Macro and Microfluidic Flows for Skeletal Regenerative Medicine
Abstract
:1. Introduction
2. Macroflows for Bone Cells
2.1. Two Dimensional Macroflows
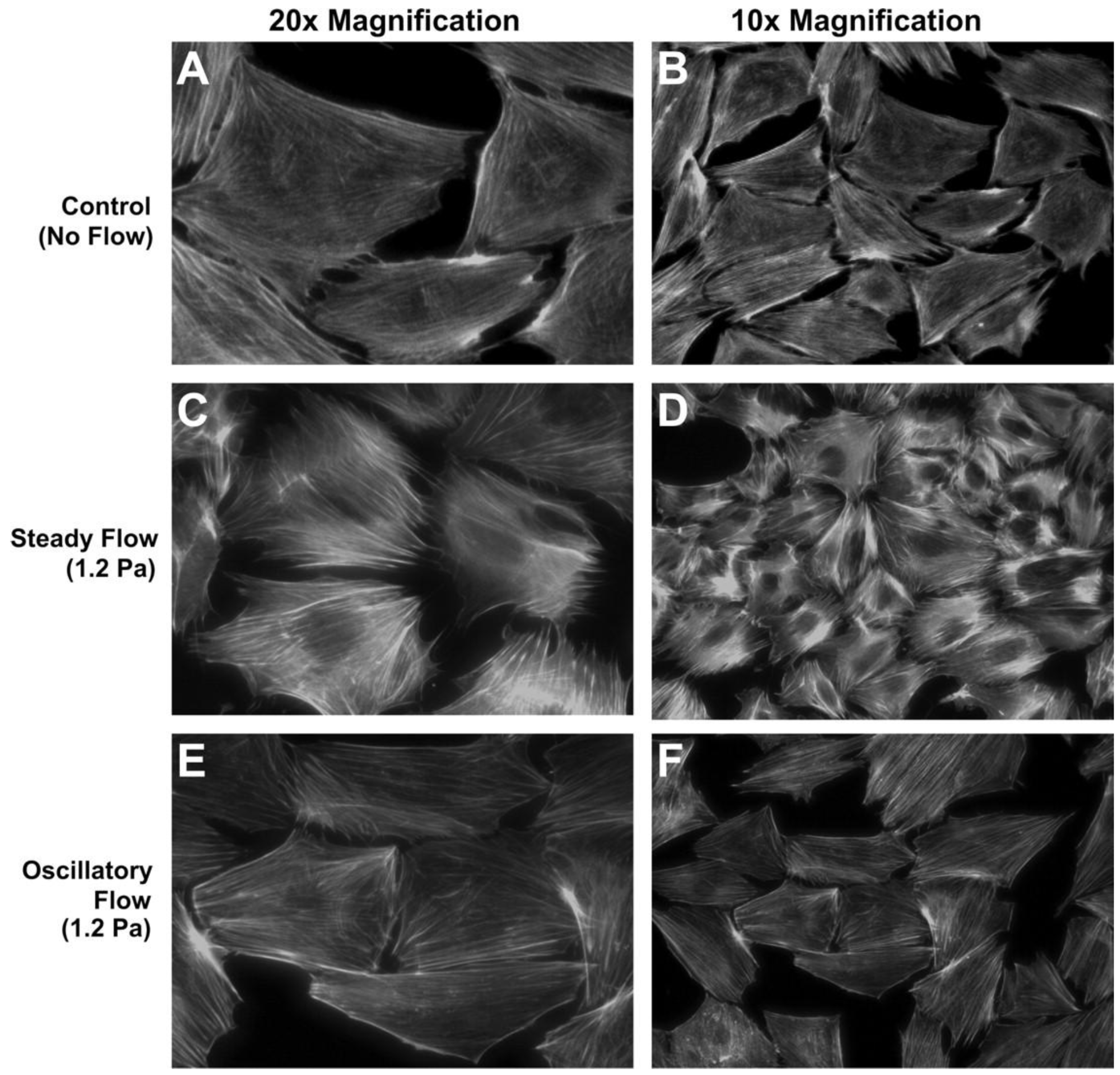
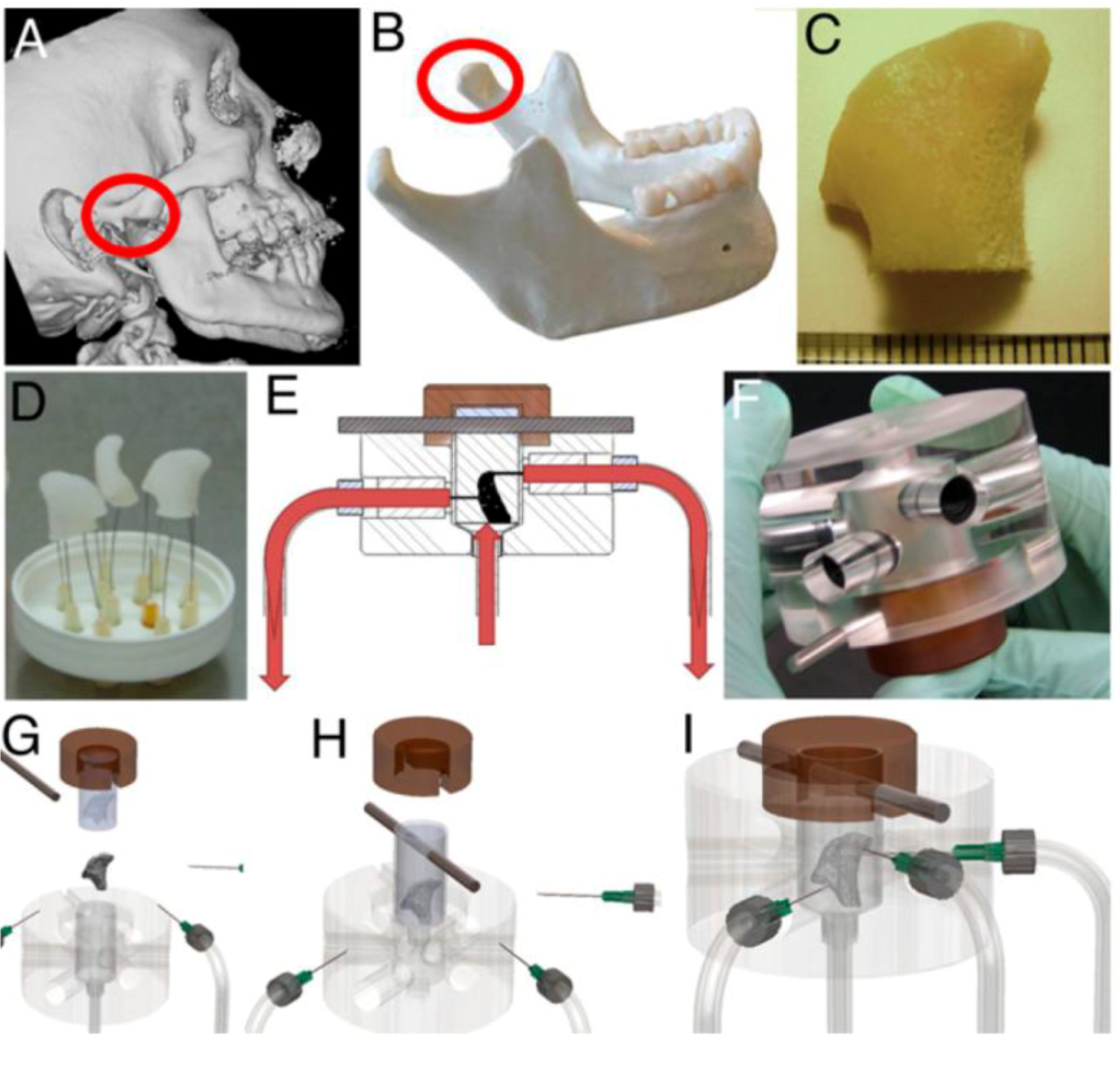
2.2. Three Dimensional Macroflows
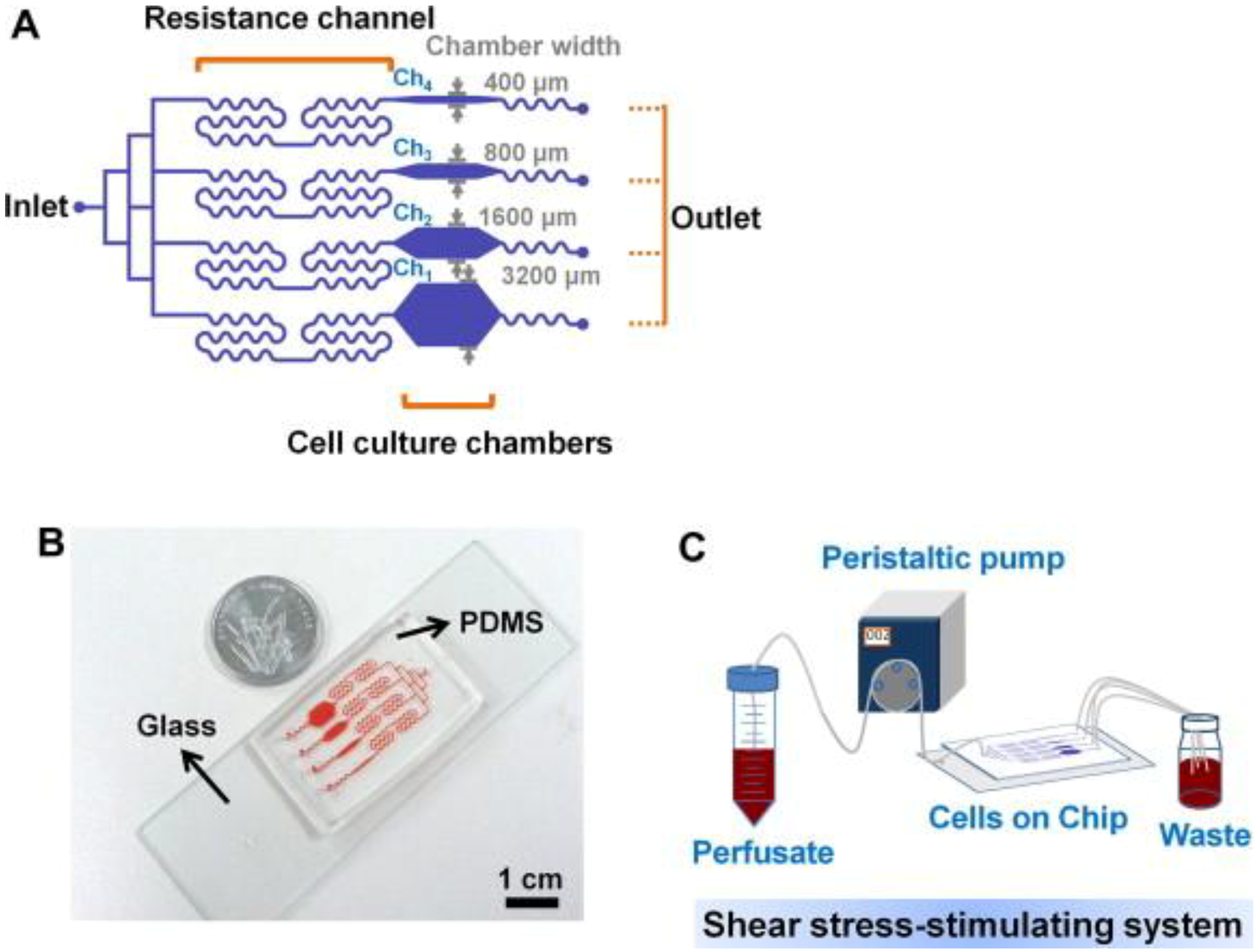
3. Microfluidics for Bone Cells
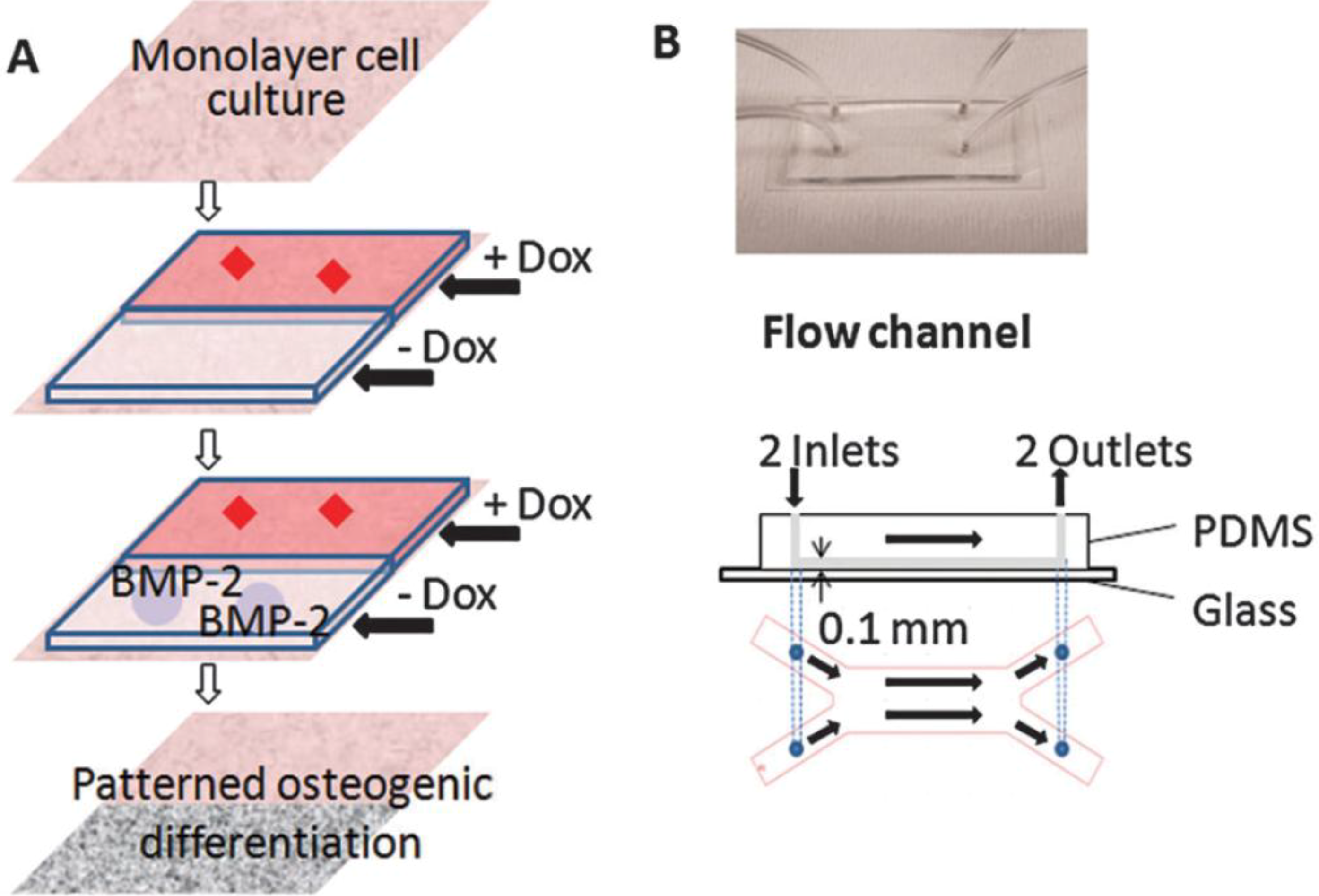
4. Implications in Skeletal Regenerative Medicine
5. Fluid Flow Effects on Other Lineages
6. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Riddle, R.C.; Donahue, H.J. From streaming-potentials to shear stress: 25 years of bone cell mechanotransduction. J. Orthop. Res. 2009, 27, 143–149. [Google Scholar]
- Wu, H.W.; Lin, C.C.; Lee, G.B. Stem cells in microfluidics. Biomicrofluidics 2011, 5, 13401. [Google Scholar] [CrossRef]
- Bennett, M.R.; Hasty, J. Microfluidic devices for measuring gene network dynamics in single cells. Nat. Rev. Genet. 2009, 10, 628–638. [Google Scholar] [CrossRef]
- Li, J.; Rose, E.; Frances, D.; Sun, Y.; You, L. Effect of oscillating fluid flow stimulation on osteocyte mRNA expression. J. Biomech. 2012, 45, 247–251. [Google Scholar] [CrossRef]
- Rumney, R.M.; Sunters, A.; Reilly, G.C.; Gartland, A. Application of multiple forms of mechanical loading to human osteoblasts reveals increased ATP release in response to fluid flow in 3D cultures and differential regulation of immediate early genes. J. Biomech. 2012, 45, 549–554. [Google Scholar] [CrossRef]
- Lu, X.L.; Huo, B.; Chiang, V.; Guo, X.E. Osteocytic network is more responsive in calcium signaling than osteoblastic network under fluid flow. J. Bone Miner. Res. 2012, 27, 563–574. [Google Scholar] [CrossRef]
- Ponik, S.M.; Triplett, J.W.; Pavalko, F.M. Osteoblasts and osteocytes respond differently to oscillatory and unidirectional fluid flow profiles. J. Cell. Biochem. 2007, 100, 794–807. [Google Scholar] [CrossRef]
- Genetos, D.C.; Kephart, C.J.; Zhang, Y.; Yellowley, C.E.; Donahue, H.J. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J. Cell. Physiol. 2007, 212, 207–214. [Google Scholar] [CrossRef]
- Gardinier, J.D.; Majumdar, S.; Duncan, R.L.; Wang, L. Cyclic hydraulic pressure and fluid flow differentially modulate cytoskeleton re-organization in MC3T3 osteoblasts. Cell. Mol. Bioeng. 2009, 2, 133–143. [Google Scholar] [CrossRef]
- Jackson, W.M.; Jaasma, M.J.; Tang, R.Y.; Keaveny, T.M. Mechanical loading by fluid shear is sufficient to alter the cytoskeletal composition of osteoblastic cells. Am. J. Physiol. Cell. Physiol. 2008, 295, C1007–C1015. [Google Scholar] [CrossRef]
