Optimization of Polycistronic Anti-CCR5 Artificial microRNA Leads to Improved Accuracy of Its Lentiviral Vector Transfer and More Potent Inhibition of HIV-1 in CD4+ T-Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Plasmid DNA
2.2. Lentiviral Particle Production
2.3. Lentiviral Transduction
2.4. Flow Cytometry
2.5. PCR Analysis of Vector Integrity
2.6. Quantitative PCR (qPCR)
2.7. HIV Challenge
3. Results
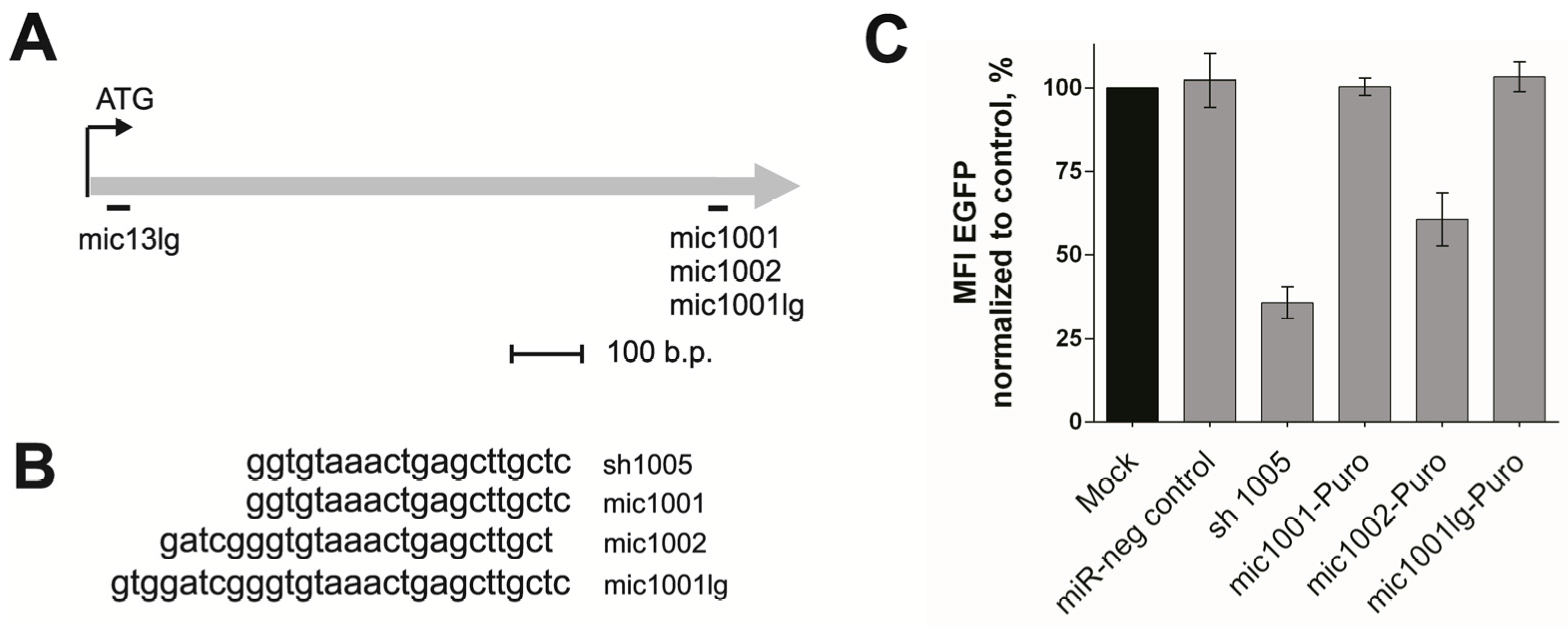
3.1. Design of the New amiRNAs and Evaluation of Their Silencing Activity
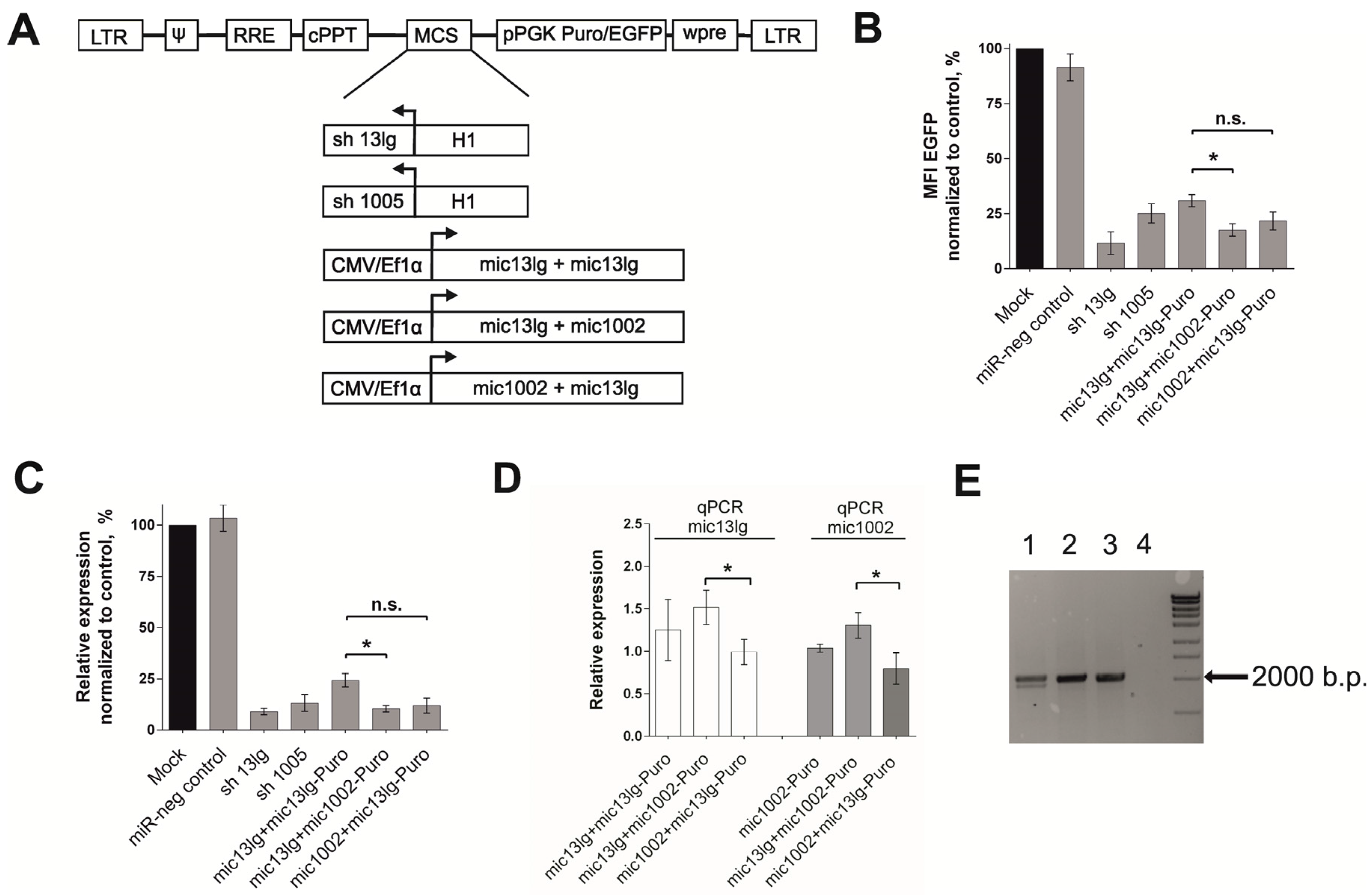
3.2. Creation of the Tandem Constructs and Evaluation of Their Silencing Activity and Integrity
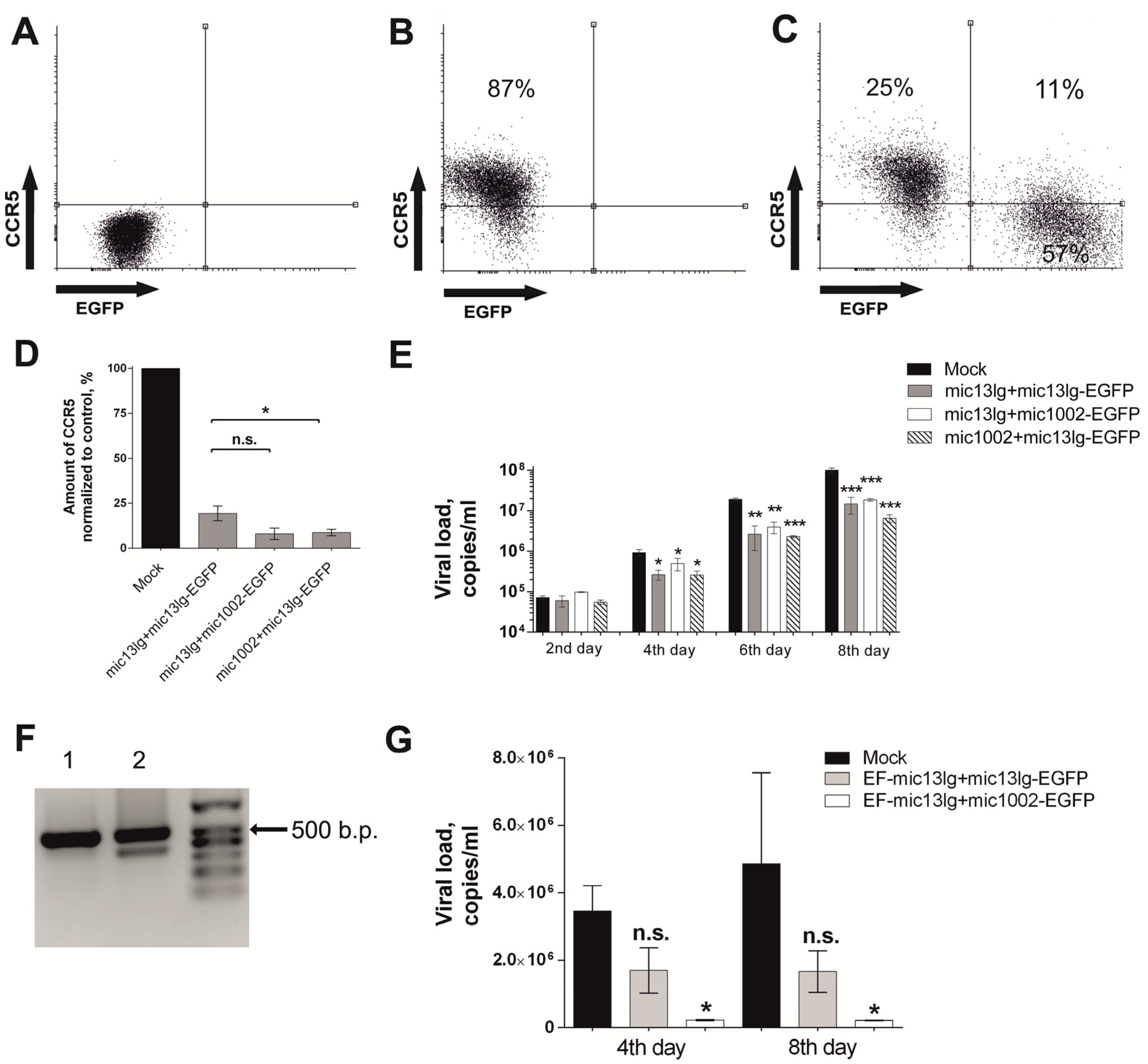
3.3. amiRNAs Suppress HIV Growth in MAGI CCR5 and Primary T Cell Cultures
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HIV | human immunodeficiency virus |
| amiRNA | artificial microRNA |
| miRNA | microRNA |
| shRNA | short hairpin RNA |
| RNAi | RNA interference |
| siRNA | short interfering RNA |
References
- Deng, H.; Liu, R.; Ellmeier, W.; Choe, S.; Unutmaz, D.; Burkhart, M.; Di Marzio, P.; Marmon, S.; Sutton, R.E.; Hill, C.M.; et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature 1996, 381, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Samson, M.; Libert, F.; Doranz, B.J.; Rucker, J.; Liesnard, C.; Farber, C.M.; Saragosti, S.; Lapoumeroulie, C.; Cognaux, J.; Forceille, C.; et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 1996, 382, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Lucotte, G. Frequencies of 32 base pair deletion of the (Delta 32) allele of the CCR5 HIV-1 co-receptor gene in Caucasians: A comparative analysis. Infect. Genet. Evol. 2002, 1, 201–205. [Google Scholar] [CrossRef]
