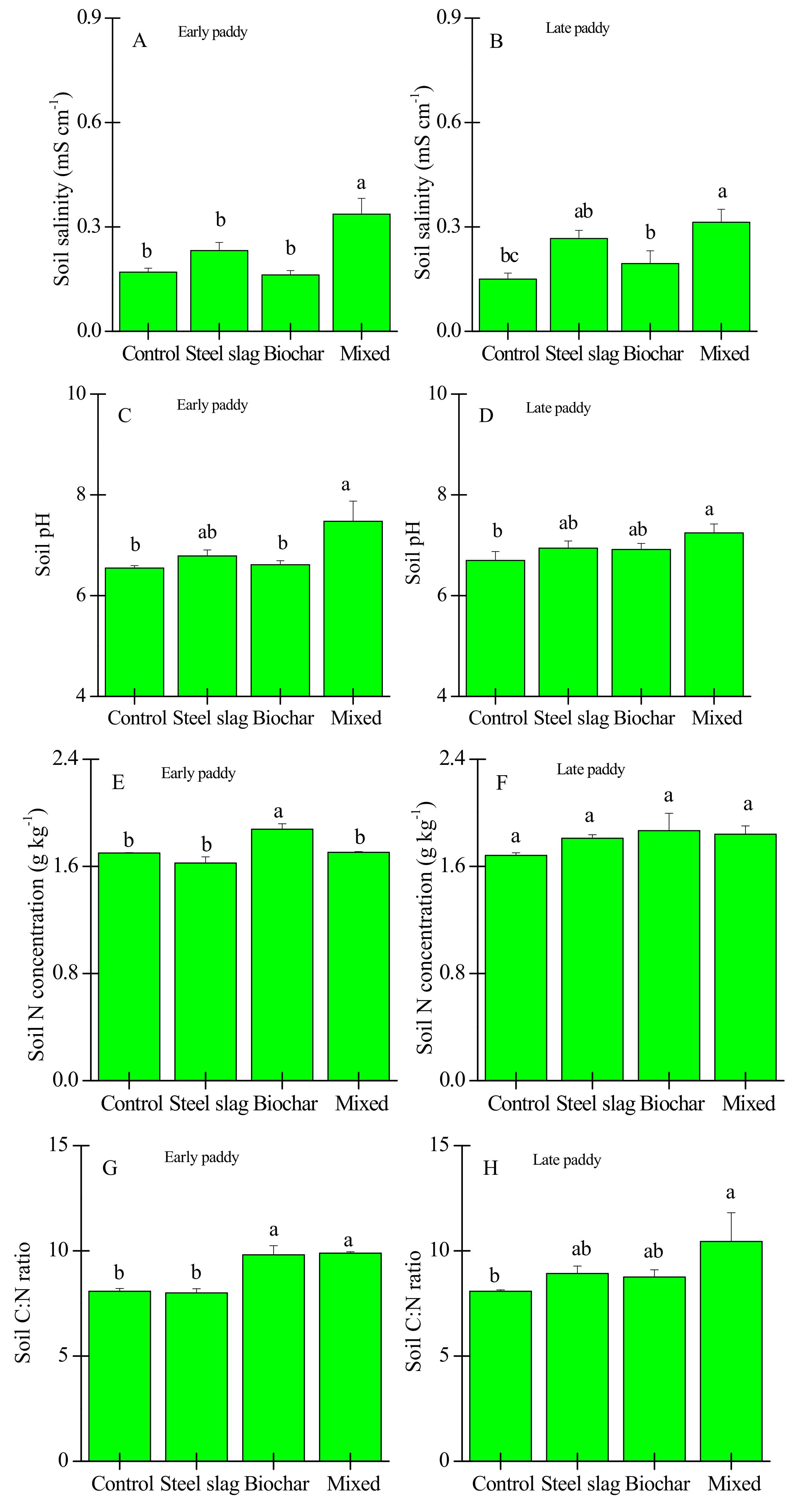
3. Results
3.1. Effect of Waste Amendment on the Concentrations of TOC, Active C Fractions, and C Release Rate
3.2. Effects of Waste Amendment on Other Soil Properties
3.3. Relationships between SOC Fractions and Soil Properties
3.4. Effects of Waste Amendment on Soil C Pool Management Index
4. Discussion
4.1. Effects of Waste Amendment on Soil Properties
4.2. Effects of Waste Amendment on Soil Active SOC Fractions and C:N Ratio
4.3. Effects of Waste Amendment on Soil CPMI
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Sardans, J.; Lai, D.Y.F.; Wang, C.; Zeng, C.; Tong, C.; Liang, Y.; Peñuelas, J. Effects of steel slag application on greenhouse gas emissions and crop yield over multiple growing seasons in a subtropical paddy field in China. Field Crop. Res. 2015, 171, 146–156. [Google Scholar] [CrossRef] [Green Version]
- Frolking, S.; Qiu, J.; Boles, S.; Xiao, X.; Liu, J.; Zhuang, Y.; Li, C.; Qin, X. Combining remote sensing and ground census data to develop new maps of the distribution of rice agriculture in China. Glob. Biogeochem. Cycle 2002, 16, 1091–1101. [Google Scholar] [CrossRef]
- Hou, H.; Peng, S.; Xu, J.; Yang, S.; Mao, Z. Seasonal variations of CH4 and N2O emissions in response to water management of paddy fields located in Southeast China. Chemosphere 2012, 89, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Fageria, N.K. Yield physiology of rice. J. Plant Nutr. 2007, 30, 843–879. [Google Scholar] [CrossRef]
- Food and Agricultural Organization of the United Nations (FAO). OECD-FAO Agricultural Outlook 2011–2030. 2009. Available online: http://aii.caas.net.cn/AgriOutlook/pdf/3-B3.Stefania%20Vannuccini_OECD-FAO%20Agricultural%20Outlook%202013-2022_China%20Fish%20Outlook.pdf (accessed on 28 May 2018).
- Pan, G.; Li, L.; Wu, L.; Zhang, X. Storage and sequestration potential of topsoil organic carbon in China’s paddy soils. Glob. Chang. Biol. 2004, 10, 79–92. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.R.; Yu, Z.L.; Zhao, S.D. arbon storage and budget of major Chinese forest types. Acta Phytoecol. Sin. 2000, 24, 518–522. [Google Scholar]
- Ni, J. Carbon storage in grasslands of China. J. Arid Environ. 2002, 50, 205–218. [Google Scholar] [CrossRef]
- Wissing, L.; Kölbl, A.; Vogelsang, V.; Fu, J.; Cao, Z.; Kögel-Knabner, I. Organic carbon accumulation in a 2000-year chronosequence of paddy soil evolution. Catena 2011, 87, 376–385. [Google Scholar] [CrossRef]
- Wang, W.; Lai, D.Y.F.; Wang, C.; Pan, T.; Zeng, C. Effects of rice straw incorporation on active soil organic carbon pools in a subtropical paddy field. Soil Till. Res. 2015, 152, 8–16. [Google Scholar] [CrossRef]
- Zhang, A.; Cui, L.; Pan, G.; Li, L.; Hussain, Q.; Zhang, X.; Zheng, J.; Crowley, D. Effect of biochar amendment on yield and methane and nitrous oxide emissions from a rice paddy from Tai Lake plain, China. Agric. Ecosyst. Environ. 2010, 139, 469–475. [Google Scholar] [CrossRef]
