The Anti-Apoptotic Role of Neurotensin
Abstract
:1. Introduction
2. Anti-Apoptotic Actions of NT in the Central Nervous System
3. Anti-Apoptotic Effects of NT in Non-Neuronal Cell Types
3.1. Cancer Cells
3.2. Gastrointestinal Tissues and Cells
3.3. Pancreatic Tissues and Cells
3.4. Immune System
4. Pro-Apoptotic Action of NT in the Central Nervous System
5. Discussion
| Site | Effect | Receptor(s) | Target | Cellular pathway | References |
|---|---|---|---|---|---|
| Brain | antagonism of pro-NGF-induced apoptosis | NTSR3/sortilin | neurons | n.d. | [33] |
| Brain | antagonism of pro-BDNF-induced apoptosis | NTSR3/sortilin | neurons | n.d. | [35] |
| Brain | reversion of ischemia-induced apoptosis | NTSR1 | neurons | Bcl-2 | [42] |
| Brain | increase of glutamate-induced apoptosis | n.d. | neurons | n.d. | [69] |
| Retina | inhibition of natural apoptosis of primary cultures | NTSR1 | corneal keratocytes | n.d. | [41] |
| Retina | reversion of light-induced apoptosis | NTSR3/sortilin | retinal cells | [37] | |
| Breast | inhibition of serum deprivation-induced apoptosis | NTSR1 | MCF-7 cells | Bcl-2 | [45] |
| Liver | inhibition of bile duct ligation-induced apoptosis | n.d. | oval cells | n.d. | [53] |
| Intestine | reduction of colitis-induced apoptosis | NTSR1 | intestinal tissue | n.d. | [54] |
| Intestine | reversion of jaundice-induced intestinal atrophy | n.d. | intestinal crypts | n.d. | [51] |
| Endocrine pancreas | protection against Il-1β-induced apoptosis | NTSR2–NTSR3/sortilin | beta cells | Akt | [13,55] |
| Immune system | reversion of pro-BDNF-induced apoptosis | NTSR3/sortilin | B-cells | n.d. | [56] |
| Immune system | reversion of serum deprivation-induced apoptosis | NTSR1–NTSR2 | B-cells | n.d. | [57] |
| Immune system | antagonism of pro-NGF-induced apoptosis | NTSR3/sortilin | NK cells | n.d. | [58] |
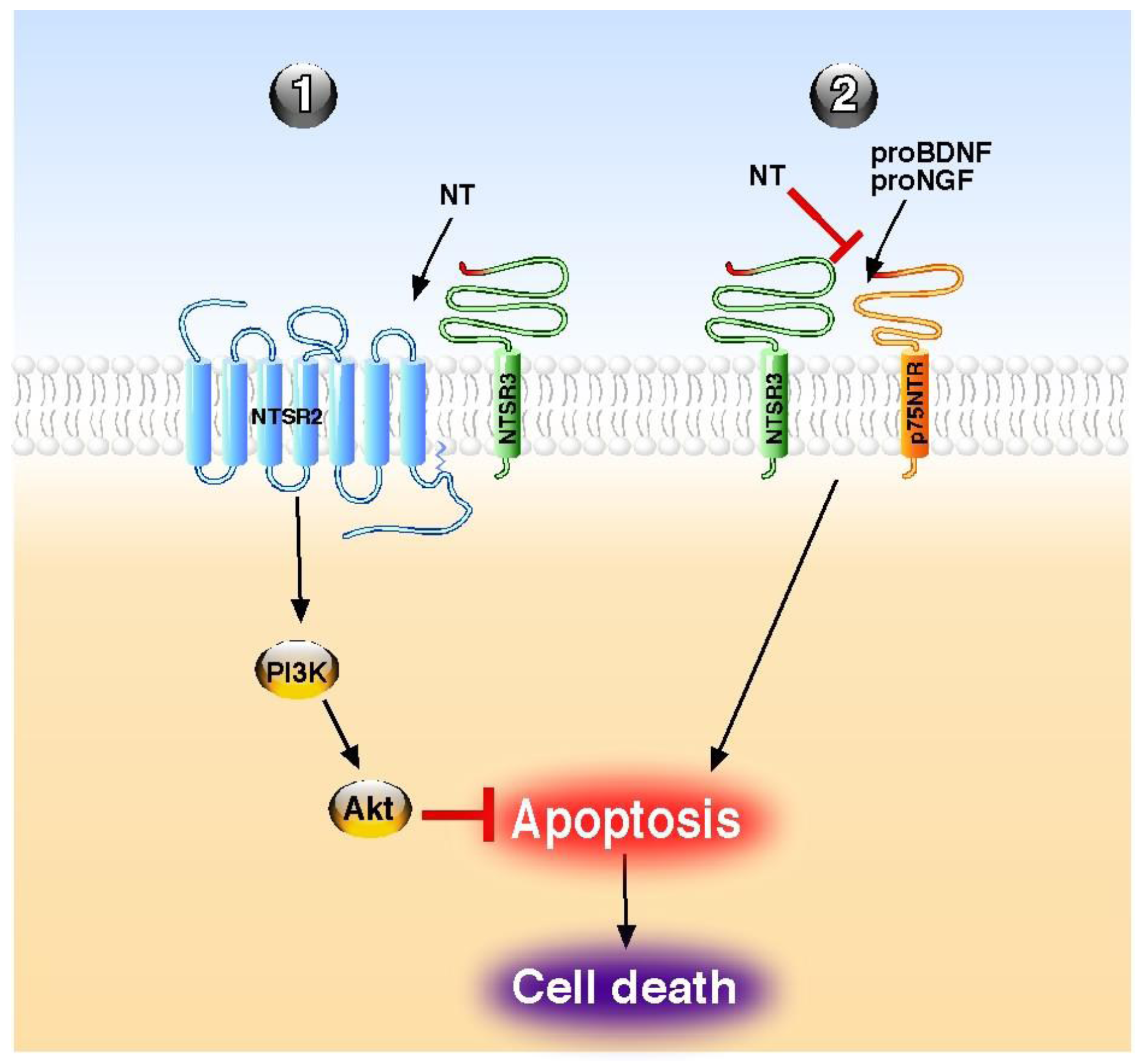
6. Conclusions
Acknowledgments
Conflict of Interest
References
- Carraway, R.; Leeman, S.E. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J. Biol. Chem. 1973, 248, 6854–6861. [Google Scholar]
- Kitabgi, P. Differential processing of pro-neurotensin/neuromedin N and relationship to pro-hormone convertases. Peptides 2006, 27, 2508–2514. [Google Scholar] [CrossRef]
- Goedert, M.; Emson, P.C. The regional distribution of neurotensin-like immunoreactivity in central and peripheral tissues of the cat. Brain Res. 1983, 272, 291–297. [Google Scholar] [CrossRef]
- Uhl, G.R.; Snyder, S.H. Regional and subcellular distributions of brain neurotensin. Life Sci. 1976, 19, 1827–1832. [Google Scholar] [CrossRef]
- Binder, E.B.; Kinkead, B.; Owens, M.J.; Nemeroff, C.B. Neurotensin and dopamine interactions. Pharmacol. Rev. 2001, 53, 453–486. [Google Scholar]