- Donahue, H.J. Gap junctions and biophysical regulation of bone cell differentiation. Bone 2000, 26, 417–422. [Google Scholar] [CrossRef]
- Hoey, D.A.; Kelly, D.J.; Jacobs, C.R. A role for the primary cilium in paracrine signaling between mechanically stimulated osteocytes and mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2011, 412, 182–187. [Google Scholar] [CrossRef]
- Taylor, A.F.; Saunders, M.M.; Shingle, D.L.; Cimbala, J.M.; Zhou, Z.; Donahue, H.J. Mechanically stimulated osteocytes regulate osteoblastic activity via gap junctions. Am. J. Physiol. Cell. Physiol. 2007, 292, C545–C552. [Google Scholar]
- Kulkarni, R.N.; Bakker, A.D.; Everts, V.; Klein-Nulend, J. Inhibition of osteoclastogenesis by mechanically loaded osteocytes: involvement of MEPE. Calcif. Tissue Int. 2010, 87, 461–468. [Google Scholar] [CrossRef]
- Kim, C.H.; You, L.; Yellowley, C.E.; Jacobs, C.R. Oscillatory fluid flow-induced shear stress decreases osteoclastogenesis through RANKL and OPG signaling. Bone 2006, 39, 1043–1047. [Google Scholar] [CrossRef]
- Malone, A.M.; Batra, N.N.; Shivaram, G.; Kwon, R.Y.; You, L.; Kim, C.H.; Rodriguez, J.; Jair, K.; Jacobs, C.R. The role of actin cytoskeleton in oscillatory fluid flow-induced signaling in MC3T3-E1 osteoblasts. Am. J. Physiol. Cell. Physiol. 2007, 292, C1830–C1836. [Google Scholar]
- Pavalko, F.M.; Chen, N.X.; Turner, C.H.; Burr, D.B.; Atkinson, S.; Hsieh, Y.F.; Qiu, J.; Duncan, R.L. Fluid shear-induced mechanical signaling in MC3T3-E1 osteoblasts requires cytoskeleton-integrin interactions. Am. J. Physiol. 1998, 275, C1591–C1601. [Google Scholar]
- Morris, H.L.; Reed, C.I.; Haycock, J.W.; Reilly, G.C. Mechanisms of fluid-flow-induced matrix production in bone tissue engineering. Proc. Inst. Mech. Eng. H 2010, 224, 1509–1521. [Google Scholar] [CrossRef]
- Kapur, S.; Baylink, D.J.; Lau, K.H. Fluid flow shear stress stimulates human osteoblast proliferation and differentiation through multiple interacting and competing signal transduction pathways. Bone 2003, 32, 241–251. [Google Scholar] [CrossRef]
- Li, P.; Ma, Y.C.; Sheng, X.Y.; Dong, H.T.; Han, H.; Wang, J.; Xia, Y.Y. Cyclic fluid shear stress promotes osteoblastic cells proliferation through ERK5 signaling pathway. Mol. Cell. Biochem. 2012, 364, 321–327. [Google Scholar] [CrossRef]
- Kreke, M.R.; Huckle, W.R.; Goldstein, A.S. Fluid flow stimulates expression of osteopontin and bone sialoprotein by bone marrow stromal cells in a temporally dependent manner. Bone 2005, 36, 1047–1055. [Google Scholar] [CrossRef]