- Wang, W.; Li, P.; Zeng, C.; Tong, C. Evaluation of silicate iron slag as a potential methane mitigating method. Adv. Mater. Res. 2012, 468, 1626–1630. [Google Scholar] [CrossRef]
- Wang, W.; Lai, D.Y.F.; Li, S.; Kim, P.J.; Zeng, C.; Li, P.; Liang, Y. Steel slag amendment reduces methane emission and increases rice productivity in subtropical paddy fields in China. Wetl. Ecol. Manag. 2014, 22, 683–691. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.A.; Lima, I.M.; Lamb, M.C.; McAloon, A.J.; et al. Biochar: A synthesis of its agronomic impact beyond carbon sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y.; Inubushi, K. Feasible suppression technique of methane emission from paddy soil by iron amendment. Nutr. Cycl. Agroecosyst. 2002, 64, 193–201. [Google Scholar] [CrossRef]
- Spokas, K.A.; Koskinen, W.C.; Baker, J.M.; Reicosky, D.C. Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere 2009, 77, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, M.; Wu, Y.; Wang, H.; Chen, Y.; Wu, W. Reducing CH4 and CO2 emissions from waterlogged paddy soil with biochar. J. Soil. Sediment. 2011, 11, 930–939. [Google Scholar] [CrossRef]
- Kimura, M.; Murase, J.; Lu, Y. Carbon cycling in rice field ecosystems in the context of input, decomposition and translocation of organic materials and the fates of their end products (CO2 and CH4). Soil. Biol. Biochem. 2004, 36, 1399–1416. [Google Scholar] [CrossRef]
- Chen, H.L.; Zhou, J.M.; Xiao, B.H. Characterization of dissolved organic matter derived from rice straw at different stages of decay. J. Soil Sediment. 2010, 10, 915–922. [Google Scholar] [CrossRef]
- Purakayastha, T.J.; Rudrappa, L.; Singh, D.; Swarup, A.; Bhadraray, S. Long-term impact of fertilizers on soil organic carbon pools and sequestration rates in maize-wheat-cowpea cropping system. Geoderma 2008, 144, 370–378. [Google Scholar] [CrossRef]
- Gong, W.; Yan, X.; Wang, J.; Hu, T.; Gong, Y. Long-term manuring and fertilization effects on soil organic carbon pools under a wheat-maize cropping system in North China Plain. Plant Soil 2009, 149, 318–324. [Google Scholar] [CrossRef]
- Xu, R.; Zhao, A.; Yuan, J.; Jiang, J. pH buffering capacity of acid soils from tropical and subtropical regions of China as influenced by incorporation of crop straw biochars. J. Soil Sediment. 2012, 12, 494–502. [Google Scholar] [CrossRef]
- Xu, M.; Lou, Y.; Sun, X.; Wang, W.; Baniyamuddin, M.; Zhao, K. Soil organic carbon active fractions as early indicators for total carbon change under straw incorporation. Biol. Fert. Soils 2011, 47, 745–752. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Nayak, A.K.; Mohanty, S.; Tripathi, R.; Mohammad, S.; Anjani, K.; Raja, R.; Panda, B.B.; Roy, K.S.; Neogi, S.; et al. Greenhouse gas emission in relation to labile soil C, N pools and functional microbial diversity as influenced by 39 years long-term fertilizer management in tropical rice. Soil Till. Res. 2013, 129, 93–105. [Google Scholar] [CrossRef]
