Decellularized Tendon Extracellular Matrix—A Valuable Approach for Tendon Reconstruction?
Abstract
:1. Introduction
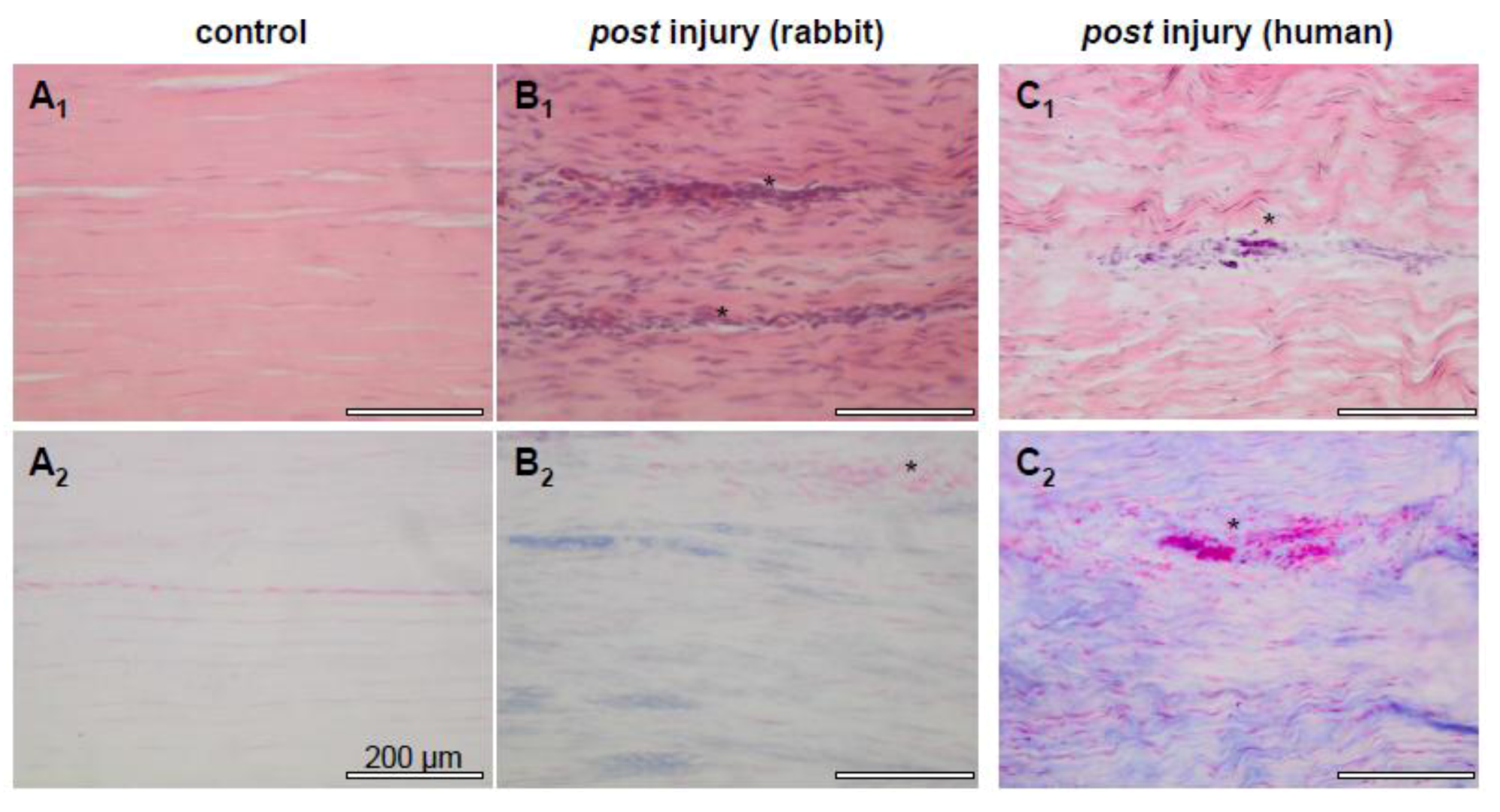
2. Results and Discussion
2.1. Tendon and Ligaments
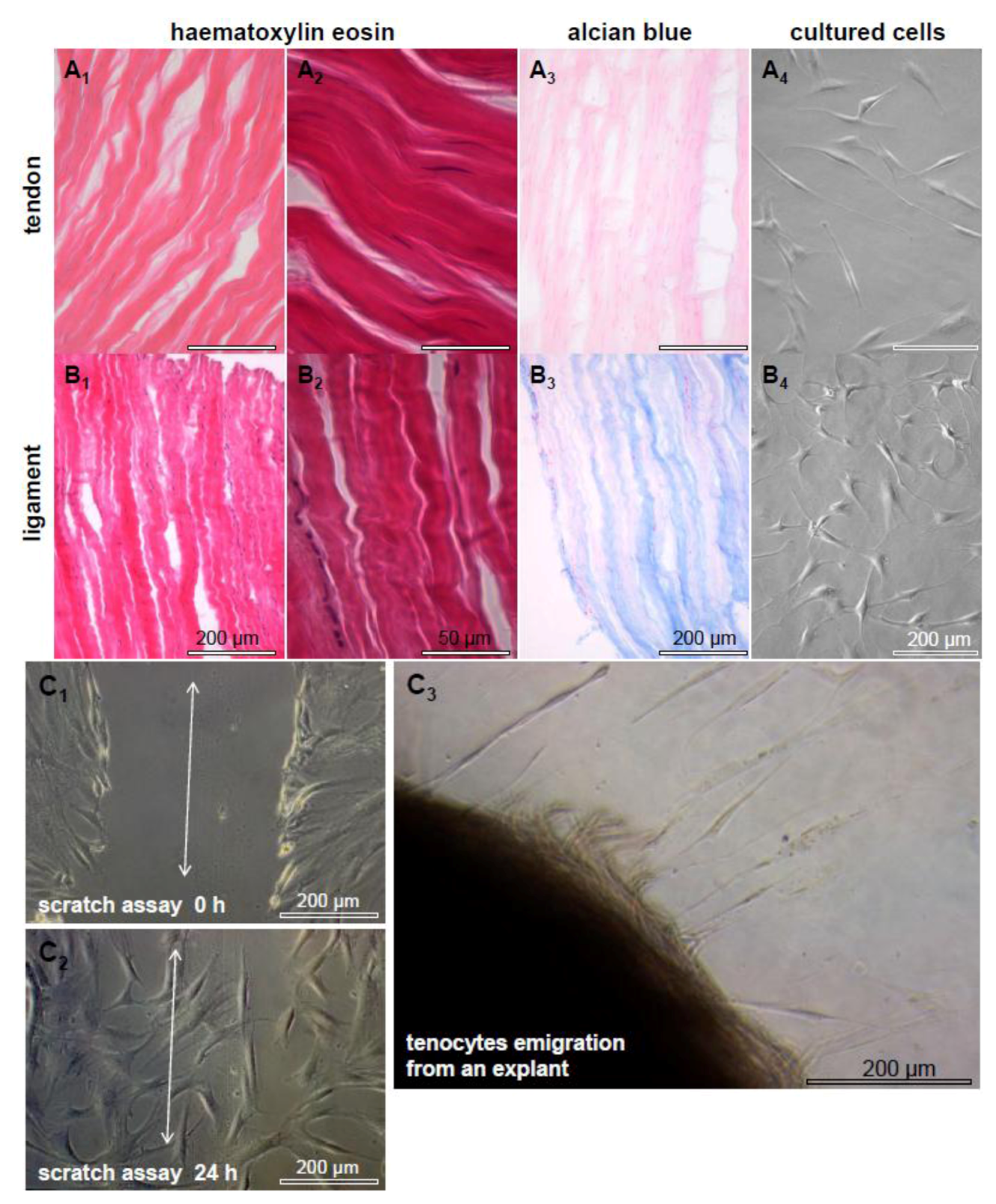
2.2. Tendon Healing and Tendon Reconstruction
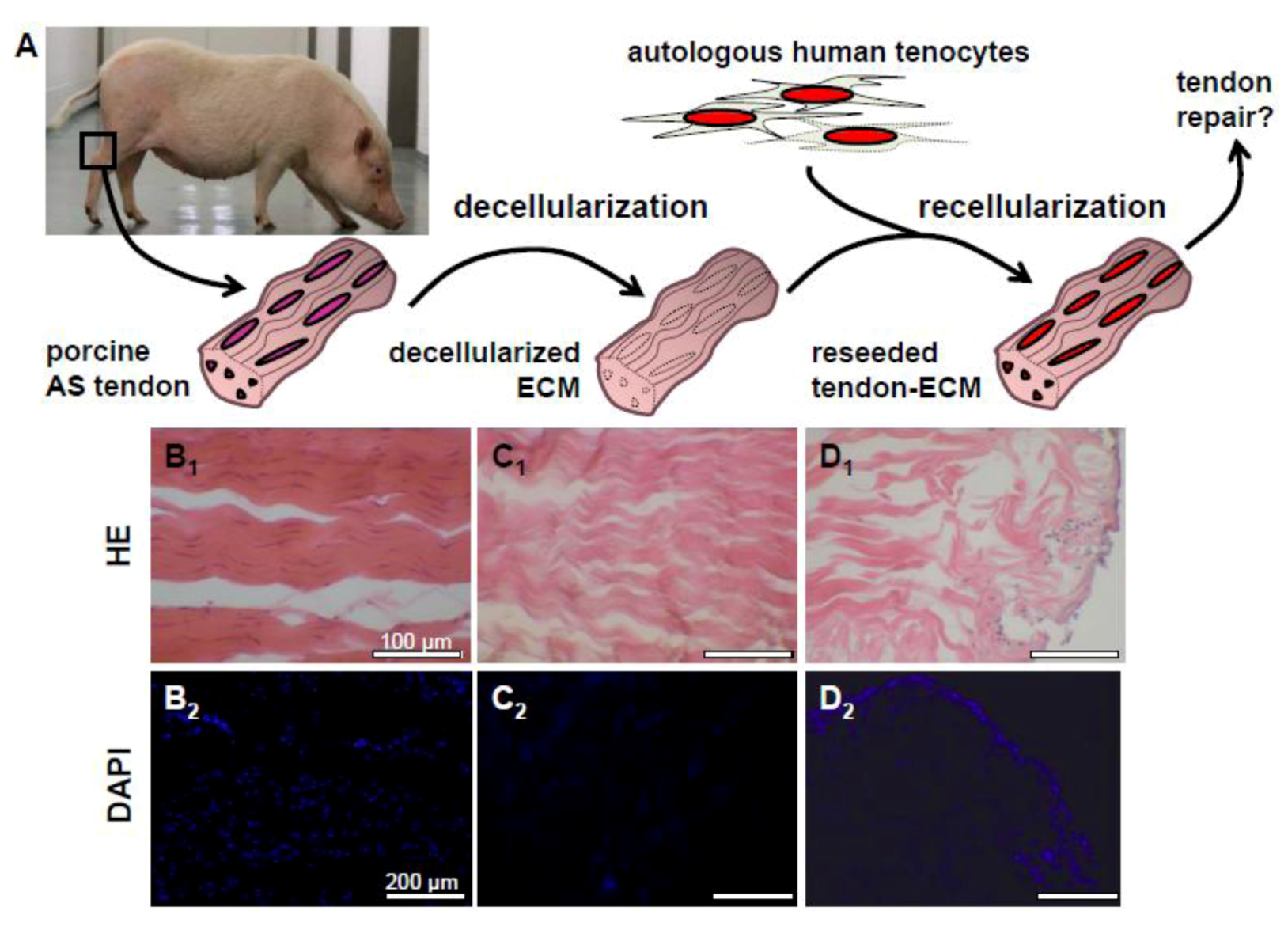
2.3. Decellularization of Tendons
2.4. Recellularization of Decellularized Tendon ECM
2.5. In Vivo Studies Using Decellularized Tendon
2.6. Blood Vessel Access in Tendon
2.7. Lymphatic Vessels and Tendon Regeneration
2.8. Novel Approaches: Tenocytes Co-Culturing with Endothelial or Other Cells
3. Limitations and Future Directions
4. Conclusions
Acknowledgements
References and Notes
- Clayton, R.A.; Court-Brown, C.M. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury 2008, 39, 1338–1344. [Google Scholar] [CrossRef]
- Olsson, N.; Nilsson-Helander, K.; Karlsson, J.; Eriksson, B.I.; Thomee, R.; Faxen, E.; Silbernagel, K.G. Major functional deficits persist 2 years after acute Achilles tendon rupture. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 1385–1393. [Google Scholar] [CrossRef]
- Woo, S.L.; Hildebrand, K.; Watanabe, N.; Fenwick, J.A.; Papageorgiou, C.D.; Wang, J.H. Tissue engineering of ligament and tendon healing. Clin. Orthop. Relat. Res. 1999, S312–S323. [Google Scholar]
- Hildebrand, K.A.; Jia, F.; Woo, S.L. Response of donor and recipient cells after transplantation of cells to the ligament and tendon. Microsc. Res. Tech. 2002, 58, 34–38. [Google Scholar] [CrossRef]
- Jung, H.J.; Fisher, M.B.; Woo, S.L. Role of biomechanics in the understanding of normal, injured, and healing ligaments and tendons. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2009, 1, 9. [Google Scholar] [CrossRef]
- Liu, Y.; Ramanath, H.S.; Wang, D.A. Tendon tissue engineering using scaffold enhancing strategies. Trends Biotechnol. 2008, 26, 201–209. [Google Scholar] [CrossRef]
- Ouyang, H.W.; Goh, J.C.; Thambyah, A.; Teoh, S.H.; Lee, E.H. Knitted poly-lactide-co-glycolide scaffold loaded with bone marrow stromal cells in repair and regeneration of rabbit Achilles tendon. Tissue Eng. 2003, 9, 431–439. [Google Scholar] [CrossRef]
- Stoll, C.; John, T.; Endres, M.; Rosen, C.; Kaps, C.; Kohl, B.; Sittinger, M.; Ertel, W.; Schulze-Tanzil, G. Extracellular matrix expression of human tenocytes in three-dimensional air-liquid and PLGA cultures compared with tendon tissue: implications for tendon tissue engineering. J. Orthop. Res. 2010, 28, 1170–1177. [Google Scholar] [CrossRef]
- Stoll, C.; John, T.; Conrad, C.; Lohan, A.; Hondke, S.; Ertel, W.; Kaps, C.; Endres, M.; Sittinger, M.; Ringe, J.; et al. Healing parameters in a rabbit partial tendon defect following tenocyte/biomaterial implantation. Biomaterials 2011, 32, 4806–4815. [Google Scholar] [CrossRef]
- Juncosa-Melvin, N.; Boivin, G.P.; Galloway, M.T.; Gooch, C.; West, J.R.; Butler, D.L. Effects of cell-to-collagen ratio in stem cell-seeded constructs for Achilles tendon repair. Tissue Eng. 2006, 12, 681–689. [Google Scholar] [CrossRef]
- Maffulli, N.; Ewen, S.W.; Waterston, S.W.; Reaper, J.; Barrass, V. Tenocytes from ruptured and tendinopathic achilles tendons produce greater quantities of type III collagen than tenocytes from normal achilles tendons. An in vitro model of human tendon healing. Am. J. Sports Med. 2000, 28, 499–505. [Google Scholar]
