Studying Autophagy in Zebrafish
Abstract
:1. Introduction
2. Zebrafish Autophagy Genes
3. Genome Editing Techniques
3.1. CRISPR/Cas9 Mutagenesis
3.2. TALENS and ZFNs
3.3. Transient Gene Knockdown by Morpholino Oligonucleotides
3.4. Mutations
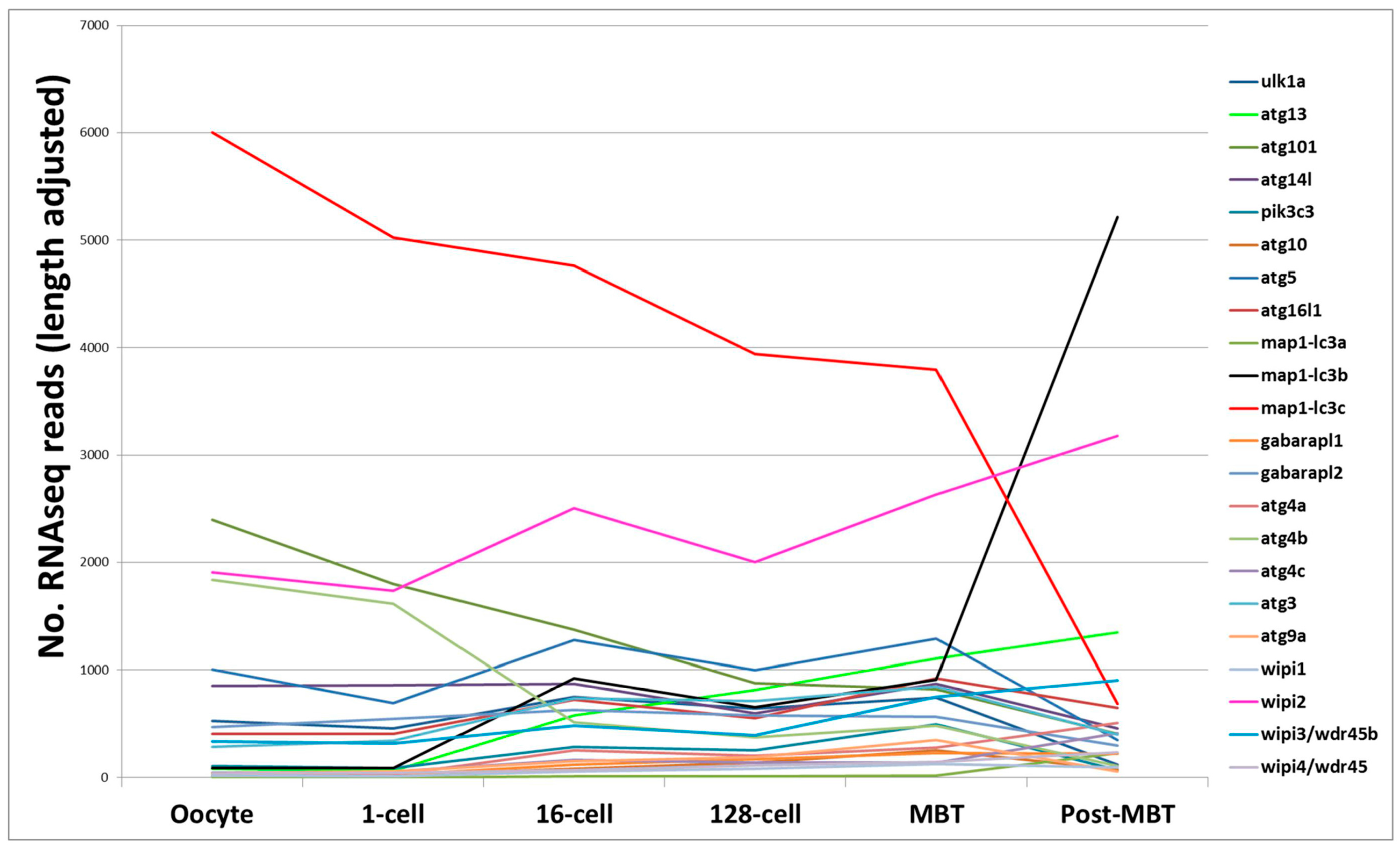
4. RNA-Based Analysis
5. Protein-based analysis
5.1. Fluorescence Microscopy
5.2. Western Blotting
5.3. Transmission Electron Microscopy (TEM)
6. Chemical/Pharmacological Modulations
7. Selective Autophagy
7.1. Mitophagy
7.2. Aggrephagy
7.3. Xenophagy
8. Future Perspective
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Boya, P.; Reggiori, F.; Codogno, P. Emerging regulation and functions of autophagy. Nat. Cell Biol. 2013, 15, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Takeshige, K.; Baba, M.; Tsuboi, S.; Noda, T.; Ohsumi, Y. Autophagy in Yeast Demonstrated with Proteinase-deficient Mutants and Conditions for its Induction. J. Cell Biol. 1992, 119, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, M.; Ohsumi, Y. Isofation and charactization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993, 333, 169–174. [Google Scholar] [CrossRef]
- Thumm, M.; Egner, R.; Koch, B.; Schlumpberger, M.; Straub, M.; Veenhuis, M.; Wolf, D.H. Isolation of autophagocytosis mutants of Saccharomyces cerevisiae. FEBS Lett. 1994, 349, 275–280. [Google Scholar] [CrossRef]
- Harding, T.M.; Morano, K.A.; Scott, S.V.; Klionsky, D.J. Isolation and characterization of yeast mutants in the cytoplasm to vacuole protein targeting pathway. J. Cell Biol. 1995, 131, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Klionsky, D.J. An Overview of the Molecular Mechanism. Curr. Top. Microbiol. Immunol. 2009, 3112–3123. [Google Scholar] [CrossRef]
- Xie, Z.; Klionsky, D.J. Autophagosome formation: core machinery and adaptations. Nat. Cell Biol. 2007, 9, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Klionsky, D.J. Regulation Mechanisms and Signalling Pathways of Autophagy. Annu. Rev. Genet. 2009, 43, 67. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Ohsumi, Y. The Role of Atg Proteins in Autophagosome Formation. Annu. Rev. Cell Dev. Biol. 2011, 27, 107–132. [Google Scholar] [CrossRef] [PubMed]
- Katheder, N.S.; Khezri, R.; O’Farrell, F.; Schultz, S.W.; Jain, A.; Rahman, M.M.; Schink, K.O.; Theodossiou, T.A.; Johansen, T.; Juhász, G.; et al. Microenvironmental autophagy promotes tumour growth. Nature 2017, 541, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Chen, N. Jayanta Debnath Autophagy and tumorigenesis. FEBS Lett. 2009, 32, 383–396. [Google Scholar] [CrossRef]
- Shibutani, S.T.; Saitoh, T.; Nowag, H.; Munz, C.; Yoshimori, T. Autophagy and autophagy-related proteins in the immune system. Nat Immunol 2015, 16, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Zabala, A.; Sierra-Torre, V.; Sierra, A. Autophagy and Microglia: Novel Partners in Neurodegeneration and Aging. Int. J. Mol. Sci. 2017, 18, 598. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V.; Saitoh, T.; Akira, S. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 2013, 13, 722–737. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth Control and Disease. Cell 2017, 149, 274–293. [Google Scholar] [CrossRef]
- Rearick Shoup, J. the Development Eyes of Pigment Type and Granules in the Eyes of Wild Type and Mutant Drosophila Melanogaster. J. Cell Biol. 1966, 29, 223–249. [Google Scholar] [CrossRef]
- Juhász, G.; Csikós, G.; Sinka, R.; Erdélyi, M.; Sass, M. The Drosophila homolog of Aut1 is essential for autophagy and development. FEBS Lett. 2003, 543, 154–158. [Google Scholar] [CrossRef]
- Meléndez, A.; Tallóczy, Z.; Seaman, M.; Eskelinen, E.-L.; Hall, D.H.; Levine, B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science 2003, 301, 1387–1391. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Kuma, A.; Kobayashi, Y.; Yamamoto, A.; Matsubae, M.; Takao, T.; Natsume, T.; Ohsumi, Y.; Yoshimori, T. Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the Apg12-Apg5 conjugate. J Cell Sci 2003, 116, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yamamoto, A.; Hatano, M.; Kobayashi, Y.; Kabey, Y.; Suzuki, K.; Tokuhis, T.; Ohsumi, Y.; Yoshimori, T. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J. Cell Biol. 2001, 152, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Moreau, K.; Segarra, A.; Tourbiez, D.; Travers, M.; Rubinsztein, D.C.; Renault, T. Autophagy plays an important role in protecting Pacific oysters from OsHV-1 and Vibrio aestuarianus infections. Autophagy 2015, 11, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Garrido, J.; Carilla-Latorre, S.; Kubohara, Y.; Santos-Rodrigo, N.; Mesquita, A.; Soldati, T.; Golstein, P.; Escalante, R. Autophagy in dictyostelium: Genes and pathways, cell death and infection. Autophagy 2010, 6, 686–701. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Sachidanandan, C. Zebrafish: A multifaceted tool for chemical biologists. Chem. Rev. 2013, 113, 7952–7980. [Google Scholar] [CrossRef] [PubMed]
- Driever, W.; Solnica-Krezel, L.; Schier, A.F.; Neuhauss, S.C.; Malicki, J.; Stemple, D.L.; Stainier, D.Y.; Zwartkruis, F.; Abdelilah, S.; Rangini, Z.; et al. A genetic screen for mutations affecting embryogenesis in zebrafish. Development 1996, 123, 37–46. [Google Scholar] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Kettleborough, R.N.; Busch-Nentwich, E.M.; Harvey, S.A.; Dooley, C.M.; de Bruijn, E.; van Eeden, F.; Sealy, I.; White, R.J.; Herd, C.; Nijman, I.J.; et al. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature 2013, 496, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Schier, A.F. Zebrafish earns its stripes. Nature 2013, 496, 443–444. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA – Guided. Science 2012, 337, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Garneau, J.E.; Dupuis, M.E.; Villion, M.; Romero, D.A.; Barrangou, R.; Boyaval, P.; Fremaux, C.; Horvath, P.; Magadan, A.H.; Moineau, S. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 2010, 468, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Gasiunas, G.; Barrangou, R.; Horvath, P.; Siksnys, V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc. Natl. Acad. Sci. USA 2012, 109, E2579–E2586. [Google Scholar] [CrossRef] [PubMed]