- Srinivasarao, C.; Venkateswarlu, B.; Lal, R.; Singh, A.K.; Kundu, S.; Vittal, K.P.R.; Patel, J.J.; Patel, M.M. Long-term manuring and fertilizer effects on depletion of soil organic carbon stocks under pearl millet-cluster bean-castor rotation in Western India. Land Degrad. Dev. 2014, 25, 173–183. [Google Scholar] [CrossRef]
- Neogi, S.; Bhattacharyya, P.; Roy, K.S.; Panda, B.B.; Nayak, A.K.; Rao, K.S.; Manna, M.C. Soil respiration, labile carbon pools and enzyme activities as affected by tillage practices in a tropical rice-maize-cowpea cropping system. Environ. Monit. Assess. 2014, 186, 4223–4236. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Qin, A.Z.; Gan, Y.T.; Yu, A.Z. Higher yield and lower carbon emission by intercropping maize with rape, pea, and wheat in arid irrigation areas. Agron. Sustain. Dev. 2013, 34, 535–543. [Google Scholar] [CrossRef]
- Gan, Y.; Liang, C.; Chai, Q.; Lemke, R.L.; Campbell, C.A.; Zentner, R.P. Improving farming practices reduces the carbon footprint of spring wheat production. Nat. Commun. 2014, 5, 6012. [Google Scholar] [CrossRef] [PubMed]
- Knoblauch, C.; Maarifat, A.A.; Pfeiffer, E.M.; Faefele, S.M. Degradability of black carbon and its impact on trace gas fluxes and carbon turnover in paddy soils. Soil Biol. Biochem. 2011, 43, 1768–1778. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Roy, K.S.; Neogi, S.; Adhya, T.K.; Rao, K.S.; Manna, M.C. Effects of rice straw and nitrogen fertilization on greenhouse gas emissions and carbon storage in tropical flooded soil planted with rice. Soil Till. Res. 2012, 124, 119–130. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Roy, K.S.; Neogi, S.; Chakravorti, S.P.; Behera, K.S.; Das, K.M.; Bardhan, S.; Rao, K.S. Effect of long term application of organic amendment on C storage in relation to global warming potential and biological activities in tropical flooded soil planted to rice. Nutr. Cycl. Agroecosyst. 2012, 94, 273–285. [Google Scholar] [CrossRef]
- Hanke, A.; Cerli, C.; Muhr, J.; Borken, W.; Kalbitz, K. Redox control on carbon mineralization and dissolved organic matter along a chronosequence of paddy soils. Eur. J. Soil Sci. 2013, 64, 476–487. [Google Scholar] [CrossRef]
- Blair, N.; Faulkner, R.D.; Till, A.R.; Poulton, P.R. Long-term management impactions on soil C, N and physical fertility. Part I: Broadbalk experiment. Soil Till. Res. 2006, 91, 30–38. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, G.; Han, X.; Feng, Y.; Ren, G. Stratification of carbon fractions and carbon management index in deep soil affected by the Grain-to-Green Program in China. PLoS ONE 2014, 9, e99657. [Google Scholar] [CrossRef] [PubMed]
- Myhre, G.; Shindell, D.; Bréon, F.M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and Natural Radiative Forcing. In Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Ali, M.A.; Oh, J.H.; Kim, P.J. Evaluation of silicate iron slag amendment on reducing methane emission from flood water rice farming. Agric. Ecosyst. Environ. 2008, 128, 21–26. [Google Scholar] [CrossRef]