- Macaulay, A.A.; Perfetti, D.C.; Levine, W.N. Anterior cruciate ligament graft choices. Sports Health 2012, 4, 63–68. [Google Scholar] [CrossRef]
- Ott, H.C.; Clippinger, B.; Conrad, C.; Schuetz, C.; Pomerantseva, I.; Ikonomou, L.; Kotton, D.; Vacanti, J.P. Regeneration and orthotopic transplantation of a bioartificial lung. Nat. Med. 2010, 16, 927–933. [Google Scholar] [CrossRef]
- Uygun, B.E.; Soto-Gutierrez, A.; Yagi, H.; Izamis, M.L.; Guzzardi, M.A.; Shulman, C.; Milwid, J.; Kobayashi, N.; Tilles, A.; Berthiaume, F.; et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 2010, 16, 814–820. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Kikuiri, T.; Akiyama, K.; Chen, C.; Xu, X.; Yang, R.; Chen, W.; Wang, S.; Shi, S. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-gamma and TNF-alpha. Nat. Med. 2011, 17, 1594–1601. [Google Scholar] [CrossRef]
- Wu, W.; Allen, R.A.; Wang, Y. Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat. Med. 2012, 18, 1148–1153. [Google Scholar] [CrossRef]
- Raghavan, S.S.; Woon, C.Y.; Kraus, A.; Megerle, K.; Choi, M.S.; Pridgen, B.C.; Pham, H.; Chang, J. Human flexor tendon tissue engineering: Decellularization of human flexor tendons reduces immunogenicity in vivo. Tissue Eng. Part A 2012, 18, 796–805. [Google Scholar] [CrossRef]
- Chen, J.M.; Willers, C.; Xu, J.; Wang, A.; Zheng, M.H. Autologous tenocyte therapy using porcine-derived bioscaffolds for massive rotator cuff defect in rabbits. Tissue Eng. 2007, 13, 1479–1491. [Google Scholar]
- Pridgen, B.C.; Woon, C.Y.; Kim, M.; Thorfinn, J.; Lindsey, D.; Pham, H.; Chang, J. Flexor tendon tissue engineering: Acellularization of human flexor tendons with preservation of biomechanical properties and biocompatibility. Tissue Eng. Part C Methods 2011, 17, 819–828. [Google Scholar]
- Kryger, G.S.; Chong, A.K.; Costa, M.; Pham, H.; Bates, S.J.; Chang, J. A comparison of tenocytes and mesenchymal stem cells for use in flexor tendon tissue engineering. J. Hand Surg. Am. 2007, 32, 597–605. [Google Scholar] [CrossRef]
- Zantop, T.; Gilbert, T.W.; Yoder, M.C.; Badylak, S.F. Extracellular matrix scaffolds are repopulated by bone marrow-derived cells in a mouse model of achilles tendon reconstruction. J. Orthop. Res. 2006, 24, 1299–1309. [Google Scholar]
- Chong, A.K.; Riboh, J.; Smith, R.L.; Lindsey, D.P.; Pham, H.M.; Chang, J. Flexor tendon tissue engineering: Acellularized and reseeded tendon constructs. Plast Reconstr. Surg. 2009, 123, 1759–1766. [Google Scholar] [CrossRef]
- Derwin, K.A.; Badylak, S.F.; Steinmann, S.P.; Iannotti, J.P. Extracellular matrix scaffold devices for rotator cuff repair. J. Shoulder Elbow Surg. 2010, 19, 467–476. [Google Scholar]
- Fini, M.; Bondioli, E.; Castagna, A.; Torricelli, P.; Giavaresi, G.; Rotini, R.; Marinelli, A.; Guerra, E.; Orlandi, C.; Carboni, A.; et al. Decellularized human dermis to treat massive rotator cuff tears: In vitro evaluations. Connect. Tissue Res. 2012, 53, 298–306. [Google Scholar] [CrossRef]
- Ricchetti, E.T.; Aurora, A.; Iannotti, J.P.; Derwin, K.A. Scaffold devices for rotator cuff repair. J. Shoulder Elbow Surg. 2012, 21, 251–265. [Google Scholar] [CrossRef]