- Allers, K.; Hütter, G.; Hofmann, J.; Loddenkemper, C.; Rieger, K.; Thiel, E.; Schneider, T. Evidence for the cure of HIV infection by CCR5 ∆32/∆32 stem cell transplantation. Blood 2011, 117, 2791–2799. [Google Scholar] [CrossRef] [PubMed]
- Ghorban, K.; Dadmanesh, M.; Hassanshahi, G.; Momeni, M.; Zare-Bidaki, M.; Arababadi, M.K.; Kennedy, D. Is the CCR5 Δ 32 mutation associated with immune system-related diseases? Inflammation 2013, 36, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Mock, U.; Machowicz, R.; Hauber, I.; Horn, S.; Abramowski, P.; Berdien, B.; Hauber, J.; Fehse, B. mRNA transfection of a novel TAL effector nuclease (TALEN) facilitates efficient knockout of HIV co-receptor CCR5. Nucleic Acids Res. 2015, 43, 5560–5571. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.N.; Wu, H.; Shankar, P. Recent advances in RNAi-based strategies for therapy and prevention of HIV-1/AIDS. Adv. Drug Deliv. Rev. 2016, 103, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Akkina, R. Complete knockdown of CCR5 by lentiviral vector-expressed siRNAs and protection of transgenic macrophages against HIV-1 infection. Gene Ther. 2007, 14, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Kamata, M.; Kittipongdaja, P.; Chen, K.N.; Kim, S.; Pang, S.; Boyer, J.; Qin, F.X.; An, D.S.; Chen, I.S. Characterization of a potent non-cytotoxic shRNA directed to the HIV-1 co-receptor CCR5. Genet. Vaccines Ther. 2009, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Symonds, G.; Bartlett, J.S.; Kiem, H.P.; Tsie, M.; Breton, L. Cell-delivered entry inhibitors for HIV-1: CCR5 downregulation and blocking virus/membrane fusion in defending the host cell population. AIDS Patient Care STDS 2016, 30, 545–550. [Google Scholar] [CrossRef] [PubMed]
