Vascular Mechanobiology: Towards Control of In Situ Regeneration
Abstract
:1. Introduction
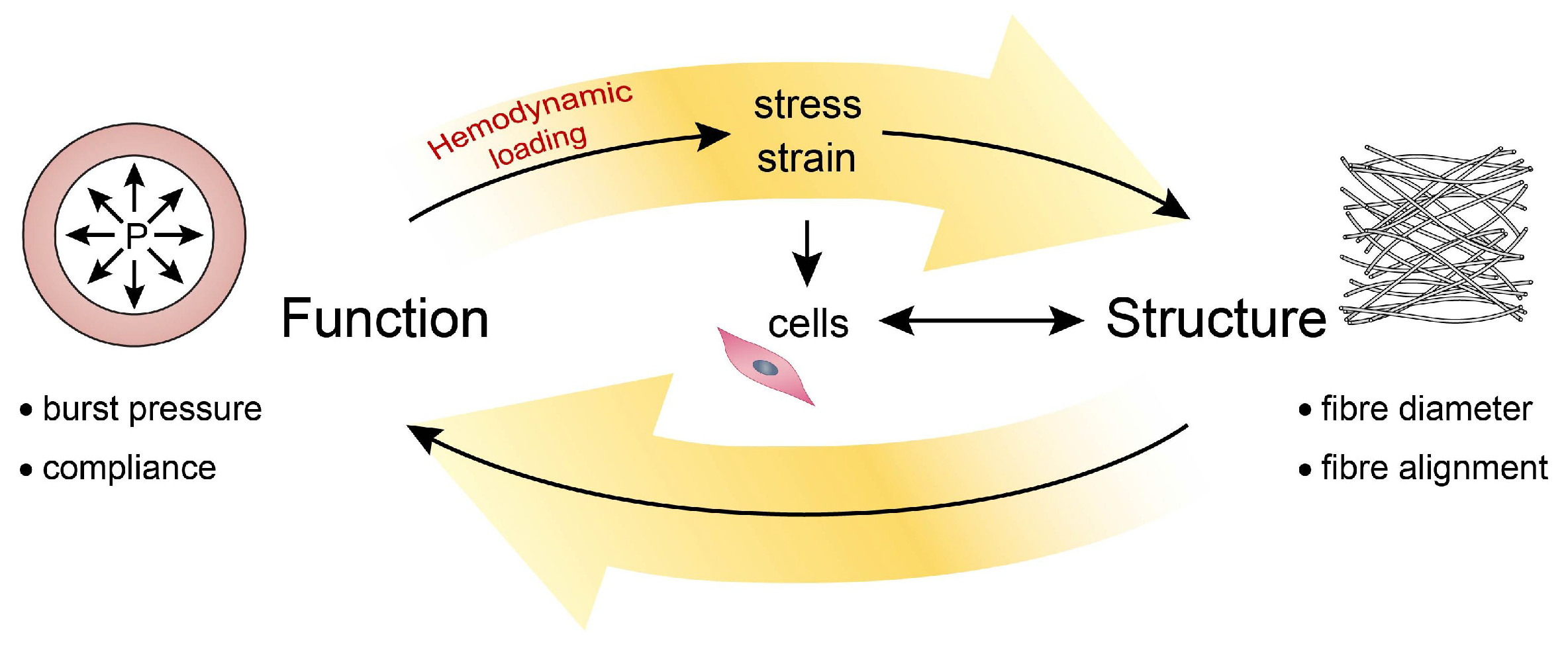
2. Mechanosensing to Mechanical Homeostasis
2.1. Mechanical Behavior of Blood Vessels
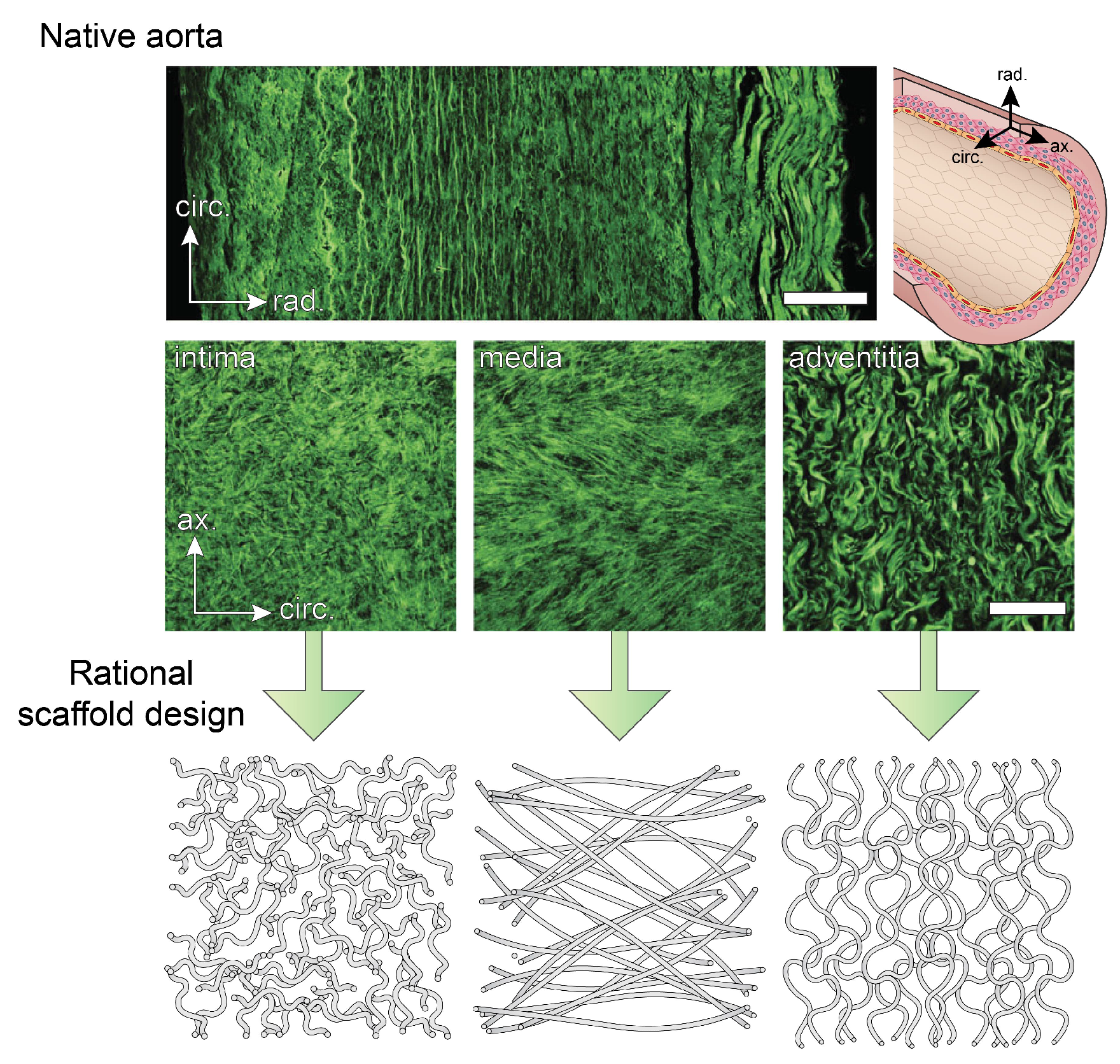
2.2. Microstructure of Blood Vessels
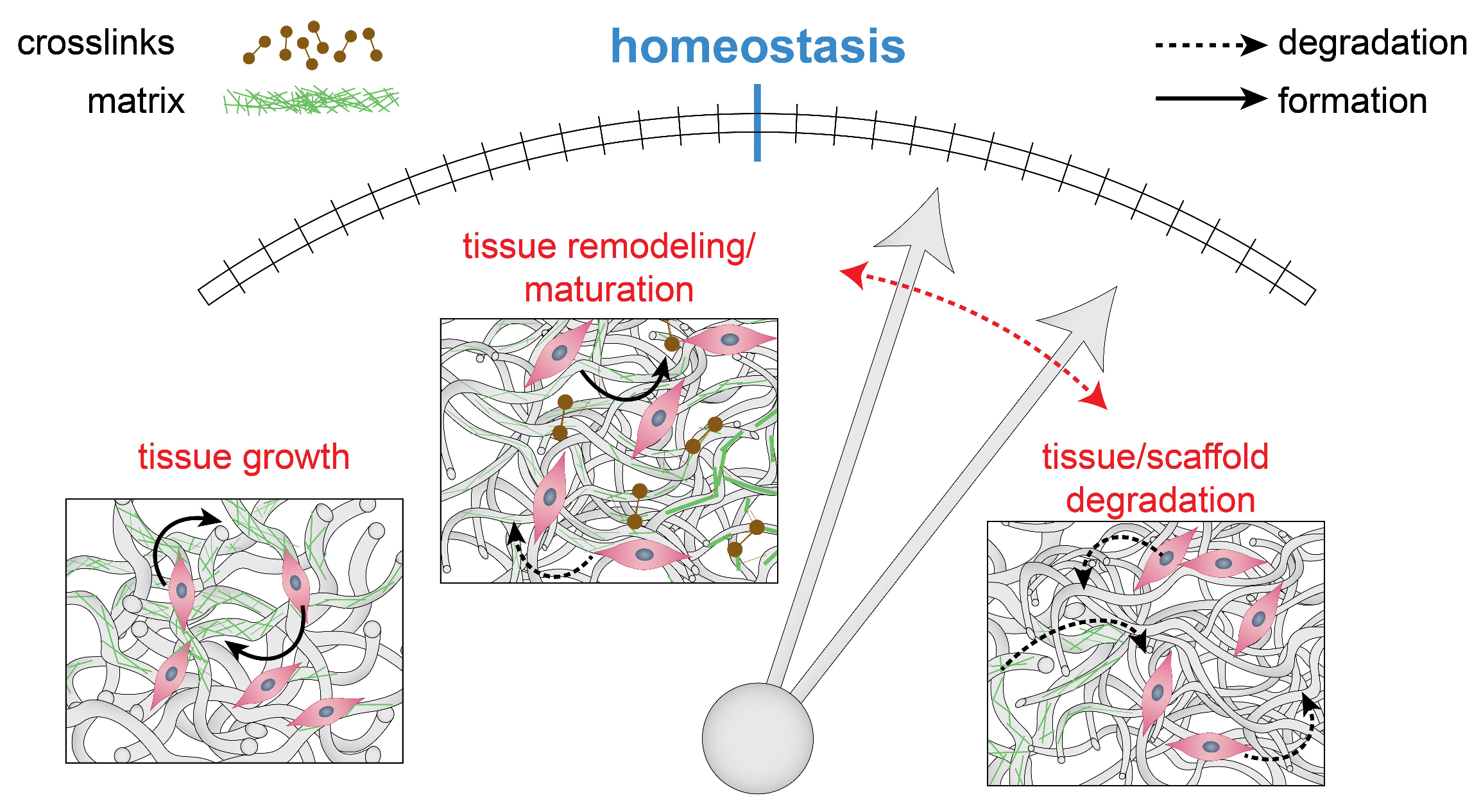
2.3. Maintaining Mechanics and Microstructure: Mechanical Homeostasis
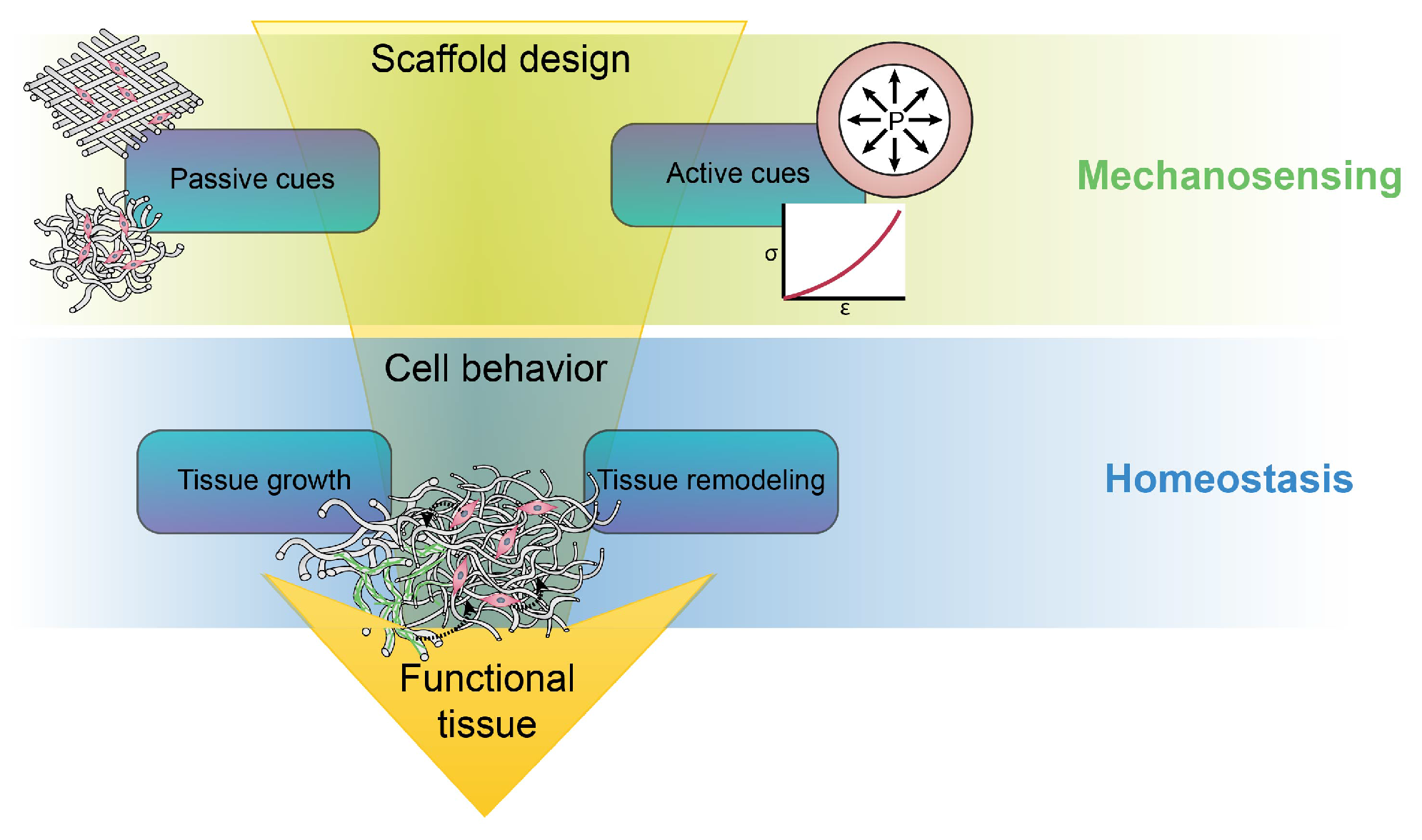
3. Passive and Active Cues in the Vessel
3.1. Passive Cues: The Cellular Micro-Environment
3.1.1. Dimension
3.1.2. Topography and Spatial Distribution of Substrate Ligands
3.1.3. Substrate Stiffness
3.2. Active Cues: Hemodynamic Loading
3.2.1. Shear Stress
3.2.2. Cyclic Stress (and Strain)
3.2.3. Residual Stress
4. Towards a Hypothesis-Driven Engineering Approach
4.1. Passive Mechanostimulation of Cells
4.2. Active Mechanostimulation of Cells
4.3. Combined Methods
5. Concluding Remarks and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- L’Heureux, N.; Dusserre, N.; Marini, A.; Garrido, S.; de la Fuente, L.; McAllister, T. Technology Insight: The evolution of tissue-engineered vascular grafts—From research to clinical practice. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.; Cleary, M.; Naito, Y.; Rocco, K.; Breuer, C. Development of tissue engineered vascular grafts and application of nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef] [PubMed]
- Putnam, A.J. Mechanobiological control of cell fate for applications in cardiovascular regenerative medicine. In Molecular and Cellular Mechanobiology; Chien, S., Engler, A.J., Wang, P.Y., Eds.; Springer: New York, NY, USA, 2016; pp. 219–253. [Google Scholar]
- Stegemann, J.P.; Kaszuba, S.N.; Rowe, S.L. Review: Advances in Vascular Tissue Engineering Using Protein-Based Biomaterials. Tissue Eng. 2007, 13, 2601–2613. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sengupta, D.; Chien, S. Vascular tissue engineering: From in vitro to in situ. Wiley Interdiscip. Rev. Syst. Biol. Med. 2014, 6, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Niklason, L.E. Vascular tissue engineering: Building perfusable vasculature for implantation. Curr. Opin. Chem. Eng. 2014, 3, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Dahl, S.L.M.; Kypson, A.P.; Lawson, J.H.; Blum, J.L.; Strader, J.T.; Li, Y.; Manson, R.J.; Tente, W.E.; DiBernardo, L.; Hensley, M.T.; et al. Readily Available Tissue-Engineered Vascular Grafts. Sci. Transl. Med. 2011, 3, 68ra9. [Google Scholar] [CrossRef] [PubMed]
- L’Heureux, N.; Dusserre, N.; Konig, G.; Victor, B.; Keire, P.; Wight, T.N.; Chronos, N.A.F.; Kyles, A.E.; Gregory, C.R.; Hoyt, G.; et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat. Med. 2006, 12, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Rothuizen, T.C.; Damanik, F.F.R.; Lavrijsen, T.; Visser, M.J.T.; Hamming, J.F.; Lalai, R.A.; Duijs, J.M.G.J.; Jan, A.; Zonneveld, V.; Hoefer, I.E.; et al. Development and evaluation of in vivo tissue engineered blood vessels in a porcine model. Biomaterials 2016, 75, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Hibino, N.; McGillicuddy, E.; Matsumura, G.; Ichihara, Y.; Naito, Y.; Breuer, C.; Shinoka, T. Late-term results of tissue-engineered vascular grafts in humans. J. Thorac. Cardiovasc. Surg. 2010, 139, 431–436.e2. [Google Scholar] [CrossRef] [PubMed]
- Cyron, C.J.; Humphrey, J.D. Vascular homeostasis and the concept of mechanobiological stability. Int. J. Eng. Sci. 2014, 85, 203–223. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Allen, R.A.; Wang, Y. Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat. Med. 2012, 18, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Dahl, S.L.M.; Rhim, C.; Song, Y.C.; Niklason, L.E. Mechanical properties and compositions of tissue engineered and native arteries. Ann. Biomed. Eng. 2007, 35, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Soletti, L.; Hong, Y.; Guan, J.; Stankus, J.J.; El-Kurdi, M.S.; Wagner, W.R.; Vorp, D.A. A bilayered elastomeric scaffold for tissue engineering of small diameter vascular grafts. Acta Biomater. 2010, 6, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Syedain, Z.H.; Meier, L.A.; Bjork, J.W.; Lee, A.; Tranquillo, R.T. Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials 2011, 32, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Suarez Bagnasco, D.; Montini Ballarin, F.; Cymberknop, L.J.; Balay, G.; Negreira, C.; Abraham, G.A.; Armentano, R.L. Elasticity assessment of electrospun nanofibrous vascular grafts: A comparison with femoral ovine arteries. Mater. Sci. Eng. C 2014, 45, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Fukunishi, T.; Best, C.A.; Sugiura, T.; Shoji, T.; Yi, T.; Udelsman, B.; Ohst, D.; Ong, C.S.; Zhang, H.; Shinoka, T.; et al. Tissue-engineered small diameter arterial vascular grafts from cell-free nanofiber PCL/chitosan scaffolds in a sheep model. PLoS ONE 2016, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tai, N.R.; Salacinski, H.J.; Edwards, A.; Hamilton, G.; Seifalian, A.M. Compliance properties of conduits used in vascular reconstruction. Br. J. Surg. 2000, 87, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Konig, G.; Mcallister, T.N.; Dusserre, N.; Garrido, S.A.; Iyican, C.; Marini, A.; Fiorillo, A.; Avila, H.; Wystrychowski, W.; Zagalski, K.; et al. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials 2009, 30, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.D.; Nelson, G.N.; Brennan, M.P.; Mirensky, T.L.; Yi, T.; Hazlett, T.F.; Tellides, G.; Sinusas, A.J.; Pober, J.S.; Saltzman, W.M.; et al. Small-diameter biodegradable scaffolds for functional vascular tissue engineering in the mouse model. Biomaterials 2008, 29, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- McClure, M.J.; Wolfe, P.S.; Simpson, D.G.; Sell, S.A.; Bowlin, G.L. The use of air-flow impedance to control fiber deposition patterns during electrospinning. Biomaterials 2012, 33, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Guan, Y.; Kim, S.H.; King, M.W. The mechanical performance of weft-knitted/electrospun bilayer small diameter vascular prostheses. J. Mech. Behav. Biomed. Mater. 2016, 61, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Montini-Ballarin, F.; Calvo, D.; Caracciolo, P.C.; Rojo, F.; Frontini, P.M.; Abraham, G.A.; Guinea, G.V. Mechanical behavior of bilayered small-diameter nanofibrous structures as biomimetic vascular grafts. J. Mech. Behav. Biomed. Mater. 2016, 60, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Motlagh, D.; Webb, A.R.; Ameer, G.A. Novel biphasic elastomeric scaffold for small-diameter blood vessel tissue engineering. Tissue Eng. 2005, 11, 1876–1886. [Google Scholar] [CrossRef] [PubMed]
- Quint, C.; Kondo, Y.; Manson, R.J.; Lawson, J.H.; Dardik, A.; Niklason, L.E. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc. Natl. Acad. Sci. USA 2011, 108, 9214–9219. [Google Scholar] [CrossRef] [PubMed]
- Stekelenburg, M.; Rutten, M.C.; Snoeckx, L.H.; Baaijens, F.P. Dynamic Straining Combined with Fibrin Gel Cell Seeding Improves Strength of Tissue-Engineered Small-Diameter Vascular Grafts. Tissue Eng. Part A 2009, 15, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- De Valence, S.; Tille, J.C.; Mugnai, D.; Mrowczynski, W.; Gurny, R.; Möller, M.; Walpoth, B.H. Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. Biomaterials 2012, 33, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Tara, S.; Kurobe, H.; Maxfield, M.W.; Rocco, K.A.; Yi, T.; Naito, Y.; Breuer, C.K.; Shinoka, T. Evaluation of remodeling process in small-diameter cell-free tissue-engineered arterial graft. J. Vasc. Surg. 2015, 62, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Salacinski, H.J.; Goldner, S.; Giudiceandrea, A.; Hamilton, G.; Seifalian, A.M.; Edwards, A.; Carson, R.J. The Mechanical Behavior of Vascular Grafts: A Review. J. Biomater. Appl. 2001, 15, 241–278. [Google Scholar] [CrossRef] [PubMed]
- Krahn, K.N.; Bouten, C.V.C.; Van Tuijl, S.; Van Zandvoort, M.A.M.J.; Merkx, M. Fluorescently labeled collagen binding proteins allow specific visualization of collagen in tissues and live cell culture. Anal. Biochem. 2006, 350, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.A.; Vesuna, S.; Dimitrievska, S.; Zhou, K.; Huang, A.; Zhao, L.; Niklason, L.E.; Levene, M.J. The use of optical clearing and multiphoton microscopy for investigation of three-dimensional tissue-engineered constructs. Tissue Eng. Part C 2014, 20, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Niestrawska, J.A.; Viertler, C.; Regitnig, P.; Cohnert, T.U.; Sommer, G.; Holzapfel, G.A. Microstructure and Mechanics of Healthy and Aneurysmatic Abdominal Aortas : Experimental Analysis and Modeling. J. R. Soc. Interface 2016, 13, 20160620. [Google Scholar] [CrossRef] [PubMed]
- Schriefl, A.J.; Zeindlinger, G.; Pierce, D.M.; Regitnig, P.; Holzapfel, G.A. Determination of the layer-specific distributed collagen fibre orientations in human thoracic and abdominal aortas and common iliac arteries. J. R. Soc. Interface 2012, 9, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Qi, N.; Gao, H.; Ogden, R.W.; Hill, N.A.; Holzapfel, G.A.; Han, H.C.; Luo, X. Investigation of the optimal collagen fibre orientation in human iliac arteries. J. Mech. Behav. Biomed. Mater. 2015, 52, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Dahl, S.L.M.; Vaughn, M.E.; Niklason, L.E. An ultrastructural analysis of collagen in tissue engineered arteries. Ann. Biomed. Eng. 2007, 35, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, Z.; Zhang, J.; Wang, L.; Yang, X.; Chen, J.; Fan, G.; Ji, S.; Xing, C.; Wang, K.; et al. Circumferentially aligned fibers guided functional neoartery regeneration in vivo. Biomaterials 2015, 61, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Zafar, F.; Hinton, R.B.; Moore, R.A.; Baker, R.S.; Bryant, R.; Narmoneva, D.A.; Taylor, M.D.; Morales, D.L. Physiological Growth, Remodeling Potential, and Preserved Function of a Novel Bioprosthetic Tricuspid Valve: Tubular Bioprosthesis Made of Small Intestinal Submucosa-Derived Extracellular Matrix. J. Am. Coll. Cariol. 2015, 66, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Menzel, A.; Kuhl, E. Frontiers in growth and remodeling. Mech. Res. Commun. 2012, 42, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, N.; Schwartz, M.A. Biomechanics of vascular mechanosensation and remodeling. Mol. Biol. Cell 2016, 27, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, D.; Ateshian, G.; Arruda, E.; Cowin, S.; Dumais, J.; Goriely, A.; Holzapfel, G.; Humphrey, J.; Kemkemer, R.; Kuhl, E.; et al. Perspectives on biological growth and remodeling. J. Mech. Phys. Solids 2011, 59, 863–883. [Google Scholar] [CrossRef] [PubMed]