- Ker, E.D.; Nain, A.S.; Weiss, L.E.; Wang, J.; Suhan, J.; Amon, C.H.; Campbell, P.G. Bioprinting of growth factors onto aligned sub-micron fibrous scaffolds for simultaneous control of cell differentiation and alignment. Biomaterials 2011, 32, 8097–8107. [Google Scholar]
- Hoganson, D.M.; O'Doherty, E.M.; Owens, G.E.; Harilal, D.O.; Goldman, S.M.; Bowley, C.M.; Neville, C.M.; Kronengold, R.T.; Vacanti, J.P. The retention of extracellular matrix proteins and angiogenic and mitogenic cytokines in a decellularized porcine dermis. Biomaterials 2010, 31, 6730–6737. [Google Scholar]
- Kannus, P. Structure of the tendon connective tissue. Scand. J. Med. Sci. Sports 2000, 10, 312–320. [Google Scholar]
- Tozer, S.; Duprez, D. Tendon and ligament: Development, repair and disease. Birth Defects Res. C Embryo Today 2005, 75, 226–236. [Google Scholar] [CrossRef]
- Rumian, A.P.; Wallace, A.L.; Birch, H.L. Tendons and ligaments are anatomically distinct but overlap in molecular and morphological features—a comparative study in an ovine model. J. Orthop. Res. 2007, 25, 458–464. [Google Scholar] [CrossRef]
- Amiel, D.; Frank, C.; Harwood, F.; Fronek, J.; Akeson, W. Tendons and ligaments: A morphological and biochemical comparison. J. Orthop. Res. 1984, 1, 257–265. [Google Scholar]
- Lippiello, L. Collagen Synthesis in tenocytes, ligament cells and chondrocytes exposed to a combination of Glucosamine HCl and chondroitin sulfate. Evid. Based Complement. Alternat. Med. 2007, 4, 219–224. [Google Scholar] [CrossRef]
- Birch, H.L. Tendon matrix composition and turnover in relation to functional requirements. Int. J. Exp. Pathol. 2007, 88, 241–248. [Google Scholar] [CrossRef]
- Smith, M.M.; Sakurai, G.; Smith, S.M.; Young, A.A.; Melrose, J.; Stewart, C.M.; Appleyard, R.C.; Peterson, J.L.; Gillies, R.M.; Dart, A.J.; et al. Modulation of aggrecan and ADAMTS expression in ovine tendinopathy induced by altered strain. Arthritis Rheum. 2008, 58, 1055–1066. [Google Scholar] [CrossRef]
- Samiric, T.; Parkinson, J.; Ilic, M.Z.; Cook, J.; Feller, J.A.; Handley, C.J. Changes in the composition of the extracellular matrix in patellar tendinopathy. Matrix Biol. 2009, 28, 230–236. [Google Scholar] [CrossRef]
- Waggett, A.D.; Ralphs, J.R.; Kwan, A.P.; Woodnutt, D.; Benjamin, M. Characterization of collagens and proteoglycans at the insertion of the human Achilles tendon. Matrix Biol. 1998, 16, 457–470. [Google Scholar] [CrossRef]
- Rigozzi, S.; Muller, R.; Snedeker, J.G. Local strain measurement reveals a varied regional dependence of tensile tendon mechanics on glycosaminoglycan content. J. Biomech. 2009, 42, 1547–1552. [Google Scholar] [CrossRef]
- Abrahamsson, S.O.; Lundborg, G.; Lohmander, L.S. Segmental variation in microstructure, matrix synthesis and cell proliferation in rabbit flexor tendon. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1989, 23, 191–198. [Google Scholar] [CrossRef]
- Meller, R.; Schiborra, F.; Brandes, G.; Knobloch, K.; Tschernig, T.; Hankemeier, S.; Haasper, C.; Schmiedl, A.; Jagodzinski, M.; Krettek, C.; et al. Postnatal maturation of tendon, cruciate ligament, meniscus and articular cartilage: A histological study in sheep. Ann. Anat. 2009, 191, 575–585. [Google Scholar] [CrossRef]