- Komor, A.C.; Badran, A.H.; Liu, D.R. CRISPR-Based Technologies for the Manipulation of Eukaryotic Genomes. Cell 2016, 168, 1–17. [Google Scholar] [CrossRef]
- Li, M.; Zhao, L.; Page-McCaw, P.S.; Chen, W. Zebrafish Genome Engineering Using the CRISPR-Cas9 System. Trends Genet. 2016, 32, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Varshney, G.K.; Pei, W.; LaFave, M.C.; Idol, J.; Xu, L.; Gallardo, V.; Carrington, B.; Bishop, K.; Jones, M.; Li, M.; et al. High-throughput gene targeting and phenotyping in zebrafish using CRISPR/Cas9. Genome Res. 2015, 25, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Mateos, M.A.; Vejnar, C.E.; Beaudoin, J.; Fernandez, J.P.; Mis, E.K.; Khokha, M.K.; Giraldez, A.J. CRISPRscan: Designing highly efficient sgRNAs for CRISPR-Cas9 targeting in vivo. Nat. Methods 2015, 12, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.; Kim, J.; Kim, H.; Lee, J.; Jeon, J. Highly efficient gene knockout in mice and zebrafish with RNA-guided endonucleases. Genome Res. 2014, 24, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Xiao, A.; Zhou, M.; Zhu, Z.; Lin, S.; Zhang, B. Heritable gene targeting in zebrafish using customized TALENs. Nat. Biotechnol. 2011, 29, 699–700. [Google Scholar] [CrossRef] [PubMed]
- Doyon, Y.; McCammon, J.M.; Miller, J.C.; Faraji, F.; Ngo, C.; Katibah, G.E.; Amora, R.; Hocking, T.D.; Zhang, L.; Rebar, E.J.; et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat. Biotechnol. 2008, 26, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.Y.; Fu, Y.; Reyon, D.; Maeder, M.L.; Tsai, S.Q.; Sander, J.D.; Peterson, R.T.; Yeh, J.R.; Joung, J.K. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol 2013, 31, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Hoshijima, K.; Jurynec, M.J.; Grunwald, D.J. Precise Editing of the Zebrafish Genome Made Simple and Efficient. Dev. Cell 2016, 36, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Jao, L.-E.; Wente, S.R.; Chen, W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc. Natl. Acad. Sci. USA 2013, 110, 13904–13909. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Maddison, L.A.; Li, M.; Kara, N.; Lafave, M.C.; Varshney, G.K.; Burgess, S.M.; Patton, J.G.; Chen, W. Multiplex conditional mutagenesis using transgenic expression of Cas9 and sgRNAs. Genetics 2015, 200, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Auer, T.O.; Duroure, K.; De Cian, A.; Concordet, J.; Del Bene, F. Highly efficient CRISPR / Cas9-mediated knock-in in zebrafish by homology-independent DNA repair. Genome Res. 2014, 24, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Jao, L.-E.; Chen, W. Generation of Targeted Mutations in Zebrafi sh Using the CRISPR/Cas System. Methods Mol. Biol. 2015, 1332, 7–8. [Google Scholar] [CrossRef]
- Gonzales, A.P. W.; Joanna Yeh, J.R. Cas9-based genome editing in Zebrafish. Methods Enzymol. 2014, 546, 377–413. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Lian, S.; Khan, A.; Llop, J.R.; Samuelson, A.V.; Chen, W.; Klionsky, D.J.; Kishi, S. Autolysosome biogenesis and developmental senescence are regulated by both Spns1 and v-ATPase. Autophagy 2016, 13, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Lian, S.; Qi, J.; Bayliss, P.E.; Carr, C.E.; Johnson, J.L.; Guha, S.; Kobler, P.; Catz, S.D.; Gill, M.; et al. Aberrant Autolysosomal Regulation Is Linked to The Induction of Embryonic Senescence: Differential Roles of Beclin 1 and p53 in Vertebrate Spns1 Deficiency. PLoS Genet. 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; McPhee, C.K.; Deng, S.; Huang, L.; Chen, L.; Liu, M.; Tracy, K.; Baehrecke, E.H.; Yu, L.; Lenardo, M.J. Spinster is required for autophagic lysosome reformation and mTOR reactivation following starvation. Proc. Natl. Acad. Sci. 2011, 108, 7826–7831. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhang, F.; Ye, Q.; Wang, H. The circadian clock regulates autophagy directly through the nuclear hormone receptor Nr1d1/Rev-erbα and indirectly via Cebpb/(C/ebpβ) in zebrafish. Autophagy 2016, 12, 1292–1309. [Google Scholar] [CrossRef] [PubMed]
- Corey, D.R.; Abrams, J.M. Morpholino antisense oligonucleotides: tools for investigating vertebrate development. Genome Biol. 2001, 2, 1015.1–1015.3. [Google Scholar] [CrossRef]
- Bill, B.R.; Petzold, A.M.; Clark, K.J.; Schimmenti, L.A.; Ekker, S.C. A primer for morpholino use in zebrafish. Zebrafish 2009, 6, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Khokha, M.K.; Yeh, J.; Grammer, T.C.; Harland, R.M. Depletion of three BMP antagonists from Spemann’s organizer leads to a catastrophic loss of dorsal structures. Dev. Cell 2005, 8, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Mir, A.; Heasman, J. How The Mother Can Help: Studying Maternal Wnt Signaling by Anti-sense-mediated Depletion of Maternal mRNAs and the Host Transfer Technique. Methods Mol. Biol. 2008, 469, 21–32. [Google Scholar] [CrossRef]
- Robu, M.E.; Larson, J.D.; Nasevicius, A.; Beiraghi, S.; Brenner, C.; Farber, S.A.; Ekker, S.C. P53 Activation By Knockdown Technologies. PLoS Genet. 2007, 3, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Kok, F.O.; Shin, M.; Ni, C.W.; Gupta, A.; Grosse, A.S.; van Impel, A.; Kirchmaier, B.C.; Peterson-Maduro, J.; Kourkoulis, G.; Male, I.; et al. Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev. Cell 2015, 32, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Kontarakis, Z.; Gerri, C.; Nolte, H.; Hölper, S.; Krüger, M.; Stainier, D.Y.R. Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 2015, 13, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, K.A.; Wu, W.-H.; Colgan, D.F.; Tsang, S.H.; Bassuk, A.G.; Mahajan, V.B. Unexpected mutations after CRISPR–Cas9 editing in vivo. Nat. Publ. Gr. 2017, 14, 547–548. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; De Robertis, E.M.; Wallingford, J.B.; Niehrs, C. Morpholinos: Antisense and Sensibility. Dev. Cell 2015, 35, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Koo, Y.; Ng, A.; Wei, Y.; Luby-Phelps, K.; Juraszek, A.; Xavier, R.J.; Cleaver, O.; Levine, B.; Amatruda, J.F. Autophagy is essential for cardiac morphogenesis during vertebrate development. Autophagy 2014, 10, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, J.; Zhang, Q. Expression pattern and functions of autophagy-related gene atg5 in zebrafish organogenesis. Autophagy 2011, 7, 1514–1527. [Google Scholar] [CrossRef] [PubMed]