- Cyron, C.J.; Wilson, J.S.; Humphrey, J.D. Mechanobiological stability: A new paradigm to understand the enlargement of aneurysms? J. R. Soc. Interface 2014, 11, 20140680. [Google Scholar] [CrossRef] [PubMed]
- Kassab, G.S.; Navia, J.A. Biomechanical Considerations in the Design of Graft: The Homeostasis Hypothesis. Annu. Rev. Biomed. Eng. 2006, 8, 499–535. [Google Scholar] [CrossRef] [PubMed]
- Rotmans, J.I.; Bezhaeva, T. The battlefield at arteriovenous crossroads: Invading arterial smooth muscle cells occupy the outflow tract of fistulas. Kidney Int. 2015, 88, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, A.B.; Sankaran, S.; Humphrey, J.D.; Marsden, A.L. Computational Simulation of the Adaptive Capacity of Vein Grafts in Response to Increased Pressure. J. Biomech. Eng. 2015, 137, 031009. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, R.; Miller, K.S.; Best, C.A.; Shih, Y.C.; Lee, Y.U.; Yi, T.; Shinoka, T.; Breuer, C.K.; Humphrey, J.D. Biomechanical diversity despite mechanobiological stability in tissue engineered vascular grafts two years post-implantation. Tissue Eng. Part A 2015, 21, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, R.; Best, C.A.; Allen, R.A.; Stowell, C.E.T.; Onwuka, E.; Zhuang, J.J.; Lee, Y.U.; Yi, T.; Bersi, M.R.; Shinoka, T.; et al. Long-Term Functional Efficacy of a Novel Electrospun Poly(Glycerol Sebacate)-Based Arterial Graft in Mice. Ann. Biomed. Eng. 2016, 44, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, G.; Isayama, N.; Matsuda, S.; Taki, K.; Sakamoto, Y.; Ikada, Y.; Yamazaki, K. Long-term results of cell-free biodegradable scaffolds for in situ tissue engineering of pulmonary artery in a canine model. Biomaterials 2013, 34, 6422–6428. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, J.D. Vascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levels. Cell Biochem. Biophys. 2008, 50, 53–78. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, J.D.; Schwartz, M.A.; Tellides, G.; Milewicz, D.M. Role of Mechanotransduction in Vascular Biology: Focus on Thoracic Aortic Aneurysms and Dissections. Circ. Res. 2015, 116, 1448–1461. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.A.; Prajapati, R.; McGrouther, D.A.; Yannas, I.V.; Eastwood, M. Tensional homeostasis in dermal fibroblasts: Mechanical responses to mechanical loading in three-dimensional substrates. J. Cell. Physiol. 1998, 175, 323–332. [Google Scholar] [CrossRef]
- Mizutani, T.; Haga, H.; Kawabata, K. Cellular stiffness response to external deformation: Tensional homeostasis in a single fibroblast. Cell. Motil. Cytoskelet. 2004, 59, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.A. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb. Perspect. Biol. 2010, 2, a005066. [Google Scholar] [CrossRef] [PubMed]
- DuFort, C.C.; Paszek, M.J.; Weaver, V. Balancing forces: Architectural control of mechanotransduction. Nat. Rev. Mol. Cell Biol. 2011, 12, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell. Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Trappmann, B.; Wang, W.Y.; Sakar, M.S.; Kim, I.L.; Shenoy, V.B.; Burdick, J.A.; Chen, C.S. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat. Mater. 2015, 14, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.F.; Haase, C.; Deguchi, S.; Kaunas, R. Cyclic stretch-induced stress fiber dynamics—Dependence on strain rate, Rho-kinase and MLCK. Biochem. Biophys. Res. Commun. 2010, 401, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Foolen, J.; Deshpande, V.S.; Kanters, F.M.; Baaijens, F.P. The influence of matrix integrity on stress-fiber remodeling in 3D. Biomaterials 2012, 33, 7508–7518. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; Te Lindert, M.; Krause, M.; Alexander, S.; Te Riet, J.; Willis, A.L.; Hoffman, R.M.; Figdor, C.G.; Weiss, S.J.; Friedl, P. Physical limits of cell migration: Control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol. 2013, 201, 1069–1084. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; Wu, Y.I.; Liu, Y.; Geiger, J.; Tam, E.; Overall, C.; Stack, M.S.; Friedl, P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat. Cell Biol. 2007, 9, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Lim, C.T.; Kurniawan, N.A. Mechanistic adaptability of cancer cells strongly affects anti-migratory drug efficacy. J. R. Soc. Interface 2014, 11, 20140638. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.H.; Trapani, L.M.; Sieminski, A.L.; Mackellar, D.; Gong, H.; Kamm, R.D.; Wells, A.; Lauffenburger, D.A.; Matsudaira, P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc. Natl. Acad. Sci. USA 2006, 103, 10889–10894. [Google Scholar] [CrossRef] [PubMed]