- Dubuc, I.; Sarret, P.; Labbe-Jullie, C.; Botto, J.M.; Honore, E.; Bourdel, E.; Martinez, J.; Costentin, J.; Vincent, J.P.; Kitabgi, P.; Mazella, J. Identification of the receptor subtype involved in the analgesic effect of neurotensin. J. Neurosci. 1999, 19, 503–510. [Google Scholar]
- Popp, E.; Schneider, A.; Vogel, P.; Teschendorf, P.; Bottiger, B.W. Time course of the hypothermic response to continuously administered neurotensin. Neuropeptides 2007, 41, 349–354. [Google Scholar]
- Nemeroff, C.B. Neurotensin: Perchance an endogenous neuroleptic? Biol. Psychiatry 1980, 15, 283–302. [Google Scholar]
- Nemeroff, C.B.; Bissette, G.; Widerlov, E.; Beckmann, H.; Gerner, R.; Manberg, P.J.; Lindstrom, L.; Prange, A.J., Jr.; Gattaz, W.F. Neurotensin-Like immunoreactivity in cerebrospinal fluid of patients with schizophrenia, depression, anorexia nervosa-bulimia, and premenstrual syndrome. J. Neuropsychiatry Clin. Neurosci. 1989, 1, 16–20. [Google Scholar]
- Breslin, N.A.; Suddath, R.L.; Bissette, G.; Nemeroff, C.B.; Lowrimore, P.; Weinberger, D.R. CSF concentrations of neurotensin in schizophrenia: An investigation of clinical and biochemical correlates. Schizophr. Res. 1994, 12, 35–41. [Google Scholar] [CrossRef]
- Leeman, S.E.; Carraway, R.E. Neurotensin: Discovery, isolation, characterization, synthesis and possible physiological roles. Ann. N Y Acad. Sci. 1982, 400, 1–16. [Google Scholar] [CrossRef]
- Gui, X.; Carraway, R.E. Enhancement of jejunal absorption of conjugated bile acid by neurotensin in rats. Gastroenterology 2001, 120, 151–60. [Google Scholar]
- Coppola, T.; Beraud-Dufour, S.; Antoine, A.; Vincent, J.P.; Mazella, J. Neurotensin protects pancreatic beta cells from apoptosis. Int. J. Biochem. Cell. Biol. 2008, 40, 2296–2302. [Google Scholar] [CrossRef]
- Dolais-Kitabgi, J.; Kitabgi, P.; Brazeau, P.; Freychet, P. Effect of neurotensin on insulin, glucagon, and somatostatin release from isolated pancreatic islets. Endocrinology 1979, 105, 256–260. [Google Scholar] [CrossRef]
- Beraud-Dufour, S.; Abderrahmani, A.; Noel, J.; Brau, F.; Waeber, G.; Mazella, J.; Coppola, T. Neurotensin is a regulator of insulin secretion in pancreatic beta-cells. Int. J. Biochem. Cell. Biol. 2010, 42, 1681–1688. [Google Scholar] [CrossRef]
- Vincent, J.P.; Mazella, J.; Kitabgi, P. Neurotensin and neurotensin receptors. Trends Pharmacol. Sci. 1999, 20, 302–309. [Google Scholar] [CrossRef]
- Myers, R.M.; Shearman, J.W.; Kitching, M.O.; Ramos-Montoya, A.; Neal, D.E.; Ley, S.V. Cancer, chemistry, and the cell: Molecules that interact with the neurotensin receptors. ACS Chem. Biol. 2009, 4, 503–525. [Google Scholar] [CrossRef]