- Scaglione, S.; Wendt, D.; Miggino, S.; Papadimitropoulos, A.; Fato, M.; Quarto, R.; Martin, I. Effects of fluid flow and calcium phosphate coating on human bone marrow stromal cells cultured in a defined 2D model system. J. Biomed. Mater. Res. A 2008, 86, 411–419. [Google Scholar]
- Arnsdorf, E.J.; Tummala, P.; Kwon, R.Y.; Jacobs, C.R. Mechanically induced osteogenic differentiation-the role of RhoA, ROCKII and cytoskeletal dynamics. J. Cell. Sci. 2009, 122, 546–553. [Google Scholar] [CrossRef]
- Yourek, G.; McCormick, S.M.; Mao, J.J.; Reilly, G.C. Shear stress induces osteogenic differentiation of human mesenchymal stem cells. Regen. Med. 2010, 5, 713–724. [Google Scholar] [CrossRef]
- Lim, J.Y.; Loiselle, A.E.; Lee, J.S.; Zhang, Y.; Salvi, J.D.; Donahue, H.J. Optimizing the osteogenic potential of adult stem cells for skeletal regeneration. J. Orthop. Res. 2011, 29, 1627–1633. [Google Scholar] [CrossRef]
- Poudel, I.; Menter, D.; Lim, J.Y. Directing cell function and fate via micropatterning: Role of cell patterning size, shape, and interconnectivity. Biomed. Eng. Lett. 2012, 2, 38–45. [Google Scholar] [CrossRef]
- Salvi, J.D.; Lim, J.Y.; Donahue, H.J. Increased mechanosensitivity of cells cultured on nanotopographies. J. Biomech. 2010, 43, 3058–3062. [Google Scholar] [CrossRef]
- Takai, E.; Landesberg, R.; Katz, R.W.; Hung, C.T.; Guo, X.E. Substrate modulation of osteoblast adhesion strength, focal adhesion kinase activation, and responsiveness to mechanical stimuli. Mol. Cell. Biomech. 2006, 3, 1–12. [Google Scholar]
- Schwartz, Z.; Denison, T.A.; Bannister, S.R.; Cochran, D.L.; Liu, Y.H.; Lohmann, C.H.; Wieland, M.; Boyan, B.D. Osteoblast response to fluid induced shear depends on substrate microarchitecture and varies with time. J. Biomed. Mater. Res. A 2007, 83, 20–32. [Google Scholar]
- Damsky, C.H.; Ilic, D. Integrin signaling: It’s where the action is. Curr. Opin. Cell. Biol. 2002, 14, 594–602. [Google Scholar] [CrossRef]
- Lim, J.Y.; Taylor, A.F.; Li, Z.; Vogler, E.A.; Donahue, H.J. Integrin expression and osteopontin regulation in human fetal osteoblastic cells mediated by substratum surface characteristics. Tissue Eng. 2005, 11, 19–29. [Google Scholar] [CrossRef]
- Lim, J.Y.; Dreiss, A.D.; Zhou, Z.; Hansen, J.C.; Siedlecki, C.A.; Hengstebeck, R.W.; Cheng, J.; Winograd, N.; Donahue, H.J. The regulation of integrin-mediated osteoblast focal adhesion and focal adhesion kinase expression by nanoscale topography. Biomaterials 2007, 28, 1787–1797. [Google Scholar] [CrossRef]