- Lamalice, L.; Le Boeuf, F.; Huot, J. Endothelial cell migration during angiogenesis. Circ. Res. 2007, 100, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Geiger, B.; Spatz, J.P.; Bershadsky, A.D. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell. Biol. 2009, 10, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Trichet, L.; Le Digabel, J.; Hawkins, R.J.; Vedula, S.R.; Gupta, M.; Ribrault, C.; Hersen, P.; Voituriez, R.; Ladoux, B. Evidence of a large-scale mechanosensing mechanism for cellular adaptation to substrate stiffness. Proc. Natl. Acad. Sci. USA 2012, 109, 6933–6938. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Sarangi, B.R.; Deschamps, J.; Nematbakhsh, Y.; Callan-Jones, A.; Margadant, F.; Mege, R.M.; Lim, C.T.; Voituriez, R.; Ladoux, B. Adaptive rheology and ordering of cell cytoskeleton govern matrix rigidity sensing. Nat. Commun. 2015, 6, 7525. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.; Bacakove, L.; Newman, C.; Hategan, A.; Griffin, M.; Discher, D. Substrate compliance versus ligand density in cell on gel responses. Biophys. J. 2004, 86, 617–628. [Google Scholar] [CrossRef]
- Yeung, T.; Georges, P.C.; Flanagan, L.A.; Marg, B.; Ortiz, M.; Funaki, M.; Zahir, N.; Ming, W.; Weaver, V.; Janmey, P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskeleton 2005, 60, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.M.; Wang, H.B.; Dembo, M.; Wang, Y.L. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000, 79, 144–152. [Google Scholar] [CrossRef]
- Hartman, C.D.; Isenberg, B.C.; Chua, S.G.; Wong, J.Y. Vascular smooth muscle cell durotaxis depends on extracellular matrix composition. Proc. Natl. Acad. Sci. USA 2016, 113, 11190–11195. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Abe, H.; Ohashi, T.; Kato, Y.; Sato, M. Local elastic modulus of atherosclerotic lesions of rabbit thoracic aortas measured by pipette aspiration method. Physiol. Meas. 2002, 23, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Buxboim, A.; Ivanovska, I.L.; Discher, D.E. Matrix elasticity, cytoskeletal forces and physics of the nucleus: How deeply do cells ’feel’ outside and in? J. Cell Sci. 2010, 123, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Trappmann, B.; Gautrot, J.E.; Connelly, J.T.; Strange, D.G.T.; Li, Y.; Oyen, M.L.; Cohen Stuart, M.A.; Boehm, H.; Li, B.; Vogel, V.; et al. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 2012, 11, 742. [Google Scholar] [CrossRef]
- Bruekers, S.M.C.; Jaspers, M.; Hendriks, J.M.A.; Kurniawan, N.A.; Koenderink, G.H.; Kouwer, P.H.J.; Rowan, A.E.; Huck, W.T.S. Fibrin-fiber architecture influences cell spreading and differentiation. Cell Adhes. Migr. 2016, 6918, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.B.; Gerecht, S. Three-dimensional culture of small-diameter vascular grafts. J. Mater. Chem. B 2016, 4, 3443–3453. [Google Scholar] [CrossRef]
- Greve, J.; Les, A. Allometric scaling of wall shear stress from mice to humans: Quantification using cine phase-contrast MRI and computational fluid dynamics. Am. J. Physiol. Heart Circ. Physiol. 2006, 5431, 1700–1708. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Helderman, F.; Tempel, D.; Segers, D.; Hierck, B.; Poelmann, R.; van Tol, A.; Duncker, D.J.; Robbers-Visser, D.; Ursem, N.T.C.; et al. Large variations in absolute wall shear stress levels within one species and between species. Atherosclerosis 2007, 195, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, N.; Nicoli, S.; Coon, B.; Ross, T.; van den Dries, K.; Han, J.; Lauridsen, H.; Mejean, C.; Eichmann, A.; Thomas, J.; et al. Vascular remodeling is governed by a VEGFR3-dependent fluid shear stress set point. eLife 2015, 4, e04645. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, J.D.; Eberth, J.F.; Dye, W.W.; Gleason, R.L. Fundamental role of axial stress in compensatory adaptations by arteries. J. Biomech. 2009, 42, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.R. Studies on the Growth of blood-vessels in the tail of the frog larva—By observation and experiment on the living animal. Am. J. Anat. 1918, 23, 37–88. [Google Scholar] [CrossRef]
- Levesque, M.J.; Nerem, R.M. The Elongation and Orientation of Cultured Endothelial Cells in Response to Shear Stress. J. Biomech. Eng. 1985, 107, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, N.; Ohashi, T.; Sato, M. Effect of shear stress on permeability of vascular endothelial monolayer cocultured with smooth muscle cells. JSME Int. J. C-Mech. Sy. 2004, 47, 992–999. [Google Scholar] [CrossRef]
- Nackman, G.B.; Fillinger, M.F.; Shafritz, R.; Wei, T.; Graham, A.M.; Freischlag, J.A.; Kent, K.C. Flow modulates endothelial regulation of smooth muscle cell proliferation: A new model. Surgery 1998, 124, 353–361. [Google Scholar] [CrossRef]
- Wang, C.; Baker, B.M.; Chen, C.S.; Schwartz, M.A. Endothelial cell sensing of flow direction. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2130–2136. [Google Scholar] [CrossRef] [PubMed]