- Mazella, J.; Zsurger, N.; Navarro, V.; Chabry, J.; Kaghad, M.; Caput, D.; Ferrara, P.; Vita, N.; Gully, D.; Maffrand, J.P.; et al. The 100-kDa neurotensin receptor is gp95/sortilin, a non-G-protein-coupled receptor. J. Biol. Chem. 1998, 273, 26273–26276. [Google Scholar]
- Petersen, C.M.; Nielsen, M.S.; Nykjaer, A.; Jacobsen, L.; Tommerup, N.; Rasmussen, H.H.; Roigaard, H.; Gliemann, J.; Madsen, P.; Moestrup, S.K. Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. J. Biol. Chem. 1997, 272, 3599–3605. [Google Scholar]
- Mazella, J. Sortilin/neurotensin receptor-3: A new tool to investigate neurotensin signaling and cellular trafficking? Cell. Signal. 2001, 13, 1–6. [Google Scholar] [CrossRef]
- Hermey, G. The Vps10p-domain receptor family. Cell. Mol. Life Sci. 2009, 66, 2677–2689. [Google Scholar] [CrossRef]
- Kerr, J.F.; Winterford, C.M.; Harmon, B.V. Apoptosis. Its significance in cancer and cancer therapy. Cancer 1994, 73, 2013–2026. [Google Scholar] [CrossRef]
- Hockenbery, D.; Nunez, G.; Milliman, C.; Schreiber, R.D.; Korsmeyer, S.J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 1990, 348, 334–336. [Google Scholar]
- Korsmeyer, S.J. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999, 59, 1693s–1700s. [Google Scholar]
- Aouacheria, A.; Brunet, F.; Gouy, M. Phylogenomics of life-or-death switches in multicellular animals: Bcl-2, BH3-Only, and BNip families of apoptotic regulators. Mol. Biol. Evol. 2005, 22, 2395–2416. [Google Scholar]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell. Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef]
- Chipuk, J.E.; Moldoveanu, T.; Llambi, F.; Parsons, M.J.; Green, D.R. The BCL-2 family reunion. Mol. Cell. 2010, 37, 299–310. [Google Scholar] [CrossRef]
- Oltvai, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993, 74, 609–619. [Google Scholar] [CrossRef]
- Shen, Y.; White, E. p53-dependent apoptosis pathways. Adv. Cancer Res. 2001, 82, 55–84. [Google Scholar] [CrossRef]
- Gahete, M.D.; Rubio, A.; Cordoba-Chacon, J.; Gracia-Navarro, F.; Kineman, R.D.; Avila, J.; Luque, R.M.; Castano, J.P. Expression of the ghrelin and neurotensin systems is altered in the temporal lobe of Alzheimer’s disease patients. J. Alzheimers Dis. 2010, 22, 819–828. [Google Scholar]
- Jansen, P.; Giehl, K.; Nyengaard, J.R.; Teng, K.; Lioubinski, O.; Sjoegaard, S.S.; Breiderhoff, T.; Gotthardt, M.; Lin, F.; Eilers, A.; et al. Roles for the pro-neurotrophin receptor sortilin in neuronal development, aging and brain injury. Nat. Neurosci. 2007, 10, 1449–1457. [Google Scholar]
- Nykjaer, A.; Willnow, T.E. Sortilin: A receptor to regulate neuronal viability and function. Trends Neurosci. 2012, 35, 261–270. [Google Scholar]