- An, D.S.; Qin, F.X.; Auyeung, V.C.; Mao, S.H.; Kung, S.K.; Baltimore, D.; Chen, I.S. Optimization and functional effects of stable short hairpin RNA expression in primary human lymphocytes via lentiviral vectors. Mol. Ther. 2006, 14, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Streetz, K.L.; Jopling, C.L.; Storm, T.A.; Pandey, K.; Davis, C.R.; Marion, P.; Salazar, F.; Kay, M.A. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature 2006, 441, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, R.L.; Martins, I.; Davidson, B.L. Artificial microRNAs as siRNA shuttles: Improved safety as compared to shRNAs in vitro and in vivo. Mol. Ther. 2009, 17, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, L.A.; Zhang, J.; von Eije, K.J.; Li, H.; Saetrom, P.; Amarzguioui, M.; Rossi, J.J. Engineering and optimization of the mir-106b-cluster for ectopic expression of multiplexed anti-HIV RNAs. Gene Ther. 2008, 15, 1536–1549. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Haasnoot, J.; ter Brake, O.; Berkhout, B.; Konstantinova, P. Inhibition of HIV-1 by multiple siRNAs expressed from a single microRNA polycistron. Nucleic Acids Res. 2008, 36, 2811–2824. [Google Scholar] [CrossRef] [PubMed]
- Myburgh, R.; Ivic, S.; Pepper, M.S.; Gers-Huber, G.; Li, D.; Audigé, A.; Rochat, M.A.; Jaquet, V.; Regenass, S.; Manz, M.G.; et al. Lentivector knockdown of CCR5 in hematopoietic stem and progenitor cells confers functional and persistent HIV-1 resistance in numanized mice. J. Virol. 2015, 89, 6761–6772. [Google Scholar] [CrossRef] [PubMed]
- Glazkova, D.V.; Vetchinova, A.S.; Bogoslovskaia, E.V.; Zhogina, I.A.; Markelov, M.L.; Shipulin, G.A. Downregulation of human CCR5 receptor gene expression using artificial microRNAs. Mol. Biol. 2013, 47, 475–485. [Google Scholar] [CrossRef]