- Schulze-Tanzil, G.; Al-Sadi, O.; Wiegand, E.; Ertel, W.; Busch, C.; Kohl, B.; Pufe, T. The role of pro-inflammatory and immunoregulatory cytokines in tendon healing and rupture: New insights. Scand. J. Med. Sci. Sports 2011, 21, 337–351. [Google Scholar] [CrossRef]
- Amiel, D.; Kuiper, S.D.; Wallace, C.D.; Harwood, F.L.; VandeBerg, J.S. Age-related properties of medial collateral ligament and anterior cruciate ligament: A morphologic and collagen maturation study in the rabbit. J. Gerontol. 1991, 46, B159–B165. [Google Scholar]
- Oryan, A.; Shoushtari, A.H. Histology and ultrastructure of the developing superficial digital flexor tendon in rabbits. Anat. Histol. Embryol. 2008, 37, 134–140. [Google Scholar] [CrossRef]
- Wagenseil, J.E.; Mecham, R.P. New insights into elastic fiber assembly. Birth Defects Res. C Embryo Today 2007, 81, 229–240. [Google Scholar] [CrossRef]
- Murray, M.M.; Weiler, A.; Spindler, K.P. Interspecies variation in the fibroblast distribution of the anterior cruciate ligament. Am. J. Sports Med. 2004, 32, 1484–1491. [Google Scholar] [CrossRef]
- Sharma, P.; Maffulli, N. Tendon injury and tendinopathy: Healing and repair. J. Bone Joint Surg. Am. 2005, 87, 187–202. [Google Scholar] [CrossRef]
- Qi, J.; Fox, A.M.; Alexopoulos, L.G.; Chi, L.; Bynum, D.; Guilak, F.; Banes, A.J. IL-1beta decreases the elastic modulus of human tenocytes. J. Appl. Physiol. 2006, 101, 189–195. [Google Scholar] [CrossRef]
- Waggett, A.D.; Benjamin, M.; Ralphs, J.R. Connexin 32 and 43 gap junctions differentially modulate tenocyte response to cyclic mechanical load. Eur. J. Cell. Biol. 2006, 85, 1145–1154. [Google Scholar] [CrossRef]
- Chiquet, M.; Renedo, A.S.; Huber, F.; Fluck, M. How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biol. 2003, 22, 73–80. [Google Scholar] [CrossRef]
- Asundi, K.R.; Rempel, D.M. MMP-1, IL-1beta, and COX-2 mRNA expression is modulated by static load in rabbit flexor tendons. Ann. Biomed. Eng. 2008, 36, 237–243. [Google Scholar] [CrossRef]
- Kjaer, M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 2004, 84, 649–698. [Google Scholar] [CrossRef]
- Heinemeier, K.M.; Kjaer, M. In vivo investigation of tendon responses to mechanical loading. J. Musculoskelet. Neuronal. Interact. 2011, 11, 115–123. [Google Scholar]
- Kapoor, A.; Caporali, E.H.; Kenis, P.J.; Stewart, M.C. Microtopographically patterned surfaces promote the alignment of tenocytes and extracellular collagen. Acta Biomater. 2010, 6, 2580–2589. [Google Scholar] [CrossRef]
- Gelberman, R.H.; Steinberg, D.; Amiel, D.; Akeson, W. Fibroblast chemotaxis after tendon repair. J. Hand Surg. Am. 1991, 16, 686–693. [Google Scholar] [CrossRef]
- Harrison, R.K.; Mudera, V.; Grobbelaar, A.O.; Jones, M.E.; McGrouther, D.A. Synovial sheath cell migratory response to flexor tendon injury: An experimental study in rats. J. Hand Surg. Am. 2003, 28, 987–993. [Google Scholar] [CrossRef]
- Koob, T.J.; Summers, A.P. Tendon—bridging the gap. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 133, 905–909. [Google Scholar] [CrossRef]
- Seyfer, A.E.; Bolger, W.E. Effects of unrestricted motion on healing: a study of posttraumatic adhesions in primate tendons. Plast. Reconstr. Surg. 1989, 83, 122–128. [Google Scholar] [CrossRef]
- Evans, R.B. Managing the injured tendon: Current concepts. J. Hand Ther. 2012, 25, 173–189, quiz 190.. [Google Scholar] [CrossRef]
