Redirection of Human Cancer Cells upon the Interaction with the Regenerating Mouse Mammary Gland Microenvironment
Abstract
:1. Introduction
2. Results and Discussion
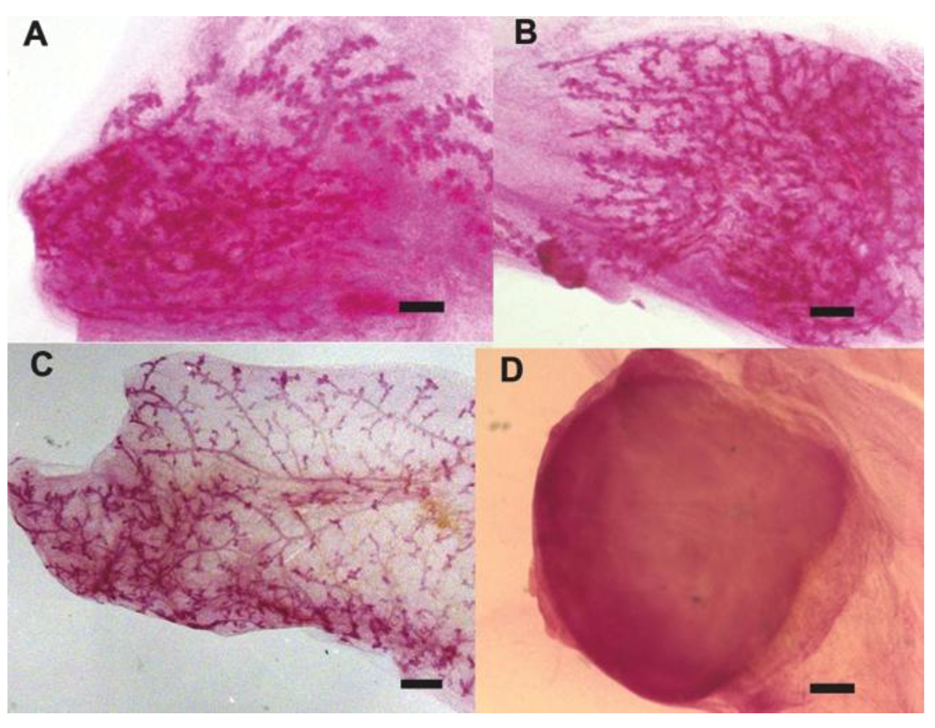
2.1. Human Cancer Cells Contribute to the Regeneration of Tumor-free Chimera (Human and Mouse) Mammary Glands and Thus Respond to Normal Tissue-specific Developmental Signals
2.2. Human Cancer Cells Contain Multipotent and Self-renewing Progenitor Cells that Respond to the Mammary Niche Differentiation Signals
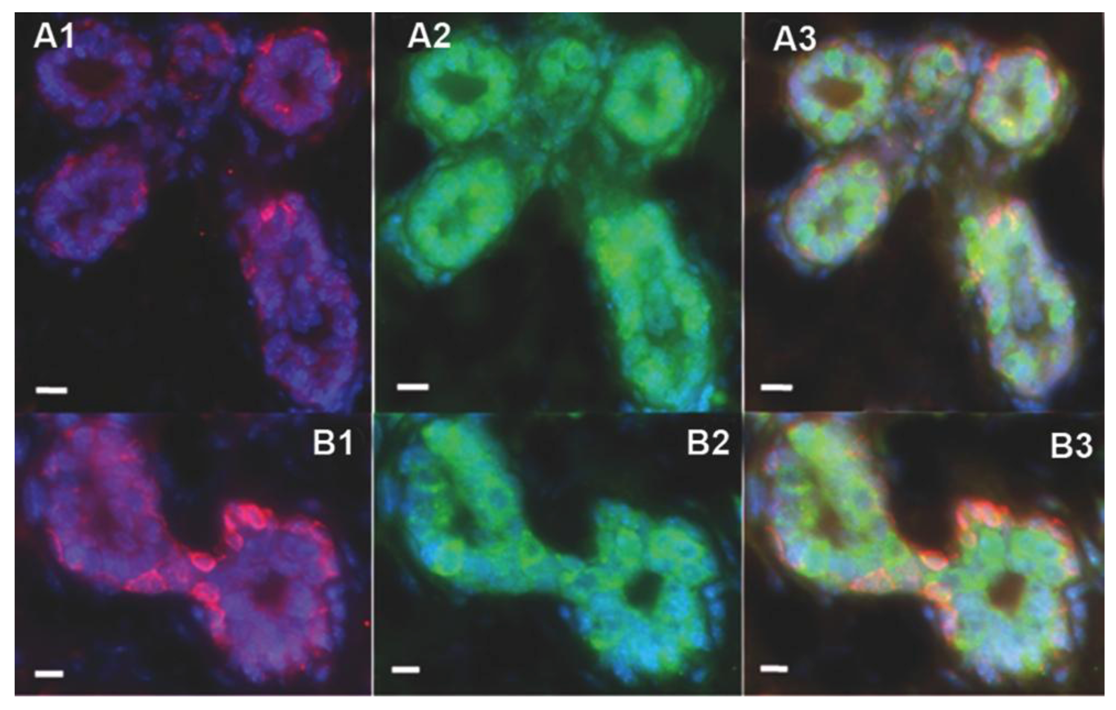
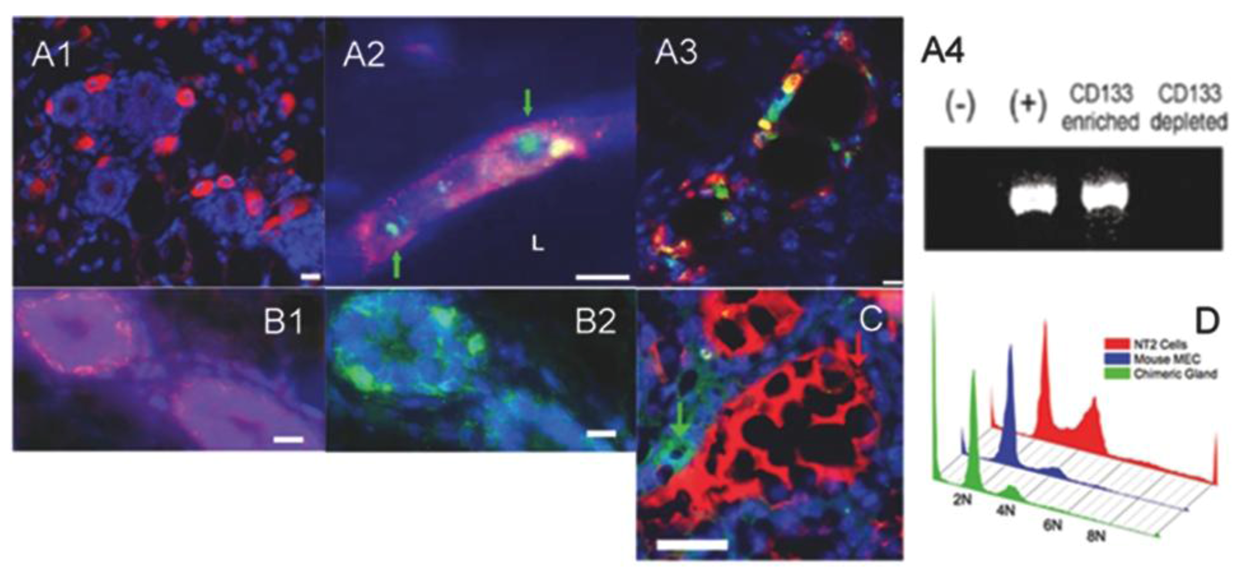
2.3. Human Cancer Cells and Host Mouse Epithelial Cells Do Not Fuse During Mammary Gland Regeneration
2.4. Tumor-initiating Cells Are Able to Obey to Normal Niche Signals
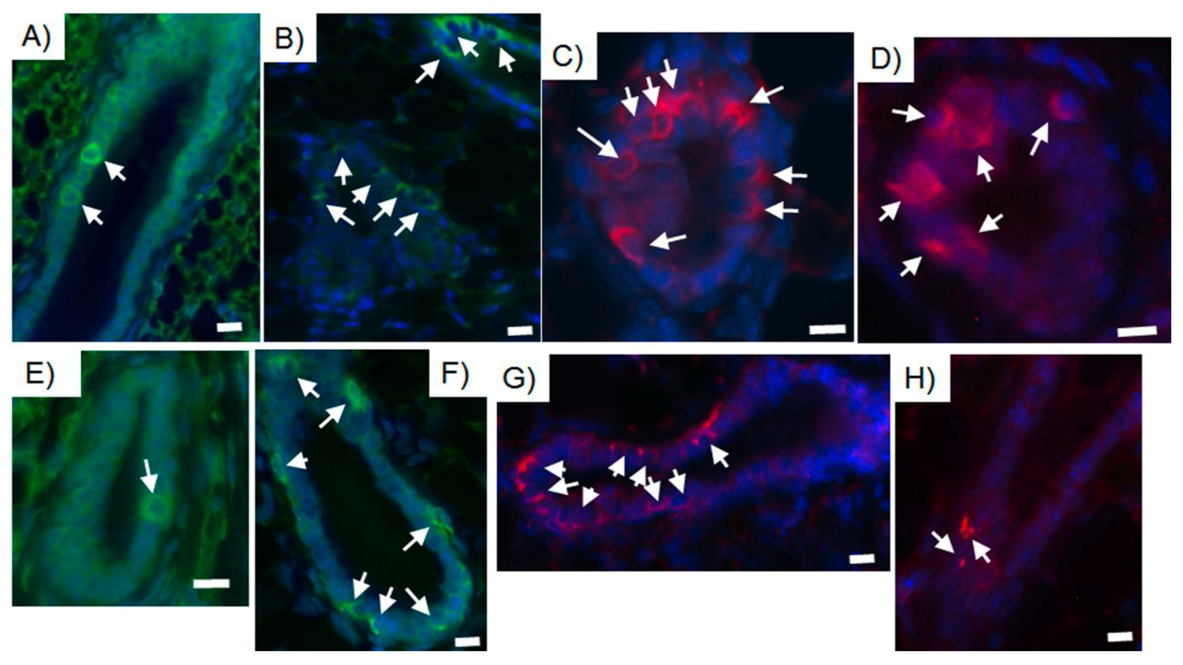
3. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Felsher, D.W. Cancer revoked: Oncogenes as therapeutic targets. Nat. Rev. Cancer 2003, 3, 375–380. [Google Scholar] [CrossRef]
- Vogelstein, B.; Kinzler, K.W. Cancer genes and the pathways they control. Nat. Med. 2004, 10, 789–799. [Google Scholar] [CrossRef]
- Jain, M.; Arvanitis, C.; Chu, K.; Dewey, W.; Leonhardt, E.; Trinh, M.; Sundberg, C.D.; Bishop, J.M.; Felsher, D.W. Sustained loss of a neoplastic phenotype by brief inactivation of myc. Science 2002, 297, 102–104. [Google Scholar]
- Hochedlinger, K.; Blelloch, R.; Brennan, C.; Yamada, Y.; Kim, M.; Chin, L.; Jaenisch, R. Reprogramming of a melanoma genome by nuclear transplantation. Genes Dev. 2004, 18, 1875–1885. [Google Scholar] [CrossRef]
- McCullough, K.D.; Coleman, W.B.; Ricketts, S.L.; Wilson, J.W.; Smith, G.J.; Grisham, J.W. Plasticity of the neoplastic phenotype in vivo is regulated by epigenetic factors. Proc. Natl. Acad. Sci. USA 1998, 95, 15333–15338. [Google Scholar]