- Young, S.R.; Gerard-O’Riley, R.; Kim, J.B.; Pavalko, F.M. Focal adhesion kinase is important for fluid shear stress-induced mechanotransduction in osteoblasts. J. Bone Miner. Res. 2009, 24, 411–424. [Google Scholar] [CrossRef]
- Young, S.R.; Hum, J.M.; Rodenberg, E.; Turner, C.H.; Pavalko, F.M. Non-overlapping functions for Pyk2 and FAK in osteoblasts during fluid shear stress-induced mechanotransduction. PLoS One 2011, 6, e16026. [Google Scholar]
- Myers, K.A.; Rattner, J.B.; Shrive, N.G.; Hart, D.A. Osteoblast-like cells and fluid flow: cytoskeleton-dependent shear sensitivity. Biochem. Biophys. Res. Commun. 2007, 364, 214–219. [Google Scholar] [CrossRef]
- Sterck, J.G.; Klein-Nulend, J.; Lips, P.; Burger, E.H. Response of normal and osteoporotic human bone cells to mechanical stress in vitro. Am. J. Physiol. 1998, 274, E1113–E1120. [Google Scholar]
- Bakker, A.D.; Klein-Nulend, J.; Tanck, E.; Heyligers, I.C.; Albers, G.H.; Lips, P.; Burger, E.H. Different responsiveness to mechanical stress of bone cells from osteoporotic versus osteoarthritic donors. Osteoporos. Int. 2006, 17, 827–833. [Google Scholar] [CrossRef]
- Zhao, F.; Chella, R.; Ma, T. Effects of shear stress on 3-D human mesenchymal stem cell construct development in a perfusion bioreactor system: Experiments and hydrodynamic modeling. Biotechnol. Bioeng. 2007, 96, 584–595. [Google Scholar] [CrossRef]
- Barron, M.J.; Tsai, C.J.; Donahue, S.W. Mechanical stimulation mediates gene expression in MC3T3 osteoblastic cells differently in 2D and 3D environments. J. Biomech. Eng. 2010, 132, 041005. [Google Scholar] [CrossRef]
- Meinel, L.; Karageorgiou, V.; Fajardo, R.; Snyder, B.; Shinde-Patil, V.; Zichner, L.; Kaplan, D.; Langer, R.; Vunjak-Novakovic, G. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann. Biomed. Eng. 2004, 32, 112–122. [Google Scholar] [CrossRef]
- Riehl, B.D.; Park, J.H.; Kwon, I.K.; Lim, J.Y. Mechanical stretching for tissue engineering: Two-dimensional and three-dimensional constructs. Tissue Eng. Part. B Rev. 2012, 18, 288–300. [Google Scholar] [CrossRef]
- Bjerre, L.; Bunger, C.; Baatrup, A.; Kassem, M.; Mygind, T. Flow perfusion culture of human mesenchymal stem cells on coralline hydroxyapatite scaffolds with various pore sizes. J. Biomed. Mater. Res. A 2011, 97, 251–263. [Google Scholar]
- Sikavitsas, V.I.; Bancroft, G.N.; Holtorf, H.L.; Jansen, J.A.; Mikos, A.G. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc. Natl. Acad. Sci. USA 2003, 100, 14683–14688. [Google Scholar] [CrossRef]
- Kim, J.; Ma, T. Bioreactor strategy in bone tissue engineering: pre-culture and osteogenic differentiation under two flow configurations. Tissue Eng. Part. A 2012, 18, 2354–2364. [Google Scholar] [CrossRef]