- Manske, P.R.; Gelberman, R.H.; Vande Berg, J.S.; Lesker, P.A. Intrinsic flexor-tendon repair. A morphological study in vitro. J. Bone Joint Surg Am. 1984, 66, 385–396. [Google Scholar]
- Martini, A.K.; Blimke, B. Animal experiment study of healing of the sutured flexor tendon. Helv. Chir. Acta 1992, 58, 431–437. [Google Scholar]
- Jakubietz, M.G.; Jakubietz, D.F.; Gruenert, J.G.; Zahn, R.; Meffert, R.H.; Jakubietz, R.G. Adequacy of palmaris longus and plantaris tendons for tendon grafting. J. Hand Surg. Am. 2011, 36, 695–698. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar]
- Ingram, J.H.; Korossis, S.; Howling, G.; Fisher, J.; Ingham, E. The use of ultrasonication to aid recellularization of acellular natural tissue scaffolds for use in anterior cruciate ligament reconstruction. Tissue Eng. 2007, 13, 1561–1572. [Google Scholar] [CrossRef]
- Deeken, C.R.; White, A.K.; Bachman, S.L.; Ramshaw, B.J.; Cleveland, D.S.; Loy, T.S.; Grant, S.A. Method of preparing a decellularized porcine tendon using tributyl phosphate. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 96, 199–206. [Google Scholar]
- Gilbert, T.W. Strategies for tissue and organ decellularization. J. Cell. Biochem. 2012, 113, 2217–2222. [Google Scholar] [CrossRef]
- Deeken, C.R.; Eliason, B.J.; Pichert, M.D.; Grant, S.A.; Frisella, M.M.; Matthews, B.D. Differentiation of biologic scaffold materials through physicomechanical, thermal, and enzymatic degradation techniques. Ann. Surg. 2012, 255, 595–604. [Google Scholar] [CrossRef]
- Keane, T.J.; Londono, R.; Turner, N.J.; Badylak, S.F. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 2012, 33, 1771–1781. [Google Scholar] [CrossRef]
- Gui, L.; Chan, S.A.; Breuer, C.K.; Niklason, L.E. Novel utilization of serum in tissue decellularization. Tissue Eng. Part. C Methods 2010, 16, 173–184. [Google Scholar] [CrossRef]
- Tischer, T.; Aryee, S.; Wexel, G.; Steinhauser, E.; Adamczyk, C.; Eichhorn, S.; Milz, S.; Martinek, V.; Gansbacher, B.; Imhoff, A.B.; et al. Tissue engineering of the anterior cruciate ligament-sodium dodecyl sulfate-acellularized and revitalized tendons are inferior to native tendons. Tissue Eng. Part. A 2010, 16, 1031–1040. [Google Scholar] [CrossRef]
- Whitlock, P.W.; Smith, T.L.; Poehling, G.G.; Shilt, J.S.; Van Dyke, M. A naturally derived, cytocompatible, and architecturally optimized scaffold for tendon and ligament regeneration. Biomaterials 2007, 28, 4321–4329. [Google Scholar] [CrossRef]
- Scott, J.E. Elasticity in extracellular matrix 'shape modules' of tendon, cartilage, etc. A sliding proteoglycan-filament model. J. Physiol. 2003, 553, 335–343. [Google Scholar] [CrossRef]
- Wolfman, N.M.; Hattersley, G.; Cox, K.; Celeste, A.J.; Nelson, R.; Yamaji, N.; Dube, J.L.; DiBlasio-Smith, E.; Nove, J.; Song, J.J.; et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J. Clin. Invest. 1997, 100, 321–330. [Google Scholar] [CrossRef]
- Hoffmann, A.; Pelled, G.; Turgeman, G.; Eberle, P.; Zilberman, Y.; Shinar, H.; Keinan-Adamsky, K.; Winkel, A.; Shahab, S.; Navon, G.; et al. Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. J. Clin. Invest. 2006, 116, 940–952. [Google Scholar] [CrossRef]
- Hou, Y.; Mao, Z.; Wei, X.; Lin, L.; Chen, L.; Wang, H.; Fu, X.; Zhang, J.; Yu, C. The roles of TGF-beta1 gene transfer on collagen formation during Achilles tendon healing. Biochem. Biophys. Res. Commun. 2009, 383, 235–239. [Google Scholar] [CrossRef]