- Nykjaer, A.; Lee, R.; Teng, K.K.; Jansen, P.; Madsen, P.; Nielsen, M.S.; Jacobsen, C.; Kliemannel, M.; Schwarz, E.; Willnow, T.E.; et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature 2004, 427, 843–848. [Google Scholar]
- Al-Shawi, R.; Hafner, A.; Olsen, J.; Chun, S.; Raza, S.; Thrasivoulou, C.; Lovestone, S.; Killick, R.; Simons, P.; Cowen, T. Neurotoxic and neurotrophic roles of proNGF and the receptor sortilin in the adult and ageing nervous system. Eur. J. Neurosci. 2008, 27, 2103–2114. [Google Scholar]
- Teng, H.K.; Teng, K.K.; Lee, R.; Wright, S.; Tevar, S.; Almeida, R.D.; Kermani, P.; Torkin, R.; Chen, Z.Y.; Lee, F.S.; et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J. Neurosci. 2005, 25, 5455–5463. [Google Scholar]
- Tauris, J.; Gustafsen, C.; Christensen, E.I.; Jansen, P.; Nykjaer, A.; Nyengaard, J.R.; Teng, K.K.; Schwarz, E.; Ovesen, T.; Madsen, P.; et al. Proneurotrophin-3 may induce Sortilin-dependent death in inner ear neurons. Eur. J. Neurosci. 2011, 33, 622–631. [Google Scholar]
- Santos, A.M.; Lopez-Sanchez, N.; Martin-Oliva, D.; de la Villa, P.; Cuadros, M.A.; Frade, J.M. Sortilin participates in light-dependent photoreceptor degeneration in vivo. PLoS One 2012, 7, e36243. [Google Scholar]
- Emmett, M.R.; Andren, P.E.; Caprioli, R.M. Specific molecular mass detection of endogenously released neuropeptides using in vivo microdialysis/mass spectrometry. J. Neurosci. Methods 1995, 62, 141–147. [Google Scholar] [CrossRef]
- Holst Pedersen, J.; Fahrenkrug, J. Neurotensin-like immunoreactivities in human plasma: Feeding responses and metabolism. Peptides 1986, 7, 15–20. [Google Scholar] [CrossRef]
- Li, H.B.; Lam, D.M. Synaptic organization of neurotensin immunoreactive amacrine cells in the chicken retina. J. Comp. Neurol. 1990, 294, 252–261. [Google Scholar] [CrossRef]
- Bourcier, T.; Rondeau, N.; Paquet, S.; Forgez, P.; Lombet, A.; Pouzaud, F.; Rostene, W.; Borderie, V.; Laroche, L. Expression of neurotensin receptors in human corneal keratocytes. Invest. Ophthalmol. Vis. Sci. 2002, 43, 1765–1771. [Google Scholar]
- Choi, K.E.; Hall, C.L.; Sun, J.M.; Wei, L.; Mohamad, O.; Dix, T.A.; Yu, S.P. A novel stroke therapy of pharmacologically induced hypothermia after focal cerebral ischemia in mice. FASEB J. 2012, 26, 2799–2810. [Google Scholar] [CrossRef]
- Hughes, F.M., Jr.; Shaner, B.E.; May, L.A.; Zotian, L.; Brower, J.O.; Woods, R.J.; Cash, M.; Morrow, D.; Massa, F.; Mazella, J.; et al. Identification and functional characterization of a stable, centrally active derivative of the neurotensin (8–13) fragment as a potential first-in-class analgesic. J. Med. Chem. 2010, 53, 4623–4632. [Google Scholar]