- Tzima, E.; Irani-Tehrani, M.; Kiosses, W.B.; Dejana, E.; Schultz, D.A.; Engelhardt, B.; Cao, G.; DeLisser, H.; Schwartz, M.A. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 2005, 437, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.H.; Farhatnia, Y.; Godarzi, F.; Tan, A.; Rajadas, J.; Cousins, B.G.; Seifalian, A.M. In situ Endothelialization: Bioengineering Considerations to Translation. Small 2015, 11, 6248–6264. [Google Scholar] [CrossRef] [PubMed]
- Keun Kwon, I.; Kidoaki, S.; Matsuda, T. Electrospun nano-to microfiber fabrics made of biodegradable copolyesters: Structural characteristics, mechanical properties and cell adhesion potential. Biomaterials 2005, 26, 3929–3939. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Almen, G.C.V.; Talacua, H.; Ippel, B.D.; Mollet, B.B.; Ramaekers, M.; Simonet, M.; Smits, A.I.P.M.; Bouten, C.V.C.; Kluin, J.; Dankers, P.Y.W. Development of Non-Cell Adhesive Vascular Grafts Using Supramolecular Building Blocks. Macromol. Biosci. 2016, 16, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.A.; Graham, D.A.; Dela Cruz, S.; Ratcliffe, A.; Karlon, W.J. Fluid shear stress-induced alignment of cultured vascular smooth muscle cells. J. Biomech. Eng. 2002, 124, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.M.; Tarbell, J.M. Modeling interstitial flow in an artery wall allows estimation of wall shear stress on smooth muscle cells. J. Biomech. Eng. 1995, 117, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.D.; Tarbell, J.M. Fluid flow mechanotransduction in vascular smooth muscle cells and fibroblasts. Ann. Biomed. Eng. 2011, 39, 1608–1619. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, J.B.; Wang, G.R.; Gregersen, H.; Kassab, G.S. Remodeling of the zero-stress state of femoral arteries in response to flow overload. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1547–H1559. [Google Scholar] [PubMed]
- Tada, S.; Tarbell, J.M. Interstitial flow through the internal elastic lamina affects shear stress on arterial smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H1589–H1597. [Google Scholar] [PubMed]
- Stella, J.A.; Liao, J.; Hong, Y.; Merryman, W.D.; Wagner, W.R.; Sacks, M.S. Tissue-to-Cellular Deformation Coupling in Cell-Microintegrated Elastomeric Scaffolds. Biomaterials 2010, 29, 83228–83236. [Google Scholar]
- Argento, G.; Simonet, M.; Oomens, C.W.J.; Baaijens, F.P.T. Multi-scale mechanical characterization of scaffolds for heart valve tissue engineering. J. Biomech. 2012, 45, 2893–2898. [Google Scholar] [CrossRef] [PubMed]
- Chanet, S.; Martin, A.C. Mechanical force sensing in tissues. Prog. Mol. Biol. Transl. Sci. 2014, 126, 317–352. [Google Scholar] [PubMed]
- De Jonge, N.; Foolen, J.; Brugmans, M.C.; Söntjens, S.H.; Baaijens, F.P.; Bouten, C.V. Degree of Scaffold [-20]Degradation Influences Collagen (re)Orientation in Engineered Tissues. Tissue Eng. Part A 2013, 20, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, N.; Kanters, F.M.W.; Baaijens, F.P.T.; Bouten, C.V.C. Strain-induced collagen organization at the micro-level in fibrin-based engineered tissue constructs. Ann. Biomed. Eng. 2013, 41, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Grood, E.S. The strain magnitude and contact guidance determine orientation response of fibroblasts to cyclic substrate strains. Connect. Tissue Res. 2000, 41, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kaunas, R.; Nguyen, P.; Usami, S.; Chien, S. Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc. Natl. Acad. Sci. USA 2005, 102, 15895–15900. [Google Scholar] [CrossRef] [PubMed]
- Foolen, J.; Janssen-van den Broek, M.W.; Baaijens, F.P. Synergy between Rho signaling and matrix density in cyclic stretch-induced stress fiber organization. Acta Biomater. 2014, 10, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Owatverot, T.B.; Oswald, S.J.; Chen, Y.; Wille, J.J.; Yin, F.C.P. Effect of combined cyclic stretch and fluid shear stress on endothelial cell morphological responses. J. Biomech. Eng. 2005, 127, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Théry, M.; Racine, V.; Pépin, A.; Piel, M.; Chen, Y.; Sibarita, J.B.; Bornens, M. The extracellular matrix guides the orientation of the cell division axis. Nat. Cell Biol. 2005, 7, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Genet, M.; Rausch, M.; Lee, L.; Choy, S.; Zhao, X.; Kassab, G.; Kozerke, S.; Guccione, J.; Kuhl, E. Heterogeneous growth-induced prestrain in the heart. J. Biomech. 2015, 48, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Rausch, M.K.; Kuhl, E. On the effect of prestrain and residual stress in thin biological membranes. J. Mech. Phys. Solids 2013, 61, 1955–1969. [Google Scholar] [CrossRef] [PubMed]