- Nyland, J.; Larsen, N.; Burden, R.; Chang, H.; Caborn, D.N. Biomechanical and tissue handling property comparison of decellularized and cryopreserved tibialis anterior tendons following extreme incubation and rehydration. Knee Surg. Sports Traumatol. Arthrosc. 2009, 17, 83–91. [Google Scholar] [CrossRef]
- Thorfinn, J.; Saber, S.; Angelidis, I.K.; Ki, S.H.; Zhang, A.Y.; Chong, A.K.; Pham, H.M.; Lee, G.K.; Chang, J. Flexor tendon tissue engineering: Temporal distribution of donor tenocytes versus recipient cells. Plast Reconstr. Surg. 2009, 124, 2019–2026. [Google Scholar] [CrossRef]
- Xu, R.; Boudreau, A.; Bissell, M.J. Tissue architecture and function: Dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis. Rev. 2009, 28, 167–176. [Google Scholar] [CrossRef]
- Zhang, A.Y.; Bates, S.J.; Morrow, E.; Pham, H.; Pham, B.; Chang, J. Tissue-engineered intrasynovial tendons: Optimization of acellularization and seeding. J. Rehabil. Res. Dev. 2009, 46, 489–498. [Google Scholar] [CrossRef]
- Petri, M.; Kruppa, C.; Haasper, C.; Broese, M.; Liodakis, E.; Krettek, C.; Hurschler, C.; Jagodzinski, M. Effects of continuous perfusion on human bone marrow stromal cells seeded on a decellularized bovine Achilles tendon matrix. Technol. Health Care 2011, 19, 223–231. [Google Scholar]
- Woon, C.Y.; Pridgen, B.C.; Kraus, A.; Bari, S.; Pham, H.; Chang, J. Optimization of human tendon tissue engineering: Peracetic acid oxidation for enhanced reseeding of acellularized intrasynovial tendon. Plast Reconstr. Surg. 2011, 127, 1107–1117. [Google Scholar] [CrossRef]
- Durgam, S.S.; Stewart, A.A.; Pondenis, H.C.; Gutierrez-Nibeyro, S.M.; Evans, R.B.; Stewart, M.C. Comparison of equine tendon- and bone marrow-derived cells cultured on tendon matrix with or without insulin-like growth factor-I supplementation. Am. J. Vet. Res. 2012, 73, 153–161. [Google Scholar] [CrossRef]
- Broese, M.; Toma, I.; Haasper, C.; Simon, A.; Petri, M.; Budde, S.; Wehmeier, M.; Krettek, C.; Jagodzinski, M. Seeding a human tendon matrix with bone marrow aspirates compared to previously isolated hBMSCs—an in vitro study. Technol. Health Care 2011, 19, 469–479. [Google Scholar]
- Omae, H.; Sun, Y.L.; An, K.N.; Amadio, P.C.; Zhao, C. Engineered tendon with decellularized xenotendon slices and bone marrow stromal cells: An in vivo animal study. J. Tissue Eng. Regen. Med. 2012, 6, 238–244. [Google Scholar] [CrossRef]
- Saber, S.; Zhang, A.Y.; Ki, S.H.; Lindsey, D.P.; Smith, R.L.; Riboh, J.; Pham, H.; Chang, J. Flexor tendon tissue engineering: Bioreactor cyclic strain increases construct strength. Tissue Eng. Part. A 2010, 16, 2085–2090. [Google Scholar] [CrossRef]
- Taylor, S.H.; Al-Youha, S.; Van Agtmael, T.; Lu, Y.; Wong, J.; McGrouther, D.A.; Kadler, K.E. Tendon is covered by a basement membrane epithelium that is required for cell retention and the prevention of adhesion formation. PLoS One 2011, 6, e16337. [Google Scholar]
- Martinello, T.; Bronzini, I.; Volpin, A.; Vindigni, V.; Maccatrozzo, L.; Caporale, G.; Bassetto, F.; Patruno, M. Successful recellularization of human tendon scaffolds using adipose-derived mesenchymal stem cells and collagen gel. J. Tissue Eng. Regen Med. 2012. [Google Scholar]