- Kyöstilä, K.; Syrjä, P.; Jagannathan, V.; Chandrasekar, G.; Jokinen, T.S.; Seppälä, E.H.; Becker, D.; Drögemüller, M.; Dietschi, E.; Drögemüller, C.; et al. A Missense Change in the ATG4D Gene Links Aberrant Autophagy to a Neurodegenerative Vacuolar Storage Disease. PLoS Genet. 2015, 11, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Skobo, T.; Benato, F.; Grumati, P.; Meneghetti, G.; Cianfanelli, V.; Castagnaro, S.; Chrisam, M.; Di Bartolomeo, S.; Bonaldo, P.; Cecconi, F.; et al. Zebrafish ambra1a and ambra1b knockdown impairs skeletal muscle development. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Benato, F.; Skobo, T.; Gioacchini, G.; Moro, I.; Ciccosanti, F.; Piacentini, M.; Fimia, G.M.; Carnevali, O.; Valle, L.D. Ambra1 knockdown in zebrafish leads to incomplete development due to severe defects in organogenesis. Autophagy 2013, 9, 476–495. [Google Scholar] [CrossRef] [PubMed]
- Nakai, A.; Yamaguchi, O.; Takeda, T.; Higuchi, Y.; Hikoso, S.; Taniike, M.; Omiya, S.; Mizote, I.; Matsumura, Y.; Asahi, M.; et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat. Med. 2007, 13, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Korac, J.; Schaeffer, V.; Kovacevic, I.; Clement, A.M.; Jungblut, B.; Behl, C.; Terzic, J.; Dikic, I. Ubiquitin-independent function of optineurin in autophagic clearance of protein aggregates. J. Cell Sci. 2013, 126, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Paulus, J.D.; Link, B.A. Loss of Optineurin in vivo results in elevated cell death and alters axonal trafficking dynamics. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Chew, T.S.; O’Shea, N.R.; Sewell, G.W.; Oehlers, S.H.; Mulvey, C.M.; Crosier, P.S.; Godovac-Zimmermann, J.; Bloom, S.L.; Smith, A.M.; Segal, A.W. Optineurin deficiency contributes to impaired cytokine secretion and neutrophil recruitment in bacteria driven colitis. Dis. Model. Mech. 2015, 6, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Mostowy, S.; Boucontet, L.; Mazon Moya, M.J.; Sirianni, A.; Boudinot, P.; Hollinshead, M.; Cossart, P.; Herbomel, P.; Levraud, J.P.; Colucci-Guyon, E. The Zebrafish as a New Model for the In Vivo Study of Shigella flexneri Interaction with Phagocytes and Bacterial Autophagy. PLoS Pathog. 2013, 9, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Van Der Vaart, M.; Korbee, C.J.; Lamers, G.E. M.; Tengeler, A.C.; Hosseini, R.; Haks, M.C.; Ottenhoff, T.H. M.; Spaink, H.P.; Meijer, A.H. The DNA damage-regulated autophagy modulator DRAM1 links mycobacterial recognition via TLP-MYD88 to authophagic defense. Cell Host Microbe 2014, 15, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Lattante, S.; De Calbiac, H.; Le Ber, I.; Brice, A.; Ciura, S.; Kabashi, E. Sqstm1 knock-down causes a locomotor phenotype ameliorated by rapamycin in a zebrafish model of ALS/FTLD. Hum. Mol. Genet. 2015, 24, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Akizu, N.; Cantagrel, V.; Zaki, M.S.; Al-Gazali, L.; Wang, X.; Rosti, R.O.; Dikoglu, E.; Gelot, A.B.; Rosti, B.; Vaux, K.K.; et al. Biallelic mutations in SNX14 cause a syndromic form of cerebellar atrophy and lysosome-autophagosome dysfunction. Nat. Genet. 2015, 47, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Dowling, J.J.; Low, S.E.; Busta, A.S.; Feldman, E.L. Zebrafish MTMR14 is required for excitation-contraction coupling, developmental motor function and the regulation of autophagy. Hum. Mol. Genet. 2010, 19, 2668–2681. [Google Scholar] [CrossRef] [PubMed]
- Vergne, I.; Roberts, E.; Elmaoued, R.A.; Tosch, V.; Delgado, M.A.; Proikas-Cezanne, T.; Laporte, J.; Deretic, V. Control of autophagy initiation by phosphoinositide 3-phosphatase Jumpy. EMBO J. 2009, 28, 2244–2258. [Google Scholar] [CrossRef] [PubMed]
- Holland, P.; Knævelsrud, H.; Søreng, K.; Mathai, B.J.; Lystad, A.H.; Pankiv, S.; Bjørndal, G.T.; Schultz, S.W.; Lobert, V.H.; Chan, R.B.; et al. HS1BP3 negatively regulates autophagy by modulation of phosphatidic acid levels. Nat. Commun. 2016, 7, 13889. [Google Scholar] [CrossRef] [PubMed]
- Ramanoudjame, L.; Rocancourt, C.; Laine, J.; Klein, A.; Joassard, L.; Gartioux, C.; Fleury, M.; Lyphout, L.; Kabashi, E.; Ciura, S.; et al. Two novel COLVI long chains in zebrafish that are essential for muscle development. Hum. Mol. Genet. 2015, 24, 6624–6639. [Google Scholar] [CrossRef] [PubMed]
- Payne, E.M.; Virgilio, M.; Narla, A.; Sun, H.; Levine, M.; Paw, B.H.; Berliner, N.; Look, A.T.; Ebert, B.L.; Khanna-Gupta, A. L-leucine improves the anemia and developmental defects associated with Diamond-Blackfan anemia and del(5q)MDSby activating themTORpathway. Blood 2012, 120, 2214–2224. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, H.F.; van Wijk, R.; Pereboom, T.C.; Goos, Y.J.; Seinen, C.W.; van Oirschot, B.A.; van Dooren, R.; Gastou, M.; Giles, R.H.; van Solinge, W.; et al. Ribosomal Protein Mutations Induce Autophagy through S6 Kinase Inhibition of the Insulin Pathway. PLoS Genet. 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Fodor, E.; Sigmond, T.; Ari, E.; Lengyel, K.; Takács-Vellai, K.; Varga, M.; Vellai, T. Methods to study autophagy in zebrafish. Methods Enzym. 2016, 587, 467–496. [Google Scholar] [CrossRef]
- Nüsslein-Volhard, C. The zebrafish issue of Development. Development 2012, 139, 4099–4103. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, G.K.; Schubiger, G. Temporal regulation in the early embryo: Is MBT too good to be true? Trends Genet. 1992, 8, 124–127. [Google Scholar] [CrossRef]