- Rausch, M.K.; Kuhl, E. On the mechanics of growing thin biological membranes. J. Mech. Phys. Solids 2014, 63, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.H.; Lee, Y.U.; Calle, E.A.; Boyle, M.; Starcher, B.C.; Humphrey, J.D.; Niklason, L.E. Design and Use of a Novel Bioreactor for Regeneration of Biaxially Stretched Tissue-Engineered Vessels. Tissue Eng. C Methods 2015, 21, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Gadegaard, N.; Oreffo, R.O.C. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat. Mater. 2014, 13, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Sathe, S.R.; Yim, E.K.F. From nano to micro: Topographical scale and its impact on cell adhesion, morphology and contact guidance. J. Phys. Condens. Matter 2016, 28, 183001. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.M.; Jiang, Z.X.; Bastmeyer, M.; Lahann, J. Physical aspects of cell culture substrates: Topography, roughness, and elasticity. Small 2012, 8, 336–355. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Provenzano, P.P.; Smith, C.L.; Levchenko, A. Matrix nanotopography as a regulator of cell function. J. Cell. Biol. 2012, 197, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, C.L.; Ruch, D.S.; Little, D.; Guilak, F. Micro-scale and meso-scale architectural cues cooperate and compete to direct aligned tissue formation. Biomaterials 2014, 35, 10015–10024. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, H.; Wei, K.; Zhao, Y. Time-dependent combinatory effects of active mechanical loading and passive topographical cues on cell orientation. Biotechnol. Bioeng. 2016, 113, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Tamiello, C.; Buskermolen, A.B.C.; Baaijens, F.P.T.; Broers, J.L.V.; Bouten, C.V.C. Heading in the right direction: Understanding cellular orientation responses to complex biophysical environments. Cell. Mol. Bioeng. 2016, 9, 12–37. [Google Scholar] [CrossRef] [PubMed]
- Théry, M. Micropatterning as a tool to decipher cell morphogenesis and functions. J. Cell. Sci. 2010, 123, 4201–4213. [Google Scholar] [CrossRef] [PubMed]
- MacNearney, D.; Mak, B.; Ongo, G.; Kennedy, T.E.; Juncker, D. Nanocontact printing of proteins on physiologically soft substrates to study cell haptotaxis. Langmuir 2016, 32, 13525–13533. [Google Scholar] [CrossRef] [PubMed]
- Denisin, A.K.; Pruitt, B.L. Tuning the range of polyacrylamide gel stiffness for mechanobiology applications. ACS Appl. Mater. Interfaces 2016, 8, 21893–21902. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; DelRio, F.W.; Ma, H.; Killaars, A.R.; Basta, L.P.; Kyburz, K.A.; Anseth, K.S. Spatially patterned matrix elasticity directs stem cell fate. Proc. Natl. Acad. Sci. USA 2016, 113, E4439–E4445. [Google Scholar] [CrossRef] [PubMed]
- Norris, S.C.P.; Tseng, P.; Kasko, A.M. Direct gradient photolithography of photodegradable hydrogels with patterned stiffness control with submicrometer resolution. ACS Biomater. Sci. Eng. 2016, 2, 1309–1318. [Google Scholar] [CrossRef]
- Davis, C.A.; Zambrano, S.; Anumolu, P.; Allen, A.C.B.; Sonoqui, L.; Moreno, M.R. Device-Based in vitro Techniques for Mechanical Stimulation of Vascular Cells: A Review. J. Biomech. Eng. 2015, 137, 040801. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Samanipour, R.; Koo, K.I.; Kim, K. Organ-on-a-Chip Platforms for Drug Delivery and Cell Characterization: A Review. Sens. Mater. 2015, 27, 487–506. [Google Scholar]
- Huh, D.; Matthews, B.; Mammoto, A.; Montoya-Zavala, M.; Yuan Hsin, H.; Inber, D. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Smits, A.I.P.M.; Ballotta, V.; Driessen-Mol, A.; Bouten, C.V.C.; Baaijens, F.P.T. Shear flow affects selective monocyte recruitment into MCP-1-loaded scaffolds. J. Cell. Mol. Med. 2014, 18, 2176–2188. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Ferdous, Z. Design considerations and challenges for mechanical stretch bioreactors in tissue engineering. Biotechnol. Prog. 2016, 32, 543–553. [Google Scholar] [CrossRef] [PubMed]
- [-20]Bilodeau, K.; Mantovani, D. Bioreactors for tissue engineering: Focus on mechanical constraints. A comparative review. Tissue Eng. 2006, 12, 2367–2383. [Google Scholar]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Solon, J.; Levental, I.; Sengupta, K.; Georges, P.C.; Janmey, P.A. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys. J. 2007, 93, 4453–4461. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.R.; Fabry, B. Cell and tissue mechanics in cell migration. Exp. Cell Res. 2013, 319, 2418–2423. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, N.A.; Chaudhuri, P.K.; Lim, C.T. Mechanobiology of cell migration in the context of dynamic two-way cell-matrix interactions. J. Biomech. 2016, 49, 1355–1368. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.N.; Califano, J.P.; Reinhart-King, C.A. Matrix Stiffness: A Regulator of Cellular Behavior and Tissue Formation. In Engineering Biomaterials for Regenerative Medicine; Bhatia, S.K., Ed.; Springer: New York, NY, USA, 2012; pp. 19–37. [Google Scholar]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Grinnell, F. Fibroblast-collagen-matrix contraction: Growth-factor signalling and mechanical loading. Trends Cell Biol. 2000, 10, 362–365. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Claridge, M.W.; Bate, G.R.; Hoskins, P.R.; Adam, D.J.; Bradbury, A.W.; Wilmink, A.B. Measurement of arterial stiffness in subjects with vascular disease: Are vessel wall changes more sensitive than increase in intima-media thickness? Atherosclerosis 2009, 205, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Maekawa, K.; Emoto, M.; Okuno, S.; Yamakawa, T.; Ishimura, E.; Inaba, M.; Nishizawa, Y. Arterial stiffness predicts cardiovascular death independent of arterial thickness in a cohort of hemodialysis patients. Atherosclerosis 2010, 210, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Abhilash, A.S.; Baker, B.M.; Trappmann, B.; Chen, C.S.; Shenoy, V.B. Remodeling of fibrous extracellular matrices by contractile cells: Predictions from discrete fiber network simulations. Biophys. J. 2014, 107, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Abhilash, A.S.; Chen, C.S.; Wells, R.G.; Shenoy, V.B. Long-range force transmission in fibrous matrices enabled by tension-driven alignment of fibers. Biophys. J. 2014, 107, 2592–2603. [Google Scholar] [CrossRef] [PubMed]