- Stoker, A.W.; Hatier, C.; Bissell, M.J. The embryonic environment strongly attenuates v-src oncogenesis in mesenchymal and epithelial tissues, but not in endothelia. J. Cell. Biol. 1990, 111, 217–228. [Google Scholar] [CrossRef]
- Rous, P. A transmissible avian neoplasm. J. Exp. Med. 1979, 150, 738–753. [Google Scholar]
- Rous, P. A transmissible avian neoplasm. (sarcoma of the common fowl.). J. Exp. Med. 1910, 12, 696–705. [Google Scholar] [CrossRef]
- Stoker, A.W.; Hatier, C.; Bissell, M.J. The embryonic environment strongly attenuates v-scr oncogenesis in mesenchymal and epithelial tissues, but not in endothelia. J. Cell. Biol. 1990, 111, 217–228. [Google Scholar] [CrossRef]
- Illmensee, K.; Mintz, B. Totipotency and normal differentiation of single teratocarcinoma cells cloned by injection into blastocysts. Proc. Natl. Acad. Sci. USA 1976, 73, 549–553. [Google Scholar]
- Gerschenson, M.; Graves, K.; Carson, S.D.; Wells, R.S.; Pierce, G.B. Regulation of melanoma by the embryonic skin. Proc. Natl. Acad. Sci. USA 1986, 83, 7307–7310. [Google Scholar]
- Mintz, B.; Illmensee, K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc. Natl. Acad. Sci. USA 1975, 72, 3585–3589. [Google Scholar]
- McCullough, K.D.; Coleman, W.B.; Ricketts, S.L.; Wilson, J.W.; Smith, G.J.; Grisham, J.W. Plasticity of the neoplastic phenotype in vivo is regulated by epigenetic factors. Proc. Natl. Acad. Sci. USA 1998, 95, 15333–15338. [Google Scholar]
- Brinster, R.L. The effect of cells transferred into the mouse blastocyst on subsequent development. J. Exp. Med. 1974, 140, 1049–1056. [Google Scholar] [CrossRef]
- Pierce, G.B.; Dixon, F.J., Jr. Testicular teratomas. Ii. Teratocarcinoma as an ascitic tumor. Cancer 1959, 12, 584–589. [Google Scholar] [CrossRef]
- Pierce, G.B. Jr.; Dixon, F.J., Jr.; Verney, E.L. Teratocarcinogenic and tissue-forming potentials of the cell types comprising neoplastic embryoid bodies. Lab. Invest. 1960, 9, 583–602. [Google Scholar]