- Korzh, V. Before maternal-zygotic transition ... There was morphogenetic function of nuclei. Zebrafish 2009, 6, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Aanes, H.; Winata, C.L.; Lin, C.H.; Chen, J.P.; Srinivasan, K.G.; Lee, S.G.P.; Lim, A.Y.M.; Hajan, H.S.; Collas, P.; Bourque, G.; et al. Zebrafish mRNA sequencing deciphers novelties in transcriptome dynamics during maternal to zygotic transition. Genome Res. 2011, 21, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Benard, E.L.; Rougeot, J.; Racz, P.I.; Spaink, H.P.; Meijer, A.H. Transcriptomic Approaches in the Zebrafish Model for Tuberculosis—Insights Into Host- and Pathogen-Specific Determinants of the Innate Immune Response; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 95, ISBN 9780128048009. [Google Scholar]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. Erratum: LC3, a mammalian homolog of yeast Apg8p, is localized in autophagosome membranes after processing (EMBO Journal (2000) 19 (5720-5728)). EMBO J. 2003, 22, 4577. [Google Scholar] [CrossRef]
- He, C.; Bartholomew, C.R.; Zhou, W.; Klionsky, D.J. Assaying autophagic activity in transgenic GFP-Lc3 and GFP-Gabarap zebrafish embryos. Autophagy 2009, 5, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Antinucci, P.; Hindges, R. OPEN A crystal -clear zebrafish for in vivo imaging. Nat. Publ. Gr. 2016, 1–10. [Google Scholar] [CrossRef]
- Kuma, A.; Matsui, M.; Mizushima, N. Caution in the Interpretation of LC3 Localization. Autophagy 2007, 34, 323–328. [Google Scholar] [CrossRef]
- Tanida, I.; Yamaji, T.; Ueno, T.; Ishiura, S.; Kominami, E.; Hanada, K. Consideration about negative controls for LC3 and expression vectors for four colored fluorescent protein-LC3 negative controls. Autophagy 2008, 4, 131–134. [Google Scholar] [CrossRef] [PubMed]
- George, A.A.; Hayden, S.; Holzhausen, L.C.; Ma, E.Y.; Suzuki, S.C.; Brockerhoff, S.E. Synaptojanin 1 is required for endolysosomal trafficking of synaptic proteins in cone photoreceptor inner segments. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Varga, M.; Sass, M.; Papp, D.; Takács-Vellai, K.; Kobolak, J.; Dinnyés, A.; Klionsky, D.J.; Vellai, T. Autophagy is required for zebrafish caudal fin regeneration. Cell Death Differ. 2014, 21, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, R.; Lamers, G.E.M.; Hodzic, Z.; Meijer, A.H.; Schaaf, M.J.M.; Spaink, H.P. Correlative light and electron microscopy imaging of autophagy in a zebrafish infection model. Autophagy 2014, 10, 1844–1857. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Wei, Y.; Zou, Z.; Tucker, K.; Rakheja, D.; Levine, B.; Amatruda, J.F. Genetic inhibition of autophagy promotes p53 loss-ofheterozygosity and tumorigenesis. Oncotarget 2016, 7, 67919–67933. [Google Scholar] [CrossRef] [PubMed]
- Saera-Vila, A.; Kish, P.E.; Louie, K.W.; Grzegorski, S.J.; Klionsky, D.J.; Kahana, A. Autophagy regulates cytoplasmic remodeling during cell reprogramming in a zebrafish model of muscle regeneration. Autophagy 2016, 12, 1864–1875. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Sim, T.H.-F.; Gong, Z.; Shen, H.-M. Generation of transgenic zebrafish with liver-specific expression of EGFP-Lc3: a new in vivo model for investigation of liver autophagy. Biochem. Biophys. Res. Commun. 2012, 422, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Noda, T.; Yoshimori, T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 2007, 3, 452–460. [Google Scholar] [CrossRef] [PubMed]
- George, A.A.; Hayden, S.; Stanton, G.R.; Brockerhoff, S.E. Arf6 and the 5’phosphatase of synaptojanin 1 regulate autophagy in cone photoreceptors. BioEssays 2016, 38, S119–S135. [Google Scholar] [CrossRef] [PubMed]
- Panic, B.; Whyte, J.R.; Munro, S.; Strochlic, T.I.; Tong, A.H.; Boone, C.; Burd, C.G.; Shemorry, A.; Varshavsky, A.; Rape, M.; et al. mTORC1 Senses Lysosomal Amino Acids. Science 2011, 678–683. [Google Scholar] [CrossRef]
- Juhász, G. Interpretation of bafilomycin, pH neutralizing or protease inhibitor treatments in autophagic flux experiments: Novel considerations. Autophagy 2012, 8, 1875–1876. [Google Scholar] [CrossRef] [PubMed]
- Florey, O.; Gammoh, N.; Kim, S.E.; Jiang, X.; Overholtzer, M. V-ATPase and osmotic imbalances activate endolysosomal LC3 lipidation. Autophagy 2015, 11, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Kaizuka, T.; Morishita, H.; Hama, Y.; Tsukamoto, S.; Matsui, T.; Toyota, Y.; Kodama, A.; Ishihara, T.; Mizushima, T.; Mizushima, N. An Autophagic Flux Probe that Releases an Internal Control. Mol. Cell 2016, 64, 835–849. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, A.; Tooze, S.A. Coordination of membrane events during autophagy by multiple class III PI3-kinase complexes. J. Cell Biol. 2009, 186, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Yabu, T.; Imamura, S.; Mizusawa, N.; Touhata, K.; Yamashita, M. Induction of autophagy by amino acid starvation in fish cells. Mar. Biotechnol. 2012, 14, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.-E.; Ma, K.; Xu, T.; Gao, L.; Wu, S.; Fu, C.; Zhang, W.; Wang, Z.; Liu, K.; Dong, M.; et al. Mutation of kri1l causes definitive hematopoiesis failure via PERK-dependent excessive autophagy induction. Cell Res. 2015, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Schiebler, M.; Brown, K.; Hegyi, K.; Newton, S.M.; Renna, M.; Hepburn, L.; Klapholz, C.; Coulter, S.; Obregón-Henao, A.; Henao Tamayo, M.; et al. Functional drug screening reveals anticonvulsants as enhancers of mTOR-independent autophagic killing of Mycobacterium tuberculosis through inositol depletion. EMBO Mol. Med. 2015, 7, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Boglev, Y.; Badrock, A.P.; Trotter, A.J.; Du, Q.; Richardson, E.J.; Parslow, A.C.; Markmiller, S.J.; Hall, N.E.; De Jong-Curtain, T.A.; Ng, A.Y.; et al. Autophagy induction is a Tor- and Tp53-independent cell survival response in a zebrafish model of disrupted ribosome biogenesis. PLoS Genet. 2013, 9, e1003279. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in mammalian autophagy research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Lee, S.E.; Wojta, K.; Ramos, E.M.; Klein, E.; Chen, J.; Boxer, A.L.; Gorno-Tempini, M.L.; Geschwind, D.H.; Schlotawa, L.; et al. A152T tau allele causes neurodegeneration that can be ameliorated in a zebrafish model by autophagy induction. Cereb. Cortex 2017, 1128–1146. [Google Scholar] [CrossRef] [PubMed]