- Ronceray, P.; Broedersz, C.P.; Lenz, M. Fiber networks amplify active stress. Proc. Natl. Acad. Sci. USA 2016, 113, 2827–2832. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.A.R.; Cibula, M.; Feng, J.C.; Krnacik, E.A.; McIntyre, D.H.; Levine, H.; Sun, B. Micromechanics of cellularized biopolymer networks. Proc. Natl. Acad. Sci. USA 2015, 112, E5117–E5122. [Google Scholar] [CrossRef] [PubMed]
- Münster, S.; Jawerth, L.M.; Leslie, B.A.; Weitz, J.I.; Fabry, B.; Weitz, D.A. Strain history dependence of the nonlinear stress response of fibrin and collagen networks. Proc. Natl. Acad. Sci. USA 2013, 110, 12197–12202. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, N.A.; Wong, L.H.; Rajagopalan, R. Early stiffening and softening of collagen: Interplay of deformation mechanisms in biopolymer networks. Biomacromolecules 2012, 13, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, N.A.; Vos, B.E.; Biebricher, A.; Wuite, G.J.L.; Peterman, E.J.G.; Koenderink, G.H. Fibrin networks support recurring mechanical loads by adapting their structure across multiple scales. Biophys. J. 2016, 111, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- [-15]Wong, L.H.; Kurniawan, N.A.; Too, H.P.; Rajagopalan, R. Spatially resolved microrheology of heterogeneous biopolymer hydrogels using covalently bound microspheres. Biomech. Model. Mechanobiol. 2014, 13, 839–849. [Google Scholar]
- Schultz, K.M.; Kyburz, K.A.; Anseth, K.S. Measuring dynamic cell-material interactions and remodeling during 3D human mesenchymal stem cell migration in hydrogels. Proc. Natl. Acad. Sci. USA 2015, 112, E3757–E3764. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Levengood, S.K.; Zhang, M. Anisotropic materials for skeletal-muscle-tissue Engineering. Adv. Mater. 2016, 28, 10588–10612. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Isolating Cells and Growing Them in Culture. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Zhao, J.; Griffin, M.; Cai, J.; Li, S.; Bulter, P.E.M.; Kalaskar, D.M. Bioreactors for tissue engineering: An update. Biochem. Eng. J. 2016, 109, 268–281. [Google Scholar] [CrossRef]
- Ryan, A.J.; Brougham, C.M.; Garciarena, C.D.; Kerrigan, S.W.; O’Brien, F.J. Towards 3D in vitro models for the study of cardiovascular tissues and disease. Drug Discov. Today 2016, 21, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Dahl, K.N.; Kalinowski, A.; Pekkan, K. Mechanobiology and the microcirculation: Cellular, nuclear and fluid mechanics. Microcirculation 2010, 17, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Swartz, M.A.; Lund, A.W. Lymphatic and interstitial flow in the tumour microenvironment: Linking mechanobiology with immunity. Nat. Rev. Cancer 2012, 12, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Sabine, A.; Syagili Demir, C.; Petrova, T.V. Endothelial cell responses to biomechanical forces in lymphatic vessels. Antioxid. Redox Signal. 2016, 25, 451–465. [Google Scholar] [CrossRef] [PubMed]

| Artery | Material | Design | Model | Compliance (%/100 mmHg) | Burst Pressure (mmHg) | Thickness (m) | Inner Diameter (mm) | T:R (-) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Native | AA | n.a. | rat | 6.7 | 3415 | 150 | 1 | 0.3 | [13] |
| CA | n.a. | porcine | 18.7 * | 3320 | 614 | n.d. | n.d. | [14] | |
| IMA | n.a. | porcine | 11.2 | 2100 | 231 | n.d. | n.d. | [15] | |
| FA | n.a. | sheep | 3.3 | 2297 | 770 | n.d. | n.d. | [16] | |
| FA | n.a. | sheep | 8.52 † | n.d. | n.d. | n.d. | n.d. | [17] | |
| CA | n.a. | sheep | 11.98 | 10,950 | 750 | 2.25 | 0.67 | [18] | |
| FA | n.a. | human | 2.6 | n.d. | n.d. | n.d. | n.d. | [19] | |
| IMA | n.a. | human | 4.5–6.2 | 2031–4225 | 350–710 | n.d. | n.d. | [9] | |
| IMA | n.a. | human | 11.5 | 3196 | 300–800 | 1.5–4.5 | 0.35–0.40 | [20] | |
| Synthetic | PGA or PLLA + PLCL | non-woven porous graft | n.a. | n.d. | 2710–2790 | 150–250 | 0.7–0.9 | 0.33–0.71 | [21] |
| PCL | e-spun microfibrous graft | n.a. | 0.58–0.92 | 850–1800 | 415 | 6 | 0.14 | [22] | |
| PLLA | e-spun microfibrous graft | n.a. | 0.93 | n.d. | 390 | 4.9 | 0.16 | [17] | |
| PLLA/PLCL | bi-layered graft with inner e-spun and outer weft-knitted layer | n.a. | 1.8 | 21,750 | 330 | 3.2 | 0.21 | [23] | |
| PLLA/PHD | bi-layered graft with different blend rations | n.a. | 1.12 | 1775 | 230 | 5 | 0.09 | [24] | |
| PEUU | bi-layered graft with large inner pores and dense outer pores | n.a. | 4.6 | 2300 | 743 | 4.7 | 0.32 | [15] | |
| poly(diol citrate) | non-woven porous graft | n.a. | 12.7 | 250 | 160 | 3.65 | 0.09 | [25] | |