- Pierce, G.B., Jr.; Verney, E.L. An in vitro and in vivo study of differentiation in teratocarcinomas. Cancer 1961, 14, 1017–1029. [Google Scholar] [CrossRef]
- Pierce, G.B.; Aguilar, D.; Hood, G.; Wells, R.S. Trophectoderm in control of murine embryonal carcinoma. Cancer Res. 1984, 44, 3987–3996. [Google Scholar]
- Papaioannou, V.E.; McBurney, M.W.; Gardner, R.L.; Evans, M.J. Fate of teratocarcinoma cells injected into early mouse embryos. Nature 1975, 258, 70–73. [Google Scholar]
- Pierce, G.B.; Pantazis, C.G.; Caldwell, J.E.; Wells, R.S. Specificity of the control of tumor formation by the blastocyst. Cancer Res. 1982, 42, 1082–1087. [Google Scholar]
- Bussard, K.M.; Boulanger, C.A.; Booth, B.W.; Bruno, R.D.; Smith, G.H. Reprogramming human cancer cells in the mouse mammary gland. Cancer Res. 2010, 70, 6336–6343. [Google Scholar]
- Boulanger, C.A.; Smith, G.H. Reprogramming cell fates in the mammary microenvironment. Cell Cycle 2009, 8, 1127–1132. [Google Scholar] [CrossRef]
- Boulanger, C.A.; Wagner, K.U.; Smith, G.H. Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to tgf-beta1 expression. Oncogene 2005, 24, 552–560. [Google Scholar] [CrossRef]
- Bruno, R.D.; Boulanger, C.A.; Smith, G.H. Notch-induced mammary tumorigenesis does not involve the lobule-limited epithelial progenitor. Oncogene 2012, 31, 60–67. [Google Scholar] [CrossRef]
- Booth, B.W.; Mack, D.L.; Androutsellis-Theotokis, A.; McKay, R.D.; Boulanger, C.A.; Smith, G.H. The mammary microenvironment alters the differentiation repertoire of neural stem cells. Proc. Natl. Acad. Sci. USA 2008, 105, 14891–14896. [Google Scholar]
- Boulanger, C.A.; Bruno, R.D.; Rosu-Myles, M.; Smith, G.H. The mouse mammary microenvironment redirects mesoderm-derived bone marrow cells to a mammary epithelial progenitor cell fate. Stem Cells Dev. 2012, 21, 948–954. [Google Scholar] [CrossRef]