- Espín-Palazón, R.; Martínez-López, A.; Roca, F.J.; López-Muñoz, A.; Tyrkalska, S.D.; Candel, S.; García-Moreno, D.; Falco, A.; Meseguer, J.; Estepa, A.; et al. TNFα Impairs Rhabdoviral Clearance by Inhibiting the Host Autophagic Antiviral Response. PLoS Pathog. 2016, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Link, V.; Shevchenko, A.; Heisenberg, C.-P. Proteomics of early zebrafish embryos. BMC Dev. Biol. 2006, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nguyen, D.T.; Olzomer, E.M.; Poon, G.P.; Cole, N.J.; Puvanendran, A.; Phillips, B.R.; Hesselson, D. Rescue of Pink1 Deficiency by Stress-Dependent Activation of Autophagy. Cell Chem. Biol. 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Sieben, C.J.; Xu, X.; Harris, P.C.; Lin, X. Autophagy activators suppress cystogenesis in an autosomal dominant polycystic kidney disease model. Hum. Mol. Genet. 2016, 26, ddw376. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, A.A.; Oorschot, V.; Ramm, G.; Bryson-Richardson, R.J. FLNC myofibrillar myopathy results from impaired autophagy and protein insufficiency. Hum. Mol. Genet. 2016, 25, 2131–2142. [Google Scholar] [CrossRef] [PubMed]
- Moreau, K.; Fleming, A.; Imarisio, S.; Lopez Ramirez, A.; Mercer, J.L.; Jimenez-Sanchez, M.; Bento, C.F.; Puri, C.; Zavodszky, E.; Siddiqi, F.; et al. PICALM modulates autophagy activity and tau accumulation. Nat. Commun. 2014, 5, 4998. [Google Scholar] [CrossRef] [PubMed]
- Catalina-Rodriguez, O.; Kolukula, V.K.; Tomita, Y.; Preet, A.; Palmieri, F.; Wellstein, A.; Byers, S.; Giaccia, A.J.; Glasgow, E.; Albanese, C.; et al. The mitochondrial citrate transporter, CIC, is essential for mitochondrial homeostasis. Oncotarget 2012, 3, 1220–1235. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Sun, X.; Huang, W.; Hoage, T.; Redfield, M.; Kushwaha, S.; Sivasubbu, S.; Lin, X.; Ekker, S.; Xu, X. Haploinsufficiency of target of rapamycin attenuates cardiomyopathies in adult zebrafish. Circ. Res. 2011, 109, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Bühler, A.; Kustermann, M.; Bummer, T.; Rottbauer, W.; Sandri, M.; Just, S. Atrogin-1 deficiency leads to myopathy and heart failure in zebrafish. Int. J. Mol. Sci. 2016, 17, 187. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Wang, Q.; Wang, S.; Wu, J.; Gao, Q.; Liu, W. Thiopeptide Antibiotics Exhibit a Dual Mode of Action against Intracellular Pathogens by Affecting Both Host and Microbe. Chem. Biol. 2015, 22, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, L.; Li, J.; Xu, G.; He, M.; Li, Y.; Huang, R. Salmonella spv locus suppresses host innate immune responses to bacterial infection. Fish Shellfish Immunol. 2016, 58, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Wang, T.; Gao, S.; Xu, G.M.; Niu, H.; Huang, R.; Wu, S.Y. Salmonella plasmid virulence gene spvB enhances bacterial virulence by inhibiting autophagy in a zebrafish infection model. Fish Shellfish Immunol. 2016, 49, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Slade, L.; Cowie, A.; Martynuik, C.J.; Kienesberger, P.C.; Pulinilkunnil, T. Dieldrin augments mTOR signaling and inhibits lysosomal acidification in the adult zebrafish heart (Danio rerio). J. Pharmacol. Exp. Ther. 2017. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, E.L.; Reggiori, F.; Baba, M.; Kovacs, A.L.; Seglen, P.O. Seeing is believing: The impact of electron microscopy on autophagy research. Autophagy 2011, 7, 935–956. [Google Scholar] [CrossRef] [PubMed]
- Lucocq, J.M.; Hacker, C. Cutting a fine figure: On the use of thin sections in electron microscopy to quantify autophagy. Autophagy 2013, 9, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Varga, M.; Fodor, E.; Vellai, T. Autophagy in zebrafish. Methods 2015, 75, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Papp, D.; Kovács, T.; Billes, V.; Varga, M.; Tarnóci, A.; Hackler, L., Jr.; Puskás, L.G.; Liliom, H.; Tárnok, K.; Schlett, K.; et al. AUTEN-67, an autophagy-enhancing drug candidate with potent antiaging and neuroprotective effects. Autophagy 2016, 12, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.L. Cellular differentiation in the kidneys of newborn mice studies with the electron microscope. J. Biophys. Biochem. Cytol. 1957, 3, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Novikoff, A.B. The proximal tubule cell in experimental hydronephrosis. J. Biophys. Biochem. Cytol. 1959, 6, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Rodriguez-Enriquez, S.; Lemasters, J.J. Selective degradation of mitochondria by mitophagy. Arch. Biochem. Biophys. 2007, 462, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Kiššová, I.; Deffieu, M.; Manon, S.; Camougrand, N. Uth1p is involved in the autophagic degradation of mitochondria. J. Biol. Chem. 2004, 279, 39068–39074. [Google Scholar] [CrossRef] [PubMed]
- Tal, R.; Winter, G.; Ecker, N.; Klionsky, D.J.; Abeliovich, H. Aup1p, a yeast mitochondrial protein phosphatase homolog, is required for efficient stationary phase mitophagy and cell survival. J. Biol. Chem. 2007, 282, 5617–5624. [Google Scholar] [CrossRef] [PubMed]
- Matic, I.; Strobbe, D.; Di Guglielmo, F.; Campanella, M. Molecular Biology Digest of Cell Mitophagy, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 332, ISBN 1937-6448. [Google Scholar]
- Mortensen, M.; Ferguson, D.J.P.; Edelmann, M.; Kessler, B.; Morten, K.J.; Komatsu, M.; Simon, A.K. Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc. Natl. Acad. Sci. 2010, 107, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Randall, M.S.; Loyd, M.R.; Dorsey, F.C.; Kundu, M.; Cleveland, J.L.; Ney, P.A. Mitochondrial clearance is regulated by Atg7-dependent and -independent mechanisms during reticulocyte maturation. Blood 2009, 114, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Narendra, D.P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 2011, 12, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010, 8. [Google Scholar] [CrossRef] [PubMed]