- Ali, M.A.; Lee, C.H.; Kim, P.J. Effect of silicate fertilizer on reducing methane emission during rice cultivation. Biol. Fert. Soils 2008, 44, 597–604. [Google Scholar] [CrossRef]
- Chung, H.; Ngo, K.J.; Plante, A.; Six, J. Evidence for carbon saturation in a highly structured and organic-matter-rich soil. Soil Sci. Soc. Am. J. 2010, 74, 130–138. [Google Scholar] [CrossRef]
- Zhang, B.; Pang, C.; Qin, J.; Liu, K.; Xu, H.; Li, H. Rice straw incorporation in winter with fertilizer-N application improves soil fertility and reduces global warming potential from a double rice paddy field. Biol. Fert. Soils 2013, 49, 1039–1052. [Google Scholar] [CrossRef]
- Wassmann, R.; Neue, H.U.; Bueno, C.; Lantin, R.S.; Alberto, M.C.R.; Buendia, L.V.; Bronson, K.; Papen, H.; Rennenberg, H. Methane production capacities of different rice soil derived from inherent and exogenous substrates. Plant and Soil 1998, 203, 227–237. [Google Scholar] [CrossRef]
- Lou, Y.; Xu, M.; Wang, W.; Sun, X.; Liang, C. Soil organic carbon fractions and management index after 20 yr of manure and fertilizer application for greenhouse vegetables. Soil Use Manag. 2011, 27, 163–169. [Google Scholar] [CrossRef]
- Wang, W.; Sardans, J.; Zeng, C.; Zhong, C.; Li, Y.; Peñuelas, J. Response of soil nutrient concentrations and stoichiometry to increased human disturbance in a subtropical tidal wetland. Geoderma 2014, 232, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.K. Analysis Methods of Soil Science and Agricultural Chemistry; Agriculture Science and Technology Press: Beijing, China, 1999. [Google Scholar]
- Wang, W.; Wang, C.; Sardans, J.; Min, Q.; Zeng, C.; Tong, C.; Peñuelas, J. Agricultural land use decouples soil nutrient cycles in a subtropical riparian wetland in China. Catena 2015, 133, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.A.; Hoque, M.A.; Kim, P.J. Mitigating global warming potentials of methane and nitrous oxide gases from rice paddies under different irrigation regimes. Ambio 2013, 42, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Steinbeiss, S.; Gleixner, G.; Antonietti, M. Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol. Biochem. 2009, 41, 1301–1310. [Google Scholar] [CrossRef]
- Spokas, K.A.; Baker, J.M.; Reicosky, D.C. Ethylene: Potential key for biochar amendment impacts. Plant Soil 2010, 333, 443–452. [Google Scholar] [CrossRef]
- Fang, Y.; Singh, B.P.; Singh, B. Temperature sensitivity of biochar and native carbon mineralization in biochar-amended soils. Agric. Ecosyst. Environ. 2014, 191, 158–167. [Google Scholar] [CrossRef]