- Carraway, R.E.; Plona, A.M. Involvement of neurotensin in cancer growth: Evidence, mechanisms and development of diagnostic tools. Peptides 2006, 27, 2445–2460. [Google Scholar] [CrossRef]
- Somai, S.; Gompel, A.; Rostene, W.; Forgez, P. Neurotensin counteracts apoptosis in breast cancer cells. Biochem. Biophys. Res. Commun. 2002, 295, 482–488. [Google Scholar] [CrossRef]
- Valerie, N.C.; Casarez, E.V.; Dasilva, J.O.; Dunlap-Brown, M.E.; Parsons, S.J.; Amorino, G.P.; Dziegielewski, J. Inhibition of neurotensin receptor 1 selectively sensitizes prostate cancer to ionizing radiation. Cancer Res. 2011, 71, 6817–6826. [Google Scholar]
- Sumitomo, M.; Shen, R.; Nanus, D.M. Involvement of neutral endopeptidase in neoplastic progression. Biochim. Biophys. Acta 2005, 1751, 52–59. [Google Scholar] [CrossRef]
- Shipp, M.A.; Tarr, G.E.; Chen, C.Y.; Switzer, S.N.; Hersh, L.B.; Stein, H.; Sunday, M.E.; Reinherz, E.L. CD10/neutral endopeptidase 24.11 hydrolyzes bombesin-like peptides and regulates the growth of small cell carcinomas of the lung. Proc. Natl. Acad. Sci. USA 1991, 88, 10662–10666. [Google Scholar]
- D'Orleans-Juste, P.; Plante, M.; Honore, J.C.; Carrier, E.; Labonte, J. Synthesis and degradation of endothelin-1. Can. J. Physiol. Pharmacol. 2003, 81, 503–510. [Google Scholar] [CrossRef]
- Checler, F.; Emson, P.C.; Vincent, J.P.; Kitabgi, P. Inactivation of neurotensin by rat brain synaptic membranes. Cleavage at the Pro10-Tyr11 bond by endopeptidase 24.11 (enkephalinase) and a peptidase different from proline-endopeptidase. J. Neurochem. 1984, 43, 1295–1301. [Google Scholar]
- Assimakopoulos, S.F.; Scopa, C.D.; Zervoudakis, G.; Mylonas, P.G.; Georgiou, C.; Nikolopoulou, V.; Vagianos, C.E. Bombesin and neurotensin reduce endotoxemia, intestinal oxidative stress, and apoptosis in experimental obstructive jaundice. Ann. Surg. 2005, 241, 159–167. [Google Scholar]
- Assimakopoulos, S.F.; Alexandris, I.H.; Scopa, C.D.; Mylonas, P.G.; Thomopoulos, K.C.; Georgiou, C.D.; Nikolopoulou, V.N.; Vagianos, C.E. Effect of bombesin and neurotensin on gut barrier function in partially hepatectomized rats. World J. Gastroenterol. 2005, 11, 6757–6764. [Google Scholar]
- Assimakopoulos, S.F.; Tsamandas, A.C.; Georgiou, C.D.; Vagianos, C.E.; Scopa, C.D. Bombesin and neurotensin exert antiproliferative effects on oval cells and augment the regenerative response of the cholestatic rat liver. Peptides 2010, 31, 2294–2303. [Google Scholar] [CrossRef]
- Akcan, A.; Muhtaroglu, S.; Akgun, H.; Akyildiz, H.; Kucuk, C.; Sozuer, E.; Yurci, A.; Yilmaz, N. Ameliorative effects of bombesin and neurotensin on trinitrobenzene sulphonic acid-induced colitis, oxidative damage and apoptosis in rats. World J. Gastroenterol. 2008, 14, 1222–1230. [Google Scholar] [CrossRef]