- Vives-Bauza, C.; Zhou, C.; Huang, Y.; Cui, M.; De Vries, R.L. A.; Kim, J.; May, J.; Tocilescu, M.A.; Liu, W.; Ko, H.S.; et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc. Natl. Acad. Sci. 2010, 107, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, N.; Sato, S.; Shiba, K.; Okatsu, K.; Saisho, K.; Gautier, C.A.; Sou, Y.S.; Saiki, S.; Kawajiri, S.; Sato, F.; et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J. Cell Biol. 2010, 189, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.X.; Ni, H.M.; Li, M.; Liao, Y.; Chen, X.; Stolz, D.B.; Dorn, G.W.; Yin, X.M. Nix is critical to two distinct phases of mitophagy, reactive oxygen species-mediated autophagy induction and Parkin-ubiquitin-p62-mediated mitochondrial priming. J. Biol. Chem. 2010, 285, 27879–27890. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H. Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol. 2008, 7, 97–109. [Google Scholar] [CrossRef]
- Anichtchik, O.; Diekmann, H.; Fleming, A.; Roach, A.; Goldsmith, P.; Rubinsztein, D.C. Loss of PINK1 Function Affects Development and Results in Neurodegeneration in Zebrafish. J. Neurosci. 2008, 28, 8199–8207. [Google Scholar] [CrossRef] [PubMed]
- Flinn, L.; Mortiboys, H.; Volkmann, K.; Kster, R.W.; Ingham, P.W.; Bandmann, O. Complex i deficiency and dopaminergic neuronal cell loss in parkin-deficient zebrafish (Danio rerio). Brain 2009, 132, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Flinn, L.J.; Keatinge, M.; Bretaud, S.; Mortiboys, H.; Matsui, H.; De Felice, E.; Woodroof, H.I.; Brown, L.; McTighe, A.; Soellner, R.; et al. TigarB causes mitochondrial dysfunction and neuronal loss in PINK1 deficiency. Ann. Neurol. 2013, 74, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Wager, K.; Russell, C. Mitophagy and neurodegeneration: The zebrafish model system. Autophagy 2013, 9, 1693–1709. [Google Scholar] [CrossRef] [PubMed]
- Plucińska, G.B. In Vivo Imaging of the Mitochondrial Life-Cycle in Zebrafish. PhD Thesis, University Library of the Technical University of Munich, Munich, Germany, 2014. [Google Scholar]
- Hyttinen, J.M.T.; Amadio, M.; Viiri, J.; Pascale, A.; Salminen, A.; Kaarniranta, K. Clearance of misfolded and aggregated proteins by aggrephagy and implications for aggregation diseases. Ageing Res. Rev. 2014, 18, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Dikic, I. Proteasomal and Autophagic Degradation Systems. Annu. Rev. Biochem. 2017, 86, 193–224. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Sarkar, S.; Cuddon, P.; Ttofi, E.K.; Siddiqi, F.H.; Jahreiss, L.; Fleming, A.; Pask, D.; Kane, C.J. O.; Floto, R.A.; et al. UKPMC Funders Group Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nat. Chem. Biol. 2009, 4, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Underwood, B.R.; Imarisio, S.; Fleming, A.; Rose, C.; Krishna, G.; Heard, P.; Quick, M.; Korolchuk, V.I.; Renna, M.; Sarkar, S.; et al. Antioxidants can inhibit basal autophagy and enhance neurodegeneration in models of polyglutamine disease. Hum. Mol. Genet. 2010, 19, 3413–3429. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.E.; Sahlender, D.A.; Graham, S.C.; Höning, S.; Robinson, M.S.; Peden, A.A.; Owen, D.J. The molecular basis for the endocytosis of small R-SNAREs by the clathrin adaptor CALM. Cell 2011, 147, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
- Arndt, V.; Dick, N.; Tawo, R.; Dreiseidler, M.; Wenzel, D.; Hesse, M.; Fürst, D.O.; Saftig, P.; Saint, R.; Fleischmann, B.K.; et al. Chaperone-Assisted Selective Autophagy Is Essential for Muscle Maintenance. Curr. Biol. 2010, 20, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, I. Autophagy Defends Cells Against Invading Group A Streptococcus. Science 2004, 306, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.G.; Master, S.S.; Singh, S.B.; Taylor, G.A.; Colombo, M.I.; Deretic, V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 2004, 119, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Brumell, J.H. Bacteria–autophagy interplay: a battle for survival. Nat. Rev. Microbiol. 2014, 12, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Henault, J.; Kolbeck, R.; Sanjuan, M.A. Noncanonical autophagy: One small step for LC3, one giant leap for immunity. Curr. Opin. Immunol. 2014, 26, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Kohler, L.J.; Roy, C.R. Autophagic targeting and avoidance in intracellular bacterial infections. Curr. Opin. Microbiol. 2017, 35, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Sprenkeler, E.G.G.; Gresnigt, M.S.; van de Veerdonk, F.L. LC3-associated phagocytosis: a crucial mechanism for antifungal host defence against Aspergillus fumigatus. Cell. Microbiol. 2016, 18, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Jagannathan, L.; Ganguli, G.; Padhi, A.; Roy, D.; Alaridah, N.; Saha, P.; Nongthomba, U.; Godaly, G.; Gopal, R.K.; et al. A mycobacterial phosphoribosyltransferase promotes bacillary survival by inhibiting oxidative stress and autophagy pathways in macrophages and zebrafish. J. Biol. Chem. 2015, 290, 13321–13343. [Google Scholar] [CrossRef] [PubMed]
- Mazon Moya, M.J.; Colucci-Guyon, E.; Mostowy, S. Use of Shigella flexneri to study autophagy-cytoskeleton interactions. J. Vis. Exp. 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Torraca, V.; Mostowy, S. Septins and Bacterial Infection. Front. Cell Dev. Biol. 2016, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Meijer, A.H.; Van Der Vaart, M. DRAM1 promotes the targeting of mycobacteria to selective autophagy. Autophagy 2014, 10, 2389–2391. [Google Scholar] [CrossRef] [PubMed]
- Crighton, D.; Wilkinson, S.; O’Prey, J.; Syed, N.; Smith, P.; Harrison, P.R.; Gasco, M.; Garrone, O.; Crook, T.; Ryan, K.M. DRAM, a p53-Induced Modulator of Autophagy, Is Critical for Apoptosis. Cell 2006, 126, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Lee, H.M.; Park, K.S.; Shin, D.M.; Kim, T.S.; Kim, Y.S.; Suh, H.W.; Kim, S.Y.; Kim, I.S.; Kim, J.M.; et al. MIR144* inhibits antimicrobial responses against Mycobacterium tuberculosis in human monocytes and macrophages by targeting the autophagy protein DRAM2. Autophagy 2017, 13, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Masud, S.; Torraca, V.; Meijer, A.H. Modeling Infectious Diseases in the Context of a Developing Immune System. Curr. Top. Dev. Biol. 2016, 124, 277–329. [Google Scholar] [CrossRef] [PubMed]