- Tischer, T.; Vogt, S.; Aryee, S.; Steinhauser, E.; Adamczyk, C.; Milz, S.; Martinek, V.; Imhoff, A.B. Tissue engineering of the anterior cruciate ligament: A new method using acellularized tendon allografts and autologous fibroblasts. Arch. Orthop. Trauma Surg. 2007, 127, 735–741. [Google Scholar] [CrossRef]
- Lang, J. On the sheath of tendons, muscles, fascia and blood vessels. Z Anat Entwicklungsgesch 1960, 122, 197–231. [Google Scholar] [CrossRef]
- Chen, T.M.; Rozen, W.M.; Pan, W.R.; Ashton, M.W.; Richardson, M.D.; Taylor, G.I. The arterial anatomy of the Achilles tendon: anatomical study and clinical implications. Clin. Anat. 2009, 22, 377–385. [Google Scholar] [CrossRef]
- Benjamin, M.; Kaiser, E.; Milz, S. Structure-function relationships in tendons: A review. J. Anat. 2008, 212, 211–228. [Google Scholar] [CrossRef]
- Cheng, N.M.; Pan, W.R.; Vally, F.; Le Roux, C.M.; Richardson, M.D. The arterial supply of the long head of biceps tendon: Anatomical study with implications for tendon rupture. Clin. Anat. 2010, 23, 683–692. [Google Scholar] [CrossRef]
- Yepes, H.; Tang, M.; Morris, S.F.; Stanish, W.D. Relationship between hypovascular zones and patterns of ruptures of the quadriceps tendon. J. Bone Joint Surg. Am. 2008, 90, 2135–2141. [Google Scholar] [CrossRef]
- Matthews, P. The fate of isolated segments of flexor tendons within the digital sheath—a study in synovial nutrition. Br. J. Plast Surg. 1976, 29, 216–224. [Google Scholar]
- Pufe, T.; Petersen, W.J.; Mentlein, R.; Tillmann, B.N. The role of vasculature and angiogenesis for the pathogenesis of degenerative tendons disease. Scand. J. Med. Sci. Sports 2005, 15, 211–222. [Google Scholar] [CrossRef]
- Al-Sadi, O.G.; Schulze-Tanzil, B.; Kohl, A.; Lohan, M.; Lemke, T.; Ertel, W.; John, T. Tenocytes, pro-inflammatory cytokines and leukocytes: A relationship? MLTJ 2011, I, 68–76. [Google Scholar]
- Pisani, D.F.; Pierson, P.M.; Massoudi, A.; Leclerc, L.; Chopard, A.; Marini, J.F.; Dechesne, C.A. Myodulin is a novel potential angiogenic factor in skeletal muscle. Exp. Cell. Res. 2004, 292, 40–50. [Google Scholar] [CrossRef]
- Zumstein, M.A.; Berger, S.; Schober, M.; Boileau, P.; Nyffeler, R.W.; Horn, M.; Dahinden, C.A. Leukocyte- and Platelet-Rich Fibrin (L-PRF) for Long-Term Delivery of Growth Factor in Rotator Cuff Repair: Review, Preliminary Results and Future Directions. Curr. Pharm. Biotechnol. 2012, 13, 1196–1206. [Google Scholar]
- Kaux, J.F.; Drion, P.V.; Colige, A.; Pascon, F.; Libertiaux, V.; Hoffmann, A.; Janssen, L.; Heyers, A.; Nusgens, B.V.; Le Goff, C.; et al. Effects of platelet-rich plasma (PRP) on the healing of Achilles tendons of rats. Wound Repair Regen. 2012, 20, 748–756. [Google Scholar] [CrossRef]
- Beck, J.; Evans, D.; Tonino, P.M.; Yong, S.; Callaci, J.J. The biomechanical and histologic effects of platelet-rich plasma on rat rotator cuff repairs. Am. J. Sports Med. 2012, 40, 2037–2044. [Google Scholar] [CrossRef]
- Sato, D.; Takahara, M.; Narita, A.; Yamakawa, J.; Hashimoto, J.; Ishikawa, H.; Ogino, T. Effect of platelet-rich plasma with fibrin matrix on healing of intrasynovial flexor tendons. J. Hand Surg. Am. 2012, 37, 1356–1363. [Google Scholar] [CrossRef]
- Maffulli, N.; Del Buono, A. Platelet plasma rich products in musculoskeletal medicine: Any evidence? Surgeon 2012, 10, 148–150. [Google Scholar] [CrossRef]
