Application of Sodium Selenite in the Prevention and Treatment of Cancers
Abstract
:1. Introduction
2. Mechanism of Recognition of Cancer Cells by Immune System
3. Unusual Properties of Selenium
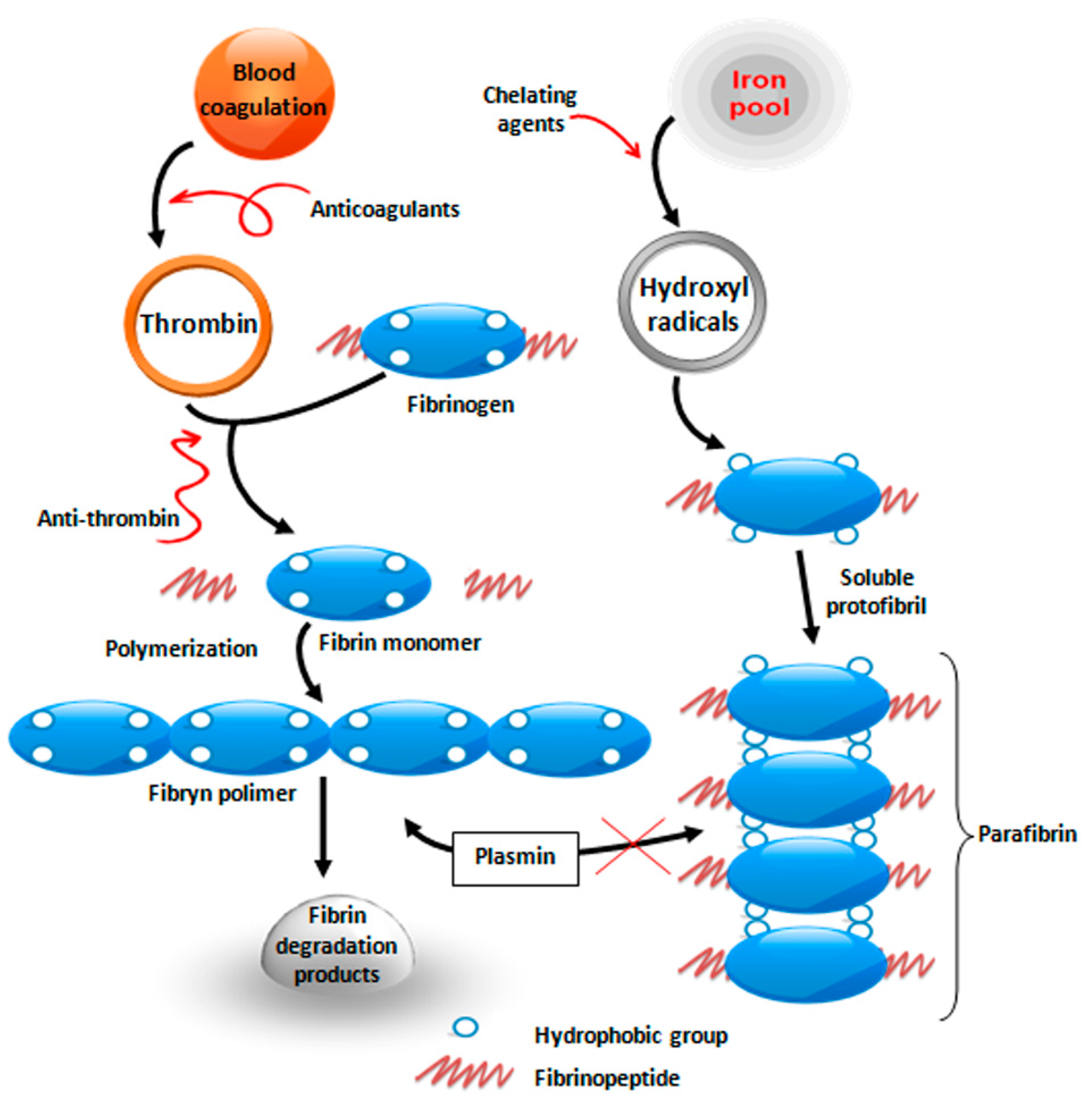
4. Prooxidative Effect of Selenite (Direct Anticancerogenic Effect)
5. Selenite as a Substrate for Synthesis of Selenoproteins with Antioxidant Properties
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Ip, C.; Hayes, C.; Budnick, M.; Ganther, H.E. Chemical form of selenium critical metabolites, and cancer prevention. Cancer Res. 1991, 51, 595–600. [Google Scholar] [PubMed]
- Duntas, L.H.; Benvenga, S. Selenium: An element for life. Endocrine 2015, 48, 756–775. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, B. Iron-induced parafibrin formation in tumors fosters immune evasion. Oncoimmunology 2014, 3, e28539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipinski, B. Rationale for the treatment of cancer with sodium selenite. Med. Hypotheses 2005, 64, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Opdenakker, G.; Van Damme, J. Cytokines and proteases in invasive processes: Molecular similarities between inflammation and cancer. Cytokine 1992, 4, 251–258. [Google Scholar] [CrossRef]
- Lipinski, B. Prostate cancer vaccines, fibrin and selenium: A conceptual review. Open Prost. Cancer J. 2010, 3, 69–73. [Google Scholar] [CrossRef]
- Lipinski, B.; Pretorius, E. Iron-induced fibrin in cardiovascular disease. Curr. Neurovasc. Res. 2013, 10, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, B.; Pretorius, E. Novel pathway of iron-induced blood coagulation: Implications for diabetes mellitus and its complications. Pol. Arch. Med. Wewn. 2012, 122, 115–122. [Google Scholar] [PubMed]
- Frenkel, G.D.; Falvey, D.; MacVicar, C. Products of the reaction of selenite with intracellular sulhydryl groups. Biol. Trace Elem. Res. 1991, 30, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Combs, G.F., Jr. Selenium in global food systems. Br. J. Nutr. 2001, 85, 517–547. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S. Selenium significance and outlook for supplementation. Nutrition 2013, 29, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S.; Bzducha-Wróbel, A. Influence of selenium content in the culture medium on protein profile of yeast cells Candida utilis ATCC 9950. Oxid. Med. Cell. Longev. 2015, 6. [Google Scholar] [CrossRef]
- Chen, J. An original discovery: Selenium deficiency and Keshan disease (an endemic heart disease). Asia Pac. J. Clin. Nutr. 2012, 21, 320–326. [Google Scholar] [PubMed]
- Schrauzer, G.N. Selenium and cancer: A review. Bioinorg. Chem. 1976, 5, 275–281. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Żbikowska, H. Selen w organizmach żywych. I. Toksyczność selenu i działanie antynowotworowe. Acta Universitatis Lodziensis. Folia Biochim. Biophys. 1997, 12, 29–37. [Google Scholar]