| Core Autophagic Process | Mammalian Protein | Zebrafish Orthologue | Refseq Id of Zebrafish DNA/Protein | Ensemble Id of Zebrafish DNA/Protein | Amino Acid Identity | Role in Autophagy | Mutant Allele Availability at the Sanger ZMP | |
|---|---|---|---|---|---|---|---|---|
| Nucleation step | ULK1 complex | ULK1 | ulk1a | NM_001130631, NP_001124103.1 | ENSDART00000090534.4 | 50% | Phosphorylated by mTORC1 (negative) and AMPK (positive). Induces autophagy by phosphorylation of ATG13 | Ulk1a—Yes |
| ulk1b | XM_005161121.3, XP_005161178.1 | ENSDART00000112407.3 | No | |||||
| ULK2 | ulk2 | XM_002664615.4, XP_002664661.3 | ENSDART00000153726 | 74% | No | |||
| ATG13/KIAA0652 | atg13 | NM_200433, NP_956727 | ENSDART00000052324.5 | 71% | Member of the ULK1 complex, phosphorylated by mTORC1 and ULK1 | No | ||
| Fip200/RB1CC1 | rb1cc1 | XM_009302198.2, XP_009300473.1 | ENSDART00000113014.3 | 59% | Scaffold for ULK1/2 and ATG13 | Yes | ||
| ATG101 | atg101 | NM_001037239, NP_001032316 | ENSDART00000063544.6 | 87% | Interacts with ATG31 | No | ||
| Class III PI3-kinase complex (PIK3C3) | ATG14L | atg14L/kiaa0831 | NM_001024812, NP_001019983 | ENSDART00000018683.10 | 67% | Autophagy-specific subunit of PIK3C3 complex I. ER binding motif | Yes | |
| PtdIns3K/VPS34 | pik3c3 | NM_001328533, NP_001315462 | ENSDART00000101265.4 | 87% | Catalytic subunit. Phosphorylates phosphatidylinositol to generated PI3-phosphate | No | ||
| Beclin1 | beclin1 | NM_200872, NP_957166 | ENSDART00000115237.3 | 79% | Subunit of PIK3C3. Regulatory function through binding to Bcl-2 | Yes | ||
| p150 | pik3r4 | XM_005158299.3, XP_001922676.1 | ENSDART00000085228.5 | 82% | Adaptor protein for VPS34 | No | ||
| Atg12 conjugation system | ATG12 | atg12 | NM_001246200, NP_001233129 | ENSDART00000101304.4 | 71% | Ubiquitin like, conjugates to ATG5 | Yes | |
| ATG7 | atg7 | XM_017358254.1, XP_017213743.1 | ENSDART00000162152 | 77% | E1-like enzyme | Yes | ||
| ATG10 | atg10 | NM_001037124, NP_001032201.1 | ENSDART00000160159.1 | 50% | E2-like enzyme | No | ||
| ATG5 | atg5/apg5L | NM_205618, NP_991181 | ENSDART00000029727.6 | 81% | Conjugated by ATG12 | Yes | ||
| ATG16L1 | atg16L1 | NM_001017854, NP_001017854 | ENSDART00000161937.1 | 69% | Interacts with ATG5 to form the ATG12-5-16L1 complex, an E3 like ligase for Atg8 conjugation | No | ||
| Atg8 conjugation system | MAP1-Lc3A | map1-lc3a | NM_214739, NP_999904 | ENSDART00000042322.3 | 96% | Ubiquitin like, conjugates to PE | No | |
| MAP1-Lc3B | map1-lc3b | NM_199604, NP_955898 | ENSDART00000163508.1 | 93% | ||||
| MAP1-Lc3C | map1-lc3c | NM_200298, NP_956592 | ENSDART00000161846.2 | 72% | ||||
| GABARAP | gabarapa | NM_001013260, NP_001013278 | ENSDART00000051547.3 | 98% | Ubiquitin like, conjugates to PE | No | ||
| GABARAPL1 | gabarapl1 | NM_001002707, NP_001002707 | ENSDART00000060037.3 | 59% | ||||
| GABARAPL2 | gabarapl2 | NM_205723, NP_991286 | ENSDART00000039485.6 | 97% | ||||
| ATG4A | atg4a | NM_001024434, NP_001019605 | ENSDART00000026666.10 | 70% | Atg8 C-terminal hydrolase, deconjugating enzyme | Yes | ||
| ATG4B | atg4b | NM_001089352, NP_001082821 | ENSDART00000121558.3 | 73% | No | |||
| ATG4C | atg4c | NM_001002103, NP_001002103 | ENSDART00000051779.3 | 59% | Yes | |||
| ATG4D | atg4da | XM_009294436.2, XP_009292711.1 | ENSDART00000152289.2 | 50% | No | |||
| atg4db | ENSDART00000172196 | 50% | No | |||||
| ATG3 | atg3 | NM_200022, NP_956316 | ENSDART00000041304.7 | 82% | E2-like enzyme | No | ||
| Other core Atg proteins during autophagosome formation | ATG2A | atg2a | XM_009307758.2, XP_009306033.1 | ENSDART00000172444.1 | 55% | Proper closure of autophagosome | No | |
| ATG2B | atg2b | XP_001340508.3 | ENSDART00000155615 | 42% | No | |||
| ATG9A | atg9a | NM_001083031, NP_001076500 | ENSDART00000065411.6 | 71% | Transmembrane protein on the autophagsome | No | ||
| ATG9B | atg9b | NM_001320078, NP_001307007 | ENSDART00000147499.3 | 49% | No | |||
| WIPI1 | wipi1 | NM_200391, NP_956685 | ENSDART00000059533.4 | 71% | Phosphatidyl-insolitol 3-phosphate PI(3)P-binding proteins | Yes | ||
| WIPI2 | wipi2 | NM_001327789, NP_001314718 | ENSDART00000134026.2 | 82% | Yes | |||
| WIPI3/WDR45B | wipi3/wdr45b | NM_200240, NP_956534 | ENSDART00000152327.2 | 96% | No | |||
| WDR45 | wipi4 | NM_200231, NP_956525 | ENSDART00000130229.2 | 90% | No | |||
| Autophagy receptor proteins | NCOA4 | ncoa4 | NM_201129, NP_957423 | ENSDART00000017052.8 | 38% | Autophagy cargo receptor required during iron homeostasis | No | |
| SQSTM1/p62 | sqstm1/p62 | NM_001312913, NP_001299842 | ENSDART00000140061.2 | 44% | Autophagy cargo receptor | No | ||
| OPTN | optn | NM_001100066, NP_001093536 | ENSDART00000014036.10 | 41% | Autophagy cargo receptor | No | ||
| CALCOCO2/NDP52 | calcoco2 | NM_001020741, NP_001018577 | ENSDART00000152964.2 | 30% | Autophagy cargo receptor during xenophagy and mitophagy | No | ||
| NBR1 | nbr1 | NM_001305595, NP_001292524 | ENSDART00000133048.2 | 38% | Autophagy cargo receptor | Yes | ||
| TAX1BP1 | tax1bp1a | NM_001346178, NP_001333107 | ENSDART00000171664.1 | 44% | Autophagy cargo receptor during mitophagy | Yes | ||
| tax1bp1b | NM_212664, NP_997829 | ENSDART00000040727.7 | 52% | Autophagy cargo receptor | Yes | |||
| Reporter | Expression | Reference |
|---|---|---|
| Tg(CMV:GFP-Lc3) | Ubiquitous | [86] |
| Tg(CMV:GFP-Gabarap) | Ubiquitous | [86] |
| Tg(pT2-mCherry-Sqstm1) | Ubiquitous | [45] |
| Tg(pT2-Lamp1-mCherry) | Ubiquitous | [45] |
| Tg(TαCP:mCherry-GFP-Map1lc3b) | Cone photoreceptors | [97] |
| Tg(TαCP:GFP-Map1lc3b) | Cone photoreceptors | [97] |
| Tg(TαCP:YFP-2XFYVE) | Cone photoreceptors | [97] |
| Tg(CMV:EGFP-Map1lc3b; CMV:mCherry-Map1lc3b) | Ubiquitous | [46] |
| Tg(CMV:EGFP-Gabarapa; CMV:mCherry-Map1lc3b) | Ubiquitous | [46] |