- Liu, L.; Yu, B.; Chen, J.; Tang, Z.; Zong, C.; Shen, D.; Zheng, Q.; Tong, X.; Gao, C.; Wang, J. Different effects of intermittent and continuous fluid shear stresses on osteogenic differentiation of human mesenchymal stem cells. Biomech. Model. Mechanobiol. 2012, 11, 391–401. [Google Scholar] [CrossRef]
- McCoy, R.J.; Jungreuthmayer, C.; O'Brien, F.J. Influence of flow rate and scaffold pore size on cell behavior during mechanical stimulation in a flow perfusion bioreactor. Biotechnol. Bioeng. 2012, 109, 1583–1594. [Google Scholar] [CrossRef]
- Jungreuthmayer, C.; Donahue, S.W.; Jaasma, M.J.; Al-Munajjed, A.A.; Zanghellini, J.; Kelly, D.J.; O'Brien, F.J. A comparative study of shear stresses in collagen-glycosaminoglycan and calcium phosphate scaffolds in bone tissue-engineering bioreactors. Tissue Eng. Part. A 2009, 15, 1141–1149. [Google Scholar] [CrossRef]
- Fritton, S.P.; Weinbaum, S. Fluid and solute transport in bone: flow-induced mechanotransduction. Annu. Rev. Fluid Mech. 2009, 41, 347–374. [Google Scholar] [CrossRef]
- Price, C.; Zhou, X.; Li, W.; Wang, L. Real-time measurement of solute transport within the lacunar-canalicular system of mechanically loaded bone: direct evidence for load-induced fluid flow. J. Bone Miner. Res. 2011, 26, 277–285. [Google Scholar] [CrossRef]
- Schmidt, S.M.; McCready, M.J.; Ostafin, A.E. Effect of oscillating fluid shear on solute transport in cortical bone. J. Biomech. 2005, 38, 2337–2343. [Google Scholar] [CrossRef]
- Porter, B.; Zauel, R.; Stockman, H.; Guldberg, R.; Fyhrie, D. 3-D computational modeling of media flow through scaffolds in a perfusion bioreactor. J. Biomech. 2005, 38, 543–549. [Google Scholar] [CrossRef]
- Kim, J.; Ma, T. Perfusion regulation of hMSC microenvironment and osteogenic differentiation in 3D scaffold. Biotechnol. Bioeng. 2012, 109, 252–261. [Google Scholar] [CrossRef]
- Li, D.; Tang, T.; Lu, J.; Dai, K. Effects of flow shear stress and mass transport on the construction of a large-scale tissue-engineered bone in a perfusion bioreactor. Tissue Eng. Part. A 2009, 15, 2773–2783. [Google Scholar] [CrossRef]
- Riddle, R.C.; Hippe, K.R.; Donahue, H.J. Chemotransport contributes to the effect of oscillatory fluid flow on human bone marrow stromal cell proliferation. J. Orthop. Res. 2008, 26, 918–924. [Google Scholar] [CrossRef]
- Cukierman, E.; Pankov, R.; Stevens, D.R.; Yamada, K.M. Taking cell-matrix adhesions to the third dimension. Science 2001, 294, 1708–1712. [Google Scholar] [CrossRef]