- Sohi, S. Carbon storage with benefits. Science 2012, 338, 1034–1035. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, L.; Zheng, J.; Pan, G.; Zhang, X.; Zheng, J.; Hussain, Q.; Han, X.; Yu, X. Sequestration of maize crop straw C in different soils: Role of oxyhydrates in chemical binding and stabilization as recalcitrance. Chemosphere 2012, 87, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.P.; Li, D.P.; Han, X.M. The fungal to bacterial ratio in soil food webs, and its measurement. Acta Ecol. Sin. 2011, 31, 4741–4748. [Google Scholar]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.A.; Davies, C.A.; Frey, S.D.; Maddox, T.R.; Melillo, J.M.; Mohan, J.E.; Reynolds, J.F.; Treseder, K.K.; Wallenstein, M.D. Thermal adaptation of soil microbial respiration to elevated temperature. Ecol. Lett. 2008, 11, 1316–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.A.; Watts, B.W.; Davies, C.A. Thermal adaptation of heterotrophic soil respiration in laboratory microcosms. Glob. Chang. Biol. 2010, 16, 1576–1588. [Google Scholar] [CrossRef]
- Singla, A.; Inubushi, K. Effect of slag-type fertilizers on N2O flux from komatsuna vegetated soil and CH4 flux from paddy vegetated soil. Paddy Water Environ. 2015, 13, 43–50. [Google Scholar] [CrossRef]
- Pitcher, M.C.; Cummings, J.H. Hydrogen sulphide: A bacterial toxin in ulcerative colitis? Gut 1996, 39, 1–4. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, V.; Mahony, T.; O’Kennedy, R.; Colleran, E. Effect of pH on growth kinetics and sulphide toxicity thresholds of a range of methaneogenic, syntrophic and sulphate-reducing bacteria. Process Biochem. 1998, 33, 555–569. [Google Scholar] [CrossRef]
- Setia, R.; Marschner, P.; Baldock, J.; Chittleborough, D. Is CO2 evolution in saline soils affected by an osmotic effect and calcium carbonate? Biol. Fert. Soils 2010, 46, 781–792. [Google Scholar] [CrossRef]
- Setia, R.; Marschner, P.; Baldock, J.; Chittleborough, D.; Verma, V. Relationships between carbon dioxide emission and soil properties in salt-affected landscapes. Soil Biol. Biochem. 2011, 43, 667–674. [Google Scholar] [CrossRef]
- Chen, B.Y.; Liu, S.Q.; Huang, J.Y.; Shiau, T.J.; Wang, Y.M. Reduction of carbon dioxide emission by using microbial fuel cells during wastewater treatment. Aerosol Air Qual. Res. 2013, 13, 266–274. [Google Scholar] [CrossRef]
- Zhu, Q.; Zeng, D.; Wang, C.; Tong, C.; Wang, W. Effects of waste applications on the distribution and stability of soil aggragates in the paddy field of Fuzhou plain. Acta Sci. Circumst. 2016, 36, 3000–3008. [Google Scholar]





| Stage | C Form | Bacteria | Fungi | Fungi: Bacteria Ratio | Salinity | pH | Total N | C:N Ratio | Bulk Density | Water Content | C Release |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Early paddy field | TOC | −0.275 | 0.443 | 0.845 ** | 0.191 | 0.314 | 0.779 ** | 0.970 ** | 0.161 | 0.346 | −0.125 |
| LOC | 0.752 ** | 0.361 | −0.347 | −0.391 | −0.381 | 0.025 | −0.115 | −0.012 | −0.436 | 0.614 * | |
| MBC | −0.479 | 0.357 | 0.227 | −0.251 | −0.106 | 0.016 | 0.188 | 0.592 * | −0.121 | −0.256 | |