- Beraud-Dufour, S.; Coppola, T.; Massa, F.; Mazella, J. Neurotensin receptor-2 and -3 are crucial for the anti-apoptotic effect of neurotensin on pancreatic beta-TC3 cells. Int. J. Biochem. Cell. Biol. 2009, 41, 2398–2402. [Google Scholar] [CrossRef]
- Fauchais, A.L.; Lalloue, F.; Lise, M.C.; Boumediene, A.; Preud'homme, J.L.; Vidal, E.; Jauberteau, M.O. Role of endogenous brain-derived neurotrophic factor and sortilin in B cell survival. J. Immunol. 2008, 181, 3027–3038. [Google Scholar]
- Saada, S.; Marget, P.; Fauchais, A.L.; Lise, M.C.; Chemin, G.; Sindou, P.; Martel, C.; Delpy, L.; Vidal, E.; Jaccard, A.; et al. Differential expression of neurotensin and specific receptors, NTSR1 and NTSR2, in Normal and malignant human B lymphocytes. J. Immunol. 2012, 189, 5293–5303. [Google Scholar] [CrossRef]
- Rogers, M.L.; Bailey, S.; Matusica, D.; Nicholson, I.; Muyderman, H.; Pagadala, P.C.; Neet, K.E.; Zola, H.; Macardle, P.; Rush, R.A. ProNGF mediates death of Natural Killer cells through activation of the p75NTR-sortilin complex. J. Neuroimmunol. 2010, 226, 93–103. [Google Scholar] [CrossRef]
- Da Silva, L.; Neves, B.M.; Moura, L.; Cruz, M.T.; Carvalho, E. Neurotensin downregulates the pro-inflammatory properties of skin dendritic cells and increases epidermal growth factor expression. Biochim. Biophys. Acta 2011, 1813, 1863–1871. [Google Scholar]
- Da Silva, L.; Carvalho, E.; Cruz, M.T. Role of neuropeptides in skin inflammation and its involvement in diabetic wound healing. Expert Opin. Biol. Ther. 2010, 10, 1427–1439. [Google Scholar] [CrossRef]
- Cheung, N.S.; Pascoe, C.J.; Giardina, S.F.; John, C.A.; Beart, P.M. Micromolar L-glutamate induces extensive apoptosis in an apoptotic-necrotic continuum of insult-dependent, excitotoxic injury in cultured cortical neurones. Neuropharmacology 1998, 37, 1419–1429. [Google Scholar] [CrossRef]
- Ferraro, L.; Tomasini, M.C.; Siniscalchi, A.; Fuxe, K.; Tanganelli, S.; Antonelli, T. Neurotensin increases endogenous glutamate release in rat cortical slices. Life Sci. 2000, 66, 927–936. [Google Scholar]
- Matsuyama, S.; Fukui, R.; Higashi, H.; Nishi, A. Regulation of DARPP-32 Thr75 phosphorylation by neurotensin in neostriatal neurons: Involvement of glutamate signalling. Eur. J. Neurosci. 2003, 18, 1247–1253. [Google Scholar] [CrossRef]
- Ferraro, L.; Tomasini, M.C.; Fernandez, M.; Bebe, B.W.; O’Connor, W.T.; Fuxe, K.; Glennon, J.C.; Tanganelli, S.; Antonelli, T. Nigral neurotensin receptor regulation of nigral glutamate and nigroventral thalamic GABA transmission: A dual-probe microdialysis study in intact conscious rat brain. Neuroscience 2001, 102, 113–120. [Google Scholar] [CrossRef]