- Jetzt, A.E.; Yu, H.; Klarmann, G.J.; Ron, Y.; Preston, B.D.; Dougherty, J.P. High rate of recombination throughout the human immunodeficiency virus type 1 genome. J. Virol. 2000, 74, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- An, W.; Telesnitsky, A. Frequency of Direct Repeat Deletion in a Human Immunodeficiency Virus Type 1 Vector during Reverse Transcription in Human Cells. Virology 2001, 286, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Ter Brake, O.; Hooft, K.; Liu, Y.P.; Centlivre, M.; von Eije, K.J.; Berkhout, B. Lentiviral vector design for multiple shRNA expression and durable HIV-1 inhibition. Mol. Ther. 2008, 16, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-time quantification of microRNAs by stem—Loop RT—PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef] [PubMed]
- Zhogina, Y.A.; Glazkova, D.V.; Vetchinova, A.S.; Bogoslovskaya, E.V.; Zyganova, G.M.; Shipulin, G.A. Comparison of activity of different gene constructions aimed at inhibition HIV1 replication. J. Biopharm. 2014, 6, 11–18. [Google Scholar]
- Salmon, P.; Kindler, V.; Ducrey, O.; Chapuis, B.; Zubler, R.H.; Trono, D. High-level transgene expression in human hematopoietic progenitors and differentiated blood lineages after transduction with improved lentiviral vectors. Blood 2000, 96, 3392–3398. [Google Scholar] [PubMed]
- Das, A.T.; Brummelkamp, T.R.; Westerhout, E.M.; Vink, M.; Madiredjo, M.; Bernards, R.; Berkhout, B. Human Immunodeficiency Virus Type 1 escapes from RNA interference-mediated inhibition. J. Virol. 2004, 78, 2601–2605. [Google Scholar] [CrossRef] [PubMed]
- Tchurikov, N.A.; Fedoseeva, D.M.; Gashnikova, N.M.; Sosin, D.V.; Gorbacheva, M.A.; Alembekov, I.R.; Chechetkin, V.R.; Kravatsky, Y.V.; Kretova, O.V. Conserved sequences in the current strains of HIV-1 subtype A in Russia are effectively targeted by artificial RNAi in vitro. Gene 2016, 583, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Barmania, F.; Potgieter, M.; Pepper, M.S. Mutations in C-C chemokine receptor type 5 (CCR5) in South African individuals. Int. J. Infect. Dis. 2013, 17, e1148–e1153. [Google Scholar] [CrossRef] [PubMed]
