Towards Improvements for Penetrating the Blood–Brain Barrier—Recent Progress from a Material and Pharmaceutical Perspective
Abstract
:1. Introduction
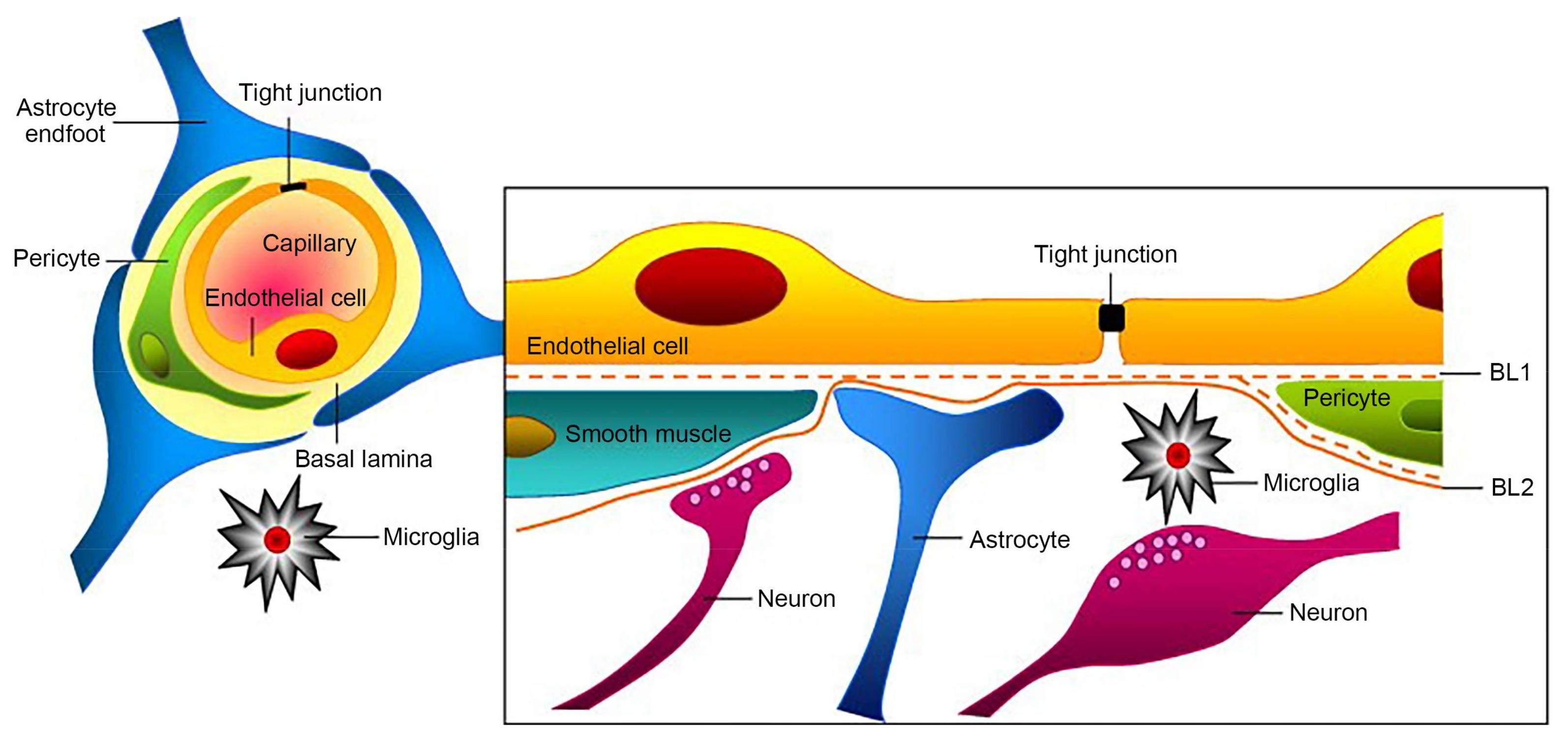