- Ferraro, L.; Beggiato, S.; Tomasini, M.C.; Fuxe, K.; Tanganelli, S.; Antonelli, T. Neurotensin regulates cortical glutamate transmission by modulating N-methyl-D-aspartate receptor functional activity: An in vivo microdialysis study. J. Neurosci. Res. 2011, 89, 1618–1626. [Google Scholar] [CrossRef]
- Allen, G.V.; Cheung, R.T.; Cechetto, D.F. Neurochemical changes following occlusion of the middle cerebral artery in rats. Neuroscience 1995, 68, 1037–1050. [Google Scholar] [CrossRef]
- Katz, L.M.; Young, A.; Frank, J.E.; Wang, Y.; Park, K. Neurotensin-induced hypothermia improves neurologic outcome after hypoxic-ischemia. Crit. Care. Med. 2004, 32, 806–810. [Google Scholar] [CrossRef]
- Kokko, K.P.; Hadden, M.K.; Price, K.L.; Orwig, K.S.; See, R.E.; Dix, T.A. In vivo behavioral effects of stable, receptor-selective neurotensin [8–13] analogues that cross the blood-brain barrier. Neuropharmacology 2005, 48, 417–425. [Google Scholar] [CrossRef]
- Antonelli, T.; Tomasini, M.C.; Fournier, J.; Mazza, R.; Tanganelli, S.; Pirondi, S.; Fuxe, K.; Ferraro, L. Neurotensin receptor involvement in the rise of extracellular glutamate levels and apoptotic nerve cell death in primary cortical cultures after oxygen and glucose deprivation. Cereb. Cortex 2008, 18, 1748–1757. [Google Scholar] [CrossRef]
- Law, I.K.; Murphy, J.E.; Bakirtzi, K.; Bunnett, N.W.; Pothoulakis, C. Neurotensin-Induced proinflammatory signaling in human colonocytes is regulated by beta-arrestins and endothelin-converting enzyme-1-dependent endocytosis and resensitization of neurotensin receptor 1. J. Biol. Chem. 2012, 287, 15066–15075. [Google Scholar]
- Martin, S.; Dicou, E.; Vincent, J.P.; Mazella, J. Neurotensin and the neurotensin receptor-3 in microglial cells. J. Neurosci. Res. 2005, 81, 322–326. [Google Scholar] [CrossRef]
- Martin, S.; Navarro, V.; Vincent, J.P.; Mazella, J. Neurotensin receptor-1 and -3 complex modulates the cellular signaling of neurotensin in the HT29 cell line. Gastroenterology 2002, 123, 1135–1143. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Devader, C.; Béraud-Dufour, S.; Coppola, T.; Mazella, J. The Anti-Apoptotic Role of Neurotensin. Cells 2013, 2, 124-135. https://doi.org/10.3390/cells2010124
Devader C, Béraud-Dufour S, Coppola T, Mazella J. The Anti-Apoptotic Role of Neurotensin. Cells. 2013; 2(1):124-135. https://doi.org/10.3390/cells2010124
Chicago/Turabian StyleDevader, Christelle, Sophie Béraud-Dufour, Thierry Coppola, and Jean Mazella. 2013. "The Anti-Apoptotic Role of Neurotensin" Cells 2, no. 1: 124-135. https://doi.org/10.3390/cells2010124