- Stiehler, M.; Bunger, C.; Baatrup, A.; Lind, M.; Kassem, M.; Mygind, T. Effect of dynamic 3-D culture on proliferation, distribution, and osteogenic differentiation of human mesenchymal stem cell. J. Biomed. Mater. Res. A 2009, 89, 96–107. [Google Scholar]
- Fröhlich, M.; Grayson, W.L.; Marolt, D.; Gimble, J.M.; Kregar-Velikonja, N.; Vunjak-Novakovic, G. Bone grafts engineered from human adipose-derived stem cells in perfusion bioreactor culture. Tissue Eng. Part. A 2010, 16, 179–189. [Google Scholar] [CrossRef]
- Grayson, W.L.; Marolt, D.; Bhumiratana, S.; Frohlich, M.; Guo, X.E.; Vunjak-Novakovic, G. Optimizing the medium perfusion rate in bone tissue engineering bioreactors. Biotechnol. Bioeng. 2011, 108, 1159–1170. [Google Scholar] [CrossRef]
- Janssen, F.W.; van Dijkhuizen-Radersma, R.; Van Oorschot, A.; Oostra, J.; de Bruijn, J.D.; Van Blitterswijk, C.A. Human tissue-engineered bone produced in clinically relevant amounts using a semi-automated perfusion bioreactor system: a preliminary study. J. Tissue Eng. Regen. Med. 2010, 4, 12–24. [Google Scholar]
- Grayson, W.L.; Frohlich, M.; Yeager, K.; Bhumiratana, S.; Chan, M.E.; Cannizzaro, C.; Wan, L.Q.; Liu, X.S.; Guo, X.E.; Vunjak-Novakovic, G. Engineering anatomically shaped human bone grafts. Proc. Natl. Acad. Sci. USA 2010, 107, 3299–3304. [Google Scholar]
- Nakamura, M.; Iwanaga, S.; Henmi, C.; Arai, K.; Nishiyama, Y. Biomatrices and biomaterials for future developments of bioprinting and biofabrication. Biofabrication. 2010, 2, 014110. [Google Scholar] [CrossRef]
- Liao, J.; Guo, X.; Nelson, D.; Kasper, F.K.; Mikos, A.G. Modulation of osteogenic properties of biodegradable polymer/extracellular matrix scaffolds generated with a flow perfusion bioreactor. Acta. Biomater. 2010, 6, 2386–2393. [Google Scholar] [CrossRef]
- Pham, Q.P.; Kasper, F.K.; Mistry, A.S.; Sharma, U.; Yasko, A.W.; Jansen, J.A.; Mikos, A.G. Analysis of the osteoinductive capacity and angiogenicity of an in vitro generated extracellular matrix. J. Biomed. Mater. Res. A 2009, 88, 295–303. [Google Scholar]
- Wheeler, A.R.; Throndset, W.R.; Whelan, R.J.; Leach, A.M.; Zare, R.N.; Liao, Y.H.; Farrell, K.; Manger, I.D.; Daridon, A. microfluidic device for single-cell analysis. Anal. Chem. 2003, 75, 3581–3586. [Google Scholar]
- Thomas, R.S.; Mitchell, P.D.; Oreffo, R.O.; Morgan, H. Trapping single human osteoblast-like cells from a heterogeneous population using a dielectrophoretic microfluidic device. Biomicrofluidics 2010, 4, 022806. [Google Scholar] [CrossRef]