- Rodeo, S.A.; Delos, D.; Williams, R.J.; Adler, R.S.; Pearle, A.; Warren, R.F. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: A prospective, randomized clinical study. Am. J. Sports Med. 2012, 40, 1234–1241. [Google Scholar] [CrossRef]
- Petersen, W.; Pufe, T.; Zantop, T.; Tillmann, B.; Mentlein, R. Hypoxia and PDGF have a synergistic effect that increases the expression of the angiogenetic peptide vascular endothelial growth factor in Achilles tendon fibroblasts. Arch. Orthop. Trauma Surg. 2003, 123, 485–488. [Google Scholar] [CrossRef]
- Edwards, D.A. The blood supply and lymphatic drainage of tendons. J. Anat. 1946, 80, 147–152. [Google Scholar]
- Setti, G.C.; Tazzi, A. Microscopical anatomy of the lymphatic circulation of the tendons. Ateneo Parmense 1 1972, 43, 115–131. [Google Scholar]
- Verdan, C. Historical development of surgery of the flexor tendons. Handchirurgie 1981, 13, 181–185. [Google Scholar]
- Kubik, S.; Kretz, O. Anatomie des Lymphgefäßsystems. In Lehrbuch der Lymphologie für Mediziner, Masseure und Physiotherapeuten; Urban & Fischer Verlag: München, Germany, 2005; Volume 6, pp. 2–150. [Google Scholar]
- Boardman, K.C.; Swartz, M.A. Interstitial flow as a guide for lymphangiogenesis. Circ. Res. 2003, 92, 801–808. [Google Scholar] [CrossRef]
- Takagi, K.; Fukunaga, S.; Nishi, A.; Shojima, T.; Yoshikawa, K.; Hori, H.; Akashi, H.; Aoyagi, S. In vivo recellularization of plain decellularized xenografts with specific cell characterization in the systemic circulation: Histological and immunohistochemical study. Artif. Organs 2006, 30, 233–241. [Google Scholar] [CrossRef]
- Niklason, L.E.; Koh, J.; Solan, A. Tissue engineering of the lymphatic system. Ann. NY Acad. Sci. 2002, 979, 27–34, discussion 35–28.. [Google Scholar] [CrossRef]
- Andres, K.H.; von During, M.; Schmidt, R.F. Sensory innervation of the Achilles tendon by group III and IV afferent fibers. Anat. Embryol. (Berl) 1985, 172, 145–156. [Google Scholar] [CrossRef]
- Yang, M.; Chen, C.Z.; Shu, Y.S.; Shi, W.P.; Cheng, S.F.; John Gu, Y. Preseeding of human vascular cells in decellularized bovine pericardium scaffold for tissue-engineered heart valve: An in vitro and in vivo feasibility study. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1654–1661. [Google Scholar]
- Wang, Z.; He, Y.; Yu, X.; Fu, W.; Wang, W.; Huang, H. Rapid vascularization of tissue-engineered vascular grafts in vivo by endothelial cells in co-culture with smooth muscle cells. J. Mater. Sci Mater. Med. 2012, 23, 1109–1117. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Schulze-Tanzil, G.; Al-Sadi, O.; Ertel, W.; Lohan, A. Decellularized Tendon Extracellular Matrix—A Valuable Approach for Tendon Reconstruction? Cells 2012, 1, 1010-1028. https://doi.org/10.3390/cells1041010
Schulze-Tanzil G, Al-Sadi O, Ertel W, Lohan A. Decellularized Tendon Extracellular Matrix—A Valuable Approach for Tendon Reconstruction? Cells. 2012; 1(4):1010-1028. https://doi.org/10.3390/cells1041010
Chicago/Turabian StyleSchulze-Tanzil, Gundula, Onays Al-Sadi, Wolfgang Ertel, and Anke Lohan. 2012. "Decellularized Tendon Extracellular Matrix—A Valuable Approach for Tendon Reconstruction?" Cells 1, no. 4: 1010-1028. https://doi.org/10.3390/cells1041010