| Tg(fabp10: EGFP-Map1lc3b) | Liver | [95] |
| Tg(TαCP:GFP-Map1lc3b) | Cone photoreceptors | [90] |
| pEGFP–Map1lc3b | Transient (embryonic cells) | [103] |
| mCherry-Lc3 mRNA | Transient | [104,105] |
| pDest(CMV:RFP.GFP.Lc3) mRNA | Transient | [105] |
| GFP-Lc3-RFP-Lc3ΔG mRNA | Transient | [101] |
| mCherry-Map1lc3b | Transient | [106] |
| hsp70l:RFP-Map1lc3b | Transient | [61] |
| Antibody | Company | Catalogue No. | Reference |
|---|---|---|---|
| LC3 | Novus biologicals | NB100-2220 | [93,108,111,112,113,114,115,116] |
| Novus biologicals | NB100-2331 | [86,94,117] | |
| Proteintech | 12135-1-AP | [118] | |
| Cell Signaling | 4108 | [45,109] | |
| Not indicated | [74,104] | ||
| 2775 | [62,114] | ||
| MBL | Not indicated | [119] | |
| PD014 | [95] | ||
| PM036 | [115] | ||
| Sigma | L7543 | [59] | |
| Abcam | ab51520 | [106] | |
| Thermo Scientific | PA1-46286 | [68] | |
| Gabarap | Non-commercial | [86] | |
| SQSTM1/p62 | Abnova | H00008878-M01 | [111] |
| Cell Signaling | 5114 | [94,112] | |
| Abcam | ab109012 | [117] | |
| ab31545 | [68] | ||
| MBL Japan | Not indicated | [119,120] | |
| Cliniscience | PM045 | [67] | |
| mTOR | Cell Signalling | 2983 | [116] |
| Phospho-mTOR, Ser2448 | Cell Signaling | 2971 | [121] |
| Akt | Cell Signaling | Not indicated | [74] |
| Phospho-Akt, Ser473 | Cell Signaling | 9271 | [74,121] |
| Phospho-S6K, Thr389 | Cell Signaling | 9205 | [121] |
| Phospho-S6K | Cell Signaling | Not indicated | [104] |
| S6k | Cell Signaling | 2708 | [121] |
| Beclin1 | R&D systems | Not indicated | [120] |
| Abcam | Not indicated | [104] | |
| Santa Cruz | H-300 11427 | [58,62] | |
| Lamp-2A | Abcam | ab18528 | [121] |
| Atg5 | Novus biologicals | NB110-53818 | [59,93] |
| Abcam | Not indicated | [108] | |
| ab540333 | [59] | ||
| Abgent | AP1812a, AP1812b | [59] | |
| Actin (loading control) | Sigma | Not indicated | [108] |
| α-Tubulin (loading control) | Sigma | T5168 | [73] |
| GAPDH (loading control) | Millipore | Not indicated | [108] |
| Reagent | Conc. | Observed Effect | Reference |
|---|---|---|---|
| Reagents increasing autophagy | |||
| Rapamycin | 400 nM | Inhibited mTOR, activated autophagy; ameliorated kidney cysts and preserved kidney function | [112] |
| 1 µM | Increased autophagy dependent release of Tumor necrosis factor α and Interleukin-8 (TNFα and IL-8) in mycobacterium-infected zebrafish larvae | [105] | |
| 10 µM | Enhanced clearance of protein aggregates in FLNCW2710X mutants | [113] | |
| 30 µM | Enhanced the clearance of A152T-tau, reduced hyperphosphorylated tau | [108] | |
| Torin1 | 0.4 µM | ATP-competitive mTOR inhibitor; increased Lc3-I and Lc3-II levels; increased resistance of zebrafish embryos to Salmonella Typhimurium infection | [119] |
| Rilmenidine | 50 µM | Imidazoline-1 receptor agonist, reduced cyclic adenosine monophosphate (cAMP) levels; enhanced the clearance of A152T-tau | [108] |
| Clonidine | 30 µM | Imidazoline-1 receptor agonist, reduced cAMP levels; enhanced the clearance of A152T-tau | [108] |
| Carbamazepine | 20 µM | mTOR-independent autophagy activator; attenuated kidney cysts | [112] |
| 50 µM | Increased autophagy-dependent cytokine release | [105] | |
| 0.5 mM | Enhanced clearance of protein aggregates in FLNCW271°X mutants | [113] | |
| Minoxidil | 400 nM | Inhibited L-type Ca2+ channel currents, thereby activating autophagy via a cyclical mTOR independent pathway; attenuated kidney cysts | [112] |
| Auten-67 | 50 µM | Upregulated autophagy by inhibiting phosphatase activity of MTMR14, which is a negative regulator of autophagic membrane formation. | [125] |
| Spermidine | 5 mM | Inhibited acetyl-transferases; enhanced clearance of protein aggregates in FLNCW2710X mutants | [113] |
| Trifluoperazine (TFP) | 1 mM | Activated Transcription Factor EB (TFEB) which is a master regulator of autophagy pathway, activated autophagy | [111] |
| Reagents blocking autophagosome—lysosome fusion | |||
| Bafilomycin A1 (BafA1) | 20 nM | Autophagosome-lysosome fusion inhibitor; slight increase in Lc3-II | [117] |
| 25 nM | Significant increase in Lc3-II | [104] | |
| 167 nM | Showed defects in autophagy flux | [112] | |
| 200 nM | Zebrafish larvae recapitulated atp6v0ca morphant, reduced yolk opacity and senescence phenotypes | [45] | |
| Chloroquine | 10 µM | Autophagosome-lysosome fusion inhibitor; blocked autophagy and increased GFP-Lc3 punctae | [73] |
| 2 mM | Reduced muscle regeneration on blocking autophagy | [94] | |
| 100 c | Decreased Lc3 accumulation, defective autophagy | [113] | |
| 5 µM | Increased Lc3 accumulation in Kri1lcas002 mutant | [104] | |
| 2.5 µM | Significant accumulation of autophagosomes in zebrafish larvae infected with mycobacterium | [105] | |
| 50 µM | Accumulation of Lc3-II and p62; no effect on zebrafish infection with Salmonella Typhimurium | [119] | |
| Omeprazole | 100 µM | Late-stage autophagy inhibitor; rescued senescence phenotype | [45] |
| Lansoprazole | 100 µM | Late-stage autophagy inhibitor; rescued senescence phenotype | [45] |
| Pantoprazole | 100 µM | Late-stage autophagy inhibitor; rescued senescence phenotype | [45] |
| Pepstatin A | 5 µg/mL | Prevented autolysosomal maturation and turnover | [45] |
| E-64d | 5 µg/mL | Prevented autolysosomal maturation and turnover | [45] |
| Ammonium chloride | 100 mM | Prevented autolysosome maturation; blocked autophagy and increased GFP-Lc3 punctae | [113] |
| 100 mM | Significant increase in Lc3-II | [117] | |
| Early autophagy inhibitor | |||
| 3-MA | 10 mM | Inhibited PIK3C3 activity; significant reduction of autophagy visualized by Lc3-II puncta | [104] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathai, B.J.; Meijer, A.H.; Simonsen, A. Studying Autophagy in Zebrafish. Cells 2017, 6, 21. https://doi.org/10.3390/cells6030021
Mathai BJ, Meijer AH, Simonsen A. Studying Autophagy in Zebrafish. Cells. 2017; 6(3):21. https://doi.org/10.3390/cells6030021
Chicago/Turabian StyleMathai, Benan John, Annemarie H. Meijer, and Anne Simonsen. 2017. "Studying Autophagy in Zebrafish" Cells 6, no. 3: 21. https://doi.org/10.3390/cells6030021