- Boulanger, C.A.; Mack, D.L.; Booth, B.W.; Smith, G.H. Interaction with the mammary microenvironment redirects spermatogenic cell fate in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 3871–3876. [Google Scholar]
- Booth, B.W.; Boulanger, C.A.; Anderson, L.H.; Smith, G.H. The normal mammary microenvironment suppresses the tumorigenic phenotype of mouse mammary tumor virus-neu-transformed mammary tumor cells. Oncogene 2011, 30, 679–689. [Google Scholar]
- Bussard, K.M.; Smith, G.H. Human breast cancer cells are redirected to mammary epithelial cells upon interaction with the regenerating mammary gland microenvironment in vivo. PLoS One 2012, 7, e49221. [Google Scholar] [CrossRef]
- Booth, B.W.; Boulanger, C.A.; Smith, G.H. Stem cells and the mammary microenvironment. Breast Dis. 2008, 29, 57–67. [Google Scholar]
- Deome, K.B.; Faulkin, L.J., Jr.; Bern, H.A.; Blair, P.B. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female c3h mice. Cancer Res. 1959, 19, 515–520. [Google Scholar]
- Bruno, R.D.; Smith, G.H. Functional characterization of stem cell activity in the mouse mammary gland. Stem Cell. Rev. 2011, 7, 238–247. [Google Scholar] [CrossRef]
- Daniel, C.W.; Deome, K.B. Growth of mouse mammary glands in vivo after monolayer culture. Science 1965, 149, 634–636. [Google Scholar]
- Faulkin, L.J., Jr.; Deome, K.B. Regulation of growth and spacing of gland elements in the mammary fat pad of the c3h mouse. J. Natl Cancer Inst. 1960, 24, 953–969. [Google Scholar]
- Brisken, C.; O'Malley, B. Hormone action in the mammary gland. Cold Spring Harb. Perspect Biol. 2010, 2, a003178. [Google Scholar] [CrossRef]
- Smith, G.H. Experimental mammary epithelial morphogenesis in an in vivo model: Evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast Cancer Res. Treat. 1996, 39, 21–31. [Google Scholar] [CrossRef]
- Chen, C.C.; Stairs, D.B.; Boxer, R.B.; Belka, G.K.; Horseman, N.D.; Alvarez, J.V.; Chodosh, L.A. Autocrine prolactin induced by the pten-akt pathway is required for lactation initiation and provides a direct link between the akt and stat5 pathways. Genes Dev. 2012, 26, 2154–2168. [Google Scholar] [CrossRef]