- Amaral, A.F.S.; Cantor, K.P.; Silverman, D.T.; Malats, N. Selenium and bladder cancer risk: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Tomblin, J.; Armistead, M.Y.; Murray, E. Selenium (sodium selenite) causes cytotoxicity and apoptotic mediated cell death in PLHC-1 fish cell line through DNA and mitochondrial membrane potential damage. Ecotoxicol. Environ. Saf. 2013, 87, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C.; Combs, G.F., Jr.; Turnbull, B.W.; Slate, E.H.; Chalker, D.K.; Chow, J.; Davis, L.S.; Glover, R.A.; Graham, G.F.; Gross, E.G.; et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. J. Am. Med. Assoc. USA 1996, 276, 1957–1963. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, J.; Jiang, C.; Deng, Y.; Özten, N.; Bosland, M.C. Cancer chemoprevention research with selenium in the post-SELECT era: Promises and challenges. Nutr. Cancer 2016, 68, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sanmartin, C.; Plano, D.; Sharma, A.K.; Palop, J.A. Selenium compounds, apoptosis and other types of cell death: An overview for cancer therapy. Int. J. Mol. Sci. 2012, 13, 9649–9672. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta 2015, 1850, 1642–1660. [Google Scholar] [CrossRef] [PubMed]
- Weekley, C.M.; Harris, H.H. Which form is that? The importance of selenium speciation in the prevention and treatment of disease. Chem. Soc. Rev. 2013, 42, 8870–8894. [Google Scholar] [CrossRef] [PubMed]
- Björnstedt, M.; Fernandes, A.P. Selenium in the prevention of human cancers. EPMA J. 2000, 1, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S.; Bzducha-Wrobel, A.; Kurcz, A. Effects of selenium on morphological changes in Candida utilis ATCC 9950 yeast cells. Biol. Trace Elem. Res. 2016, 169, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Paolicchi, E.; Gemignani, F.; Krstic-Demonacos, M.; Dedhar, S.; Mutti, L.; Landi, S. Targeting hypoxic response for cancer therapy. Oncotarget 2016, 7, 13464–13478. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S. Current knowlwdge on the importance of selenium in food for living organsims: A review. Molecules 2016, 21, E609. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak, P.; Lipińska, A. Rola transportera glukozy 1 (GLUT1) w diagnostyce i terapii nowotworów. Postep. Hig. Med. Dosw. 2012, 66, 165–174. [Google Scholar]
- Misra, S.; Boylan, M.; Selvam, A.; Spallholz, J.E.; Björnstedt, M. Redox-active selenium compounds—From toxicity and cell death to cancer treatment. Nutrients 2015, 7, 3536–3556. [Google Scholar] [CrossRef] [PubMed]
- Paskett, E.D.; Dean, J.A.; Oliveri, J.M.; Harrop, J.P. Cancer-related lymphedema risk factors, diagnosis, treatment, and impact: A review. J. Clin. Oncol. 2012, 30, 3726–3733. [Google Scholar] [CrossRef] [PubMed]
- Pfister, C.; Dawzcynski, H.; Schingale, F.-J. Sodium selenite and cancer related lymphedema: Biological and pharmacological effects. J. Trace Elem. Med. Biol. 2016, 37, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, T.; Leonhardt, H.; Kersting, S.; Albrecht, S.; Range, U.; Eckelt, U. Reduction of postoperative lymphedema after oral tumor surgery with sodium selenite. Biol. Trace Elem. Res. 2005, 106, 193–203. [Google Scholar] [CrossRef]