- Kou, S.; Pan, L.; van Noort, D.; Meng, G.; Wu, X.; Sun, H.; Xu, J.; Lee, I. A multishear microfluidic device for quantitative analysis of calcium dynamics in osteoblasts. Biochem. Biophys. Res. Commun. 2011, 408, 350–355. [Google Scholar] [CrossRef]
- Leclerc, E.; David, B.; Griscom, L.; Lepioufle, B.; Fujii, T.; Layrolle, P.; Legallaisa, C. Study of osteoblastic cells in a microfluidic environment. Biomaterials 2006, 27, 586–595. [Google Scholar] [CrossRef]
- Jang, K.; Sato, K.; Igawa, K.; Chung, U.I.; Kitamori, T. Development of an osteoblast-based 3D continuous-perfusion microfluidic system for drug screening. Anal. Bioanal. Chem. 2008, 390, 825–832. [Google Scholar] [CrossRef]
- Song, S.H.; Choi, J.; Jung, H.I. A microfluidic magnetic bead impact generator for physical stimulation of osteoblast cell. Electrophoresis 2010, 31, 2762–2770. [Google Scholar] [CrossRef]
- Kirchhof, K.; Andar, A.; Yin, H.B.; Gadegaard, N.; Riehle, M.O.; Groth, T. Polyelectrolyte multilayers generated in a microfluidic device with pH gradients direct adhesion and movement of cells. Lab. Chip 2011, 11, 3326–3335. [Google Scholar] [CrossRef]
- Zhang, Y.; Gazit, Z.; Pelled, G.; Gazit, D.; Vunjak-Novakovic, G. Patterning osteogenesis by inducible gene expression in microfluidic culture systems. Integr. Biol. 2011, 3, 39–47. [Google Scholar] [CrossRef]
- Numthuam, S.; Ginoza, T.; Zhu, M.; Suzuki, H.; Fukuda, J. Gold-black micropillar electrodes for microfluidic ELISA of bone metabolic markers. Analyst 2011, 136, 456–458. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, Y.; Tan, Q.; Shojaei-Baghini, E.; Zhang, Y.L.; Li, J.; Prasad, P.; You, L.; Wu, X.Y.; Sun, Y. Classification of cell types using a microfluidic device for mechanical and electrical measurement on single cells. Lab. Chip 2011, 11, 3174–3181. [Google Scholar] [CrossRef]
- Lee, J.H.; Gu, Y.; Wang, H.; Lee, W.Y. Microfluidic 3D bone tissue model for high-throughput evaluation of wound-healing and infection-preventing biomaterials. Biomaterials 2012, 33, 999–1006. [Google Scholar] [CrossRef]
- Lee, J.H.; Wang, H.; Kaplan, J.B.; Lee, W.Y. Effects of Staphylococcus epidermidis on osteoblast cell adhesion and viability on a Ti alloy surface in a microfluidic co-culture environment. Acta. Biomater. 2010, 6, 4422–4429. [Google Scholar] [CrossRef]
- Wei, C.W.; Cheng, J.Y.; Young, T.H. Elucidating in vitro cell-cell interaction using a microfluidic coculture system. Biomed. Microdevices. 2006, 8, 65–71. [Google Scholar] [CrossRef]