- Chen, E.H.; Grote, E.; Mohler, W.; Vignery, A. Cell-cell fusion. FEBS Lett. 2007, 581, 2181–2193. [Google Scholar] [CrossRef]
- Vignery, A. Macrophage fusion: Are somatic and cancer cells possible partners? Med. Sci. (Paris) 2005, 21, 1070–1075. [Google Scholar] [CrossRef]
- Azuma, H.; Paulk, N.; Ranade, A.; Dorrell, C.; Al-Dhalimy, M.; Ellis, E.; Strom, S.; Kay, M.A.; Finegold, M.; Grompe, M. Robust expansion of human hepatocytes in fah-/-/rag2-/-/il2rg-/- mice. Nat. Biotechnol. 2007, 25, 903–910. [Google Scholar] [CrossRef]
- Okamura, K.; Asahina, K.; Fujimori, H.; Ozeki, R.; Shimizu-Saito, K.; Tanaka, Y.; Teramoto, K.; Arii, S.; Takase, K.; Kataoka, M.; et al. Generation of hybrid hepatocytes by cell fusion from monkey embryoid body cells in the injured mouse liver. Histochem. Cell. Biol 2006, 125, 247–257. [Google Scholar] [CrossRef]
- Pajcini, K.V.; Pomerantz, J.H.; Alkan, O.; Doyonnas, R.; Blau, H.M. Myoblasts and macrophages share molecular components that contribute to cell-cell fusion. J. Cell. Biol. 2008, 180, 1005–1019. [Google Scholar]
- Kordon, E.C.; Smith, G.H. An entire functional mammary gland may comprise the progeny from a single cell. Development 1998, 125, 1921–1930. [Google Scholar]
- Smith, G.H.; Gallahan, D.; Zwiebel, J.A.; Freeman, S.M.; Bassin, R.H.; Callahan, R. Long-term in vivo expression of genes introduced by retrovirus-mediated transfer into mammary epithelial cells. J. Virol. 1991, 65, 6365–6370. [Google Scholar]
- Dalerba, P.; Kalisky, T.; Sahoo, D.; Rajendran, P.S.; Rothenberg, M.E.; Leyrat, A.A.; Sim, S.; Okamoto, J.; Johnston, D.M.; Qian, D.; et al. Single-cell dissection of transcriptional heterogeneity in human colon tumors. Nat. Biotechnol. 2011, 29, 1120–1127. [Google Scholar]