- Kasseroller, R. Sodium selenite as prophylaxis against erysipelas in secondary lymphedema. Anticancer Res. 1998, 18, 2227–2230. [Google Scholar] [PubMed]
- Sinha, R.; El-Bayoumy, K. Apoptosis is a critical cellular event in cancer chemoprevention and chemotherapy by selenium compounds. Curr. Cancer Drug Target 2004, 4, 13–28. [Google Scholar] [CrossRef]
- Patrick, L. Selenium biochemistry and cancer: A review of the literature. Altern. Med. Rev. 2004, 9, 239–258. [Google Scholar] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Brodin, O.; Eksborg, S.; Wallenberg, M.; Asker-Hagelberg, C.; Larsen, E.H.; Mohlkert, D.; Lenneby-Helleday, C.; Jacobsson, H.; Linder, S.; Misra, S.; et al. Pharmacokinetics and toxicity of sodium selenite in the treatment of patients with carcinoma in a phase I clinical trial: The SECAR study. Nutrients 2015, 7, 4978–4994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsonne, G.; Sun, X.; Nyström, C.; Rundlöf, A.K.; Potamitou Fernandes, A.; Björnstedt, M.; Dobra, K. Selenite induces apoptosis in sarcomatoid malignant mesothelioma cells through oxidative stress. Free Radic. Biol. Med. 2006, 41, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Lener, M.R.; Gupta, S.; Scott, R.J.; Tootsi, M.; Kulp, M.; Tammesoo, M.L.; Viitak, A.; Metspalu, A.; Serrano-Fernández, P.; Kładny, J.; et al. Can selenium levels act as a marker of colorectal cancer risk? BMC Cancer 2014, 13, 214. [Google Scholar] [CrossRef] [PubMed]
- Xiang, N.; Zhao, R.; Zhong, W. Sodium selenite induces apoptosis by generation of superoxide via the mitochondrial-dependent pathway in human prostate cancer cells. Cancer Chemother. Pharmacol. 2009, 63, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, W.; Decock, J.; Mulholland, F.; Bao, Y.; Fairweather-Tait, S. Selenium biomarkers in prostate cancer cell lines and influence of selenium on invasive potential of PC3 cells. Front. Oncol. 2013, 3, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S.; Gientka, I.; Bzducha-Wróbel, A. Accumulation and metabolism of selenium by yeast cells. Appl. Microbiol. Biotechnol. 2015, 99, 5373–5382. [Google Scholar] [CrossRef] [PubMed]
- Ganther, H.E. Selenium metabolism, selenoproteins and mechanisms of cancer prevention: Complexities with thioredoxin reductase. Carcinogenesis 1999, 20, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Zacharski, L.R. Anticoagulation, ferrotoxicity and the future of translational lung cancer research. Transl. Lung Cancer Res. 2016, 5, 280–287. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kieliszek, M.; Lipinski, B.; Błażejak, S. Application of Sodium Selenite in the Prevention and Treatment of Cancers. Cells 2017, 6, 39. https://doi.org/10.3390/cells6040039
Kieliszek M, Lipinski B, Błażejak S. Application of Sodium Selenite in the Prevention and Treatment of Cancers. Cells. 2017; 6(4):39. https://doi.org/10.3390/cells6040039
Chicago/Turabian StyleKieliszek, Marek, Boguslaw Lipinski, and Stanisław Błażejak. 2017. "Application of Sodium Selenite in the Prevention and Treatment of Cancers" Cells 6, no. 4: 39. https://doi.org/10.3390/cells6040039