- Scherberich, A.; Galli, R.; Jaquiery, C.; Farhadi, J.; Martin, I. Three-dimensional perfusion culture of human adipose tissue-derived endothelial and osteoblastic progenitors generates osteogenic constructs with intrinsic vascularization capacity. Stem Cells 2007, 25, 1823–1829. [Google Scholar] [CrossRef]
- Yuan, L.; Sakamoto, N.; Song, G.; Sato, M. Migration of human mesenchymal stem cells under low shear stress mediated by mitogen-activated protein kinase signaling. Stem Cells Dev. 2012, 21, 2520–2530. [Google Scholar] [CrossRef]
- Gurkan, U.A.; Akkus, O. The mechanical environment of bone marrow: A review. Ann. Biomed. Eng. 2008, 36, 1978–1991. [Google Scholar] [CrossRef]
- Kshitiz; Park, J.; Kim, P.; Helen, W.; Engler, A.J.; Levchenko, A.; Kim, D.H. Control of stem cell fate and function by engineering physical microenvironments. Integr. Biol. 2012, 4, 1008–1018. [Google Scholar] [CrossRef]
- Gonçalves, A.; Costa, P.; Rodrigues, M.T.; Dias, I.R.; Reis, R.L.; Gomes, M.E. Effect of flow perfusion conditions in the chondrogenic differentiation of bone marrow stromal cells cultured onto starch based biodegradable scaffolds. Acta. Biomater. 2011, 7, 1644–1652. [Google Scholar] [CrossRef] [Green Version]
- Alves da Silva, M.L.; Martins, A.; Costa-Pinto, A.R.; Correlo, V.M.; Sol, P.; Bhattacharya, M.; Faria, S.; Reis, R.L.; Neves, N.M. Chondrogenic differentiation of human bone marrow mesenchymal stem cells in chitosan-based scaffolds using a flow-perfusion bioreactor. J. Tissue Eng. Regen. Med. 2011, 5, 722–732. [Google Scholar] [CrossRef]
- Kinney, M.A.; Sargent, C.Y.; McDevitt, T.C. The multiparametric effects of hydrodynamic environments on stem cell culture. Tissue Eng. Part. B Rev. 2011, 17, 249–262. [Google Scholar]
- Patwari, P.; Lee, R.T. Mechanical control of tissue morphogenesis. Circ. Res. 2008, 103, 234–243. [Google Scholar] [CrossRef]
- Barron, M.J.; Goldman, J.; Tsai, C.J.; Donahue, S.W. Perfusion flow enhances osteogenic gene expression and the infiltration of osteoblasts and endothelial cells into three-dimensional calcium phosphate scaffolds. Int. J. Biomater. 2012, 2012, 915620. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Riehl, B.D.; Lim, J.Y. Macro and Microfluidic Flows for Skeletal Regenerative Medicine. Cells 2012, 1, 1225-1245. https://doi.org/10.3390/cells1041225
Riehl BD, Lim JY. Macro and Microfluidic Flows for Skeletal Regenerative Medicine. Cells. 2012; 1(4):1225-1245. https://doi.org/10.3390/cells1041225
Chicago/Turabian StyleRiehl, Brandon D., and Jung Yul Lim. 2012. "Macro and Microfluidic Flows for Skeletal Regenerative Medicine" Cells 1, no. 4: 1225-1245. https://doi.org/10.3390/cells1041225