- Fearon, E.R.; Hamilton, S.R.; Vogelstein, B. Clonal analysis of human colorectal tumors. Science 1987, 238, 193–197. [Google Scholar]
- Fialkow, P.J. Clonal origin of human tumors. Annu. Rev. Med. 1979, 30, 135–143. [Google Scholar] [CrossRef]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar]
- Marx, J. Cancer research. Mutant stem cells may seed cancer. Science 2003, 301, 1308–1310. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar]
- Louderbough, J.M.; Schroeder, J.A. Understanding the dual nature of cd44 in breast cancer progression. Mol. Cancer Res. 2011, 9, 1573–1586. [Google Scholar] [CrossRef]
- Smith, G.H. Experimental mammary epithelial morphogenesis in an in vivo model: Evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast Cancer Res. Treat. 1996, 39, 21–31. [Google Scholar] [CrossRef]
- Boulanger, C.A.; Wagner, K.U.; Smith, G.H. Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to tgf-beta1 expression. Oncogene 2005, 24, 552–560. [Google Scholar] [CrossRef]
- Wagner, K.U.; Boulanger, C.A.; Henry, M.D.; Sgagias, M.; Hennighausen, L.; Smith, G.H. An adjunct mammary epithelial cell population in parous females: Its role in functional adaptation and tissue renewal. Development 2002, 129, 1377–1386. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rosenfield, S.M.; Smith, G.H. Redirection of Human Cancer Cells upon the Interaction with the Regenerating Mouse Mammary Gland Microenvironment. Cells 2013, 2, 43-56. https://doi.org/10.3390/cells2010043
Rosenfield SM, Smith GH. Redirection of Human Cancer Cells upon the Interaction with the Regenerating Mouse Mammary Gland Microenvironment. Cells. 2013; 2(1):43-56. https://doi.org/10.3390/cells2010043
Chicago/Turabian StyleRosenfield, Sonia M., and Gilbert H. Smith. 2013. "Redirection of Human Cancer Cells upon the Interaction with the Regenerating Mouse Mammary Gland Microenvironment" Cells 2, no. 1: 43-56. https://doi.org/10.3390/cells2010043