| In vitro | PGA | non-woven porous graft | 10 weeks under pulsatile conditions | n.d. | 1300–1337 | 442 | 3 | 0.29 | [26] |
| PGA | non-woven porous graft | 1 week static, 4 weeks dynamic strain (1%) | n.d. | 906 | 1000 | 3 | 0.67 | [27] | |
| human fibroblast sheets | sheet-based | 8 weeks static, 10 weeks maturation | 1.5 | 3468 | 407 | 4.2 | 0.19 | [9] | |
| fibrin | fibroblast-seeded fibrin gel | 2 weeks static, 5–7 weeks dynamic strain (7%) | 2.4–4.4 | 1366–1542 | 280–430 | 2–4 | 0.22–0.28 | [16] | |
| PGA | non-woven porous graft | 7–10 weeks dynamic strain (2.5%) | 3.3 | 3337 | 1000 | 6 | 0.33 | [8] | |
| PGA | non-woven porous graft | 7–8 weeks dynamic strain (1.5%) | 3.5 * | 800 | 220 | 3 | 0.15 | [14] | |
| human fibroblast sheets | sheet-based | 6–8 weeks static, 12 weeks maturation | 3.54 | 3490 | 200–600 | 2.4–6.6 | 0.18 | [20] | |
| In situ | PCL/CS | e-spun nanofibrous graft | sheep (CA), 6 months | 6.58 | 10,275 | 1180 | 2.9 | 0.81 | [18] |
| PEOT/BPT/PCL | PEOT/BPT solid rod with external e-spun PCL sheet | porcine (SC), 4 weeks | 7.46 | 3947 | 700 | 2 | 0.70 | [10] | |
| PCL | e-spun nano/microfibrous graft | rat (AA), 1.5–18 months | 7.8 | 3280 | 650 | 2 | 0.65 | [28] | |
| PGS/PCL | porous PGS reinforced with PCL sheet | rat (AA), 3 months | 11 | 2360 | 290 | 0.72 | 0.81 | [13] |
| In-Vivo Model | Material | Design | Implantation Time | Main Outcome | Ref. |
|---|---|---|---|---|---|
| human | PLCL/PGA or PLLA | knitted PGA or PLLA fibers with PLCL sponge * | 4.3–7.3 years | no graft related deaths, TEVGs are technically feasible | [11] |
| mouse | PGS/PCL | microfibrous PGS core with PCL outer sheet | 12 months | perfect patency, progressive luminal enlargement due to PGS degradation | [47] |
| mouse | PGA/PLCL | non-woven porous graft with outer PLCL sheet | 24 months | biomechanical diversity among implanted vascular grafts due to variations in the ratio collagen type I/III | [46] |
| rat | PCL | e-spun nano/microfibrous graft | 18 months | perfect patency with excellent structural integrity, but calcifications appeared in the IH layers | [28] |
| mouse | PLCL/PLA | non-woven porous graft | 12 months | well-organized neotissue formation, but mos mice developed aneurysms | [29] |
| dog | PGA/PLCL/ P(GA-CL) | knitted PGA fibres with PLCL sponge and outer P(GA/CL) reinforcement | 12 months | no aneurysmal change or stenosis, but underdeveloped VSMCs | [48] |
| Key Scaffold Properties | Mechanostimulation | Technique to Study | Variables | Current Limitations |
|---|---|---|---|---|
| Passive | ||||
| fibre diameter, fibre topography | • microgrooves | • groove width (nm–m) • shape | groove-depth as confounding parameter | |
| • dimension/topography | ||||
| • micropatterning | • pattern size (m) • shape • protein gradients | range of pattern-size | ||
| fibre stiffness, macroscopic stiffness, scaffold density | • substrate stiffness | • polyacrylamide gels (2D) [119] | • 1 Pa–100 kPa | unable to capture fibrous 3D morphology |
| • hydrogels (3D) [120,121] | • <1 Pa–few kPa • stiffness gradients (2D) • non-linearity | low stiffness magnitude | ||
| Active | ||||
| anisotropy, geometry | • shear stress | • parallel plates [122] | • shear stress (<1 Pa–few Pa) | pressure as confounding parameter |
| • orbital shaker [122] | • shear stress (<1 Pa–few Pa) | temporal and spatial variations in shear stress | ||
| anisotropy, geometry, macroscopic stiffness | • strain | • motor/pressure driven distensible membrane [122] | • strain (1–20%) | spatial variations in strain |
| anisotropy, geometry, macroscopic stiffness | • shear stress & strain | • mock artery [122] | • shear stress (<1 Pa) • strain (1–10%) | no independent control of variables |
| • microfluidic device [123,124,125] | • shear stress (<1 Pa–few Pa) • strain (1–10%) | lack of 3D environment | ||
| Passive and active | ||||
| fibre diameter, anisotropy, pore size | • scaffold + shear stress | • parallel plates in mesofluidic device [126] | • shear stress (<1 Pa–few Pa) • scaffold properties | pressure as confounding parameter |
| anisotropy, pore size, connectivity, macroscopicstiffness, degradation rate | • scaffold + strain | • motor/pressure driven distensible membrane [99,127] | • strain (1–20%) • scaffold properties | spatial variations in strain |
| fibre diameter, anisotropy, pore size, connectivity, macroscopic stiffness, degradation rate | • scaffold + shear stress & strain | • perfusion bioreactor [128] | • shear stress (<1 Pa–few Pa) • strain (1–5%) • scaffold properties | no independent control of active variables |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Haaften, E.E.; Bouten, C.V.C.; Kurniawan, N.A. Vascular Mechanobiology: Towards Control of In Situ Regeneration. Cells 2017, 6, 19. https://doi.org/10.3390/cells6030019
Van Haaften EE, Bouten CVC, Kurniawan NA. Vascular Mechanobiology: Towards Control of In Situ Regeneration. Cells. 2017; 6(3):19. https://doi.org/10.3390/cells6030019
Chicago/Turabian StyleVan Haaften, Eline E., Carlijn V. C. Bouten, and Nicholas A. Kurniawan. 2017. "Vascular Mechanobiology: Towards Control of In Situ Regeneration" Cells 6, no. 3: 19. https://doi.org/10.3390/cells6030019