| DOC | −0.367 | −0.028 | 0.150 | 0.849 ** | 0.791 ** | −0.422 | 0.196 | −0.069 | −0.264 | −0.614 * | |
| Late paddy field | TOC | −0.518 * | −0.332 | 0.320 | 0.838 ** | 0.566 * | 0.442 | 0.921 ** | −0.340 | 0.235 | 0.436 |
| LOC | 0.296 | 0.382 | −0.159 | −0.084 | 0.011 | −0.636 * | 0.205 | 0.374 | −0.260 | −0.056 | |
| MBC | −0.352 | −0.582 * | 0.175 | 0.467 | 0.357 | 0.410 | 0.252 | −0.279 | 0.161 | 0.379 | |
| DOC | −0.318 | −0.418 | 0.323 | 0.313 | 0.139 | 0.328 | 0.262 | 0.138 | 0.301 | −0.071 | |
| Combined paddy field | TOC | −0.301 | 0.063 | 0.302 | 0.520 ** | 0.407 * | 0.583 ** | 0.933 ** | −0.145 | 0.243 | 0.244 |
| LOC | 0.605 ** | 0.506 ** | 0.102 | −0.197 | −0.147 | −0.202 | 0.091 | −0.202 | 0.101 | 0.358 * | |
| MBC | −0.570 ** | −0.675 ** | −0.250 | 0.060 | −0.014 | −0.008 | 0.150 | 0.553 ** | −0.434 * | −0.308 | |
| DOC | −0.434 * | −0.308 | −0.058 | 0.601 ** | 0.565 ** | −0.216 | 0.170 | 0.214 | −0.244 | −0.416 * |
| Stage | Treatment | CA (mg·g−1) | CNA (mg·g−1) | CPA | CPI | CPAI | CPMI | Crop Yield (Mg ha−1) |
|---|---|---|---|---|---|---|---|---|
| Early Paddy field | Control | 7.02 ± 0.68 a | 6.80 ± 0.66 b | 1.09 ± 0.22 a | 1.00 ± 0.00 c | 1.00 ± 0.00 a | 100.00 ± 0.00 a | 4.63 ± 0.64 a |
| Steel slag | 4.91 ± 0.21 b | 8.41 ± 0.86 b | 0.78 ± 0.10 ab | 0.94 ± 0.02 c | 0.71 ± 0.06 a | 63.30 ± 6.76 b | 4.67 ± 0.20 a | |
| Biochar | 5.52 ± 0.65 ab | 13.36 ± 0.62 a | 0.47 ± 0.06 b | 1.34 ± 0.06 a | 0.45 ± 0.10 b | 58.36 ± 10.61 b | 4.91 ± 0.52 a | |
| Steel slag + biochar | 5.21 ± 0.39 b | 12.57 ± 0.40 a | 0.54 ± 0.08 b | 1.22 ± 0.03 b | 0.49 ± 0.02 b | 54.55 ± 4.47 b | 5.06 ± 0.26 a | |
| Late Paddy field | Control | 8.00 ± 0.41 a | 5.67 ± 0.46 b | 1.50 ± 0.21 a | 1.00 ± 0.00 b | 1.00 ± 0.00 a | 100.00 ± 0.00 a | 6.73 ± 0.94 a |
| Steel slag | 6.41 ± 0.75 ab | 9.84 ± 0.30 a | 0.91 ± 0.35 ab | 1.20 ± 0.03 ab | 0.67 ± 0.33 a | 82.30 ± 42.28 a | 6.83 ± 0.19 a | |
| Biochar | 5.56 ± 0.63 b | 10.89 ± 1.92 a | 0.68 ± 0.22 b | 1.22 ± 0.14 ab | 0.45 ± 0.14 a | 53.90 ± 15.01 a | 6.97 ± 0.21 a | |
| Steel slag + biochar | 6.58 ± 0.69 ab | 12.86 ± 1.41 a | 0.71 ± 0.14 b | 1.40 ± 0.12 a | 0.50 ± 0.16 a | 67.91 ± 24.04 a | 7.20 ± 0.14 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Lai, D.Y.F.; Abid, A.A.; Neogi, S.; Xu, X.; Wang, C. Effects of Steel Slag and Biochar Incorporation on Active Soil Organic Carbon Pools in a Subtropical Paddy Field. Agronomy 2018, 8, 135. https://doi.org/10.3390/agronomy8080135
Wang W, Lai DYF, Abid AA, Neogi S, Xu X, Wang C. Effects of Steel Slag and Biochar Incorporation on Active Soil Organic Carbon Pools in a Subtropical Paddy Field. Agronomy. 2018; 8(8):135. https://doi.org/10.3390/agronomy8080135
Chicago/Turabian StyleWang, Weiqi, Derrick Yuk Fo Lai, Abbas Ali Abid, Suvadip Neogi, Xuping Xu, and Chun Wang. 2018. "Effects of Steel Slag and Biochar Incorporation on Active Soil Organic Carbon Pools in a Subtropical Paddy Field" Agronomy 8, no. 8: 135. https://doi.org/10.3390/agronomy8080135