- Al Balwi, M.A.; Hadadi, A.I.; Alharbi, W.; Ballow, M.; AlAsiri, A.; AlAbdulrahman, A.; Udayaraja, G.K.; Aldrees, M.; AlAbdulkareem, I.; Hajeer, A.H. Analysis of CCR5 gene polymorphisms in 321 healthy Saudis using Next Generation Sequencing. Hum. Immunol. 2017, 78, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Myburgh, R.; Cherpin, O.; Schlaepfer, E.; Rehrauer, H.; Speck, R.F.; Krause, K.-H.; Salmon, P. Optimization of сritical hairpin features allows miRNA-based gene knockdown upon single-copy transduction. Mol. Ther. Nucleic Acids. 2014, 3, e207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urusov, F.; Glazkova, D.; Omelchenko, D.; Bogoslovskaya, E.; Tsyganova, G.; Kersting, K.; Shipulin, G.; Pokrovsky, V. Optimization of Polycistronic Anti-CCR5 Artificial microRNA Leads to Improved Accuracy of Its Lentiviral Vector Transfer and More Potent Inhibition of HIV-1 in CD4+ T-Cells. Cells 2018, 7, 10. https://doi.org/10.3390/cells7020010
Urusov F, Glazkova D, Omelchenko D, Bogoslovskaya E, Tsyganova G, Kersting K, Shipulin G, Pokrovsky V. Optimization of Polycistronic Anti-CCR5 Artificial microRNA Leads to Improved Accuracy of Its Lentiviral Vector Transfer and More Potent Inhibition of HIV-1 in CD4+ T-Cells. Cells. 2018; 7(2):10. https://doi.org/10.3390/cells7020010
Chicago/Turabian StyleUrusov, Felix, Dina Glazkova, Denis Omelchenko, Elena Bogoslovskaya, Galina Tsyganova, Katerina Kersting, German Shipulin, and Vadim Pokrovsky. 2018. "Optimization of Polycistronic Anti-CCR5 Artificial microRNA Leads to Improved Accuracy of Its Lentiviral Vector Transfer and More Potent Inhibition of HIV-1 in CD4+ T-Cells" Cells 7, no. 2: 10. https://doi.org/10.3390/cells7020010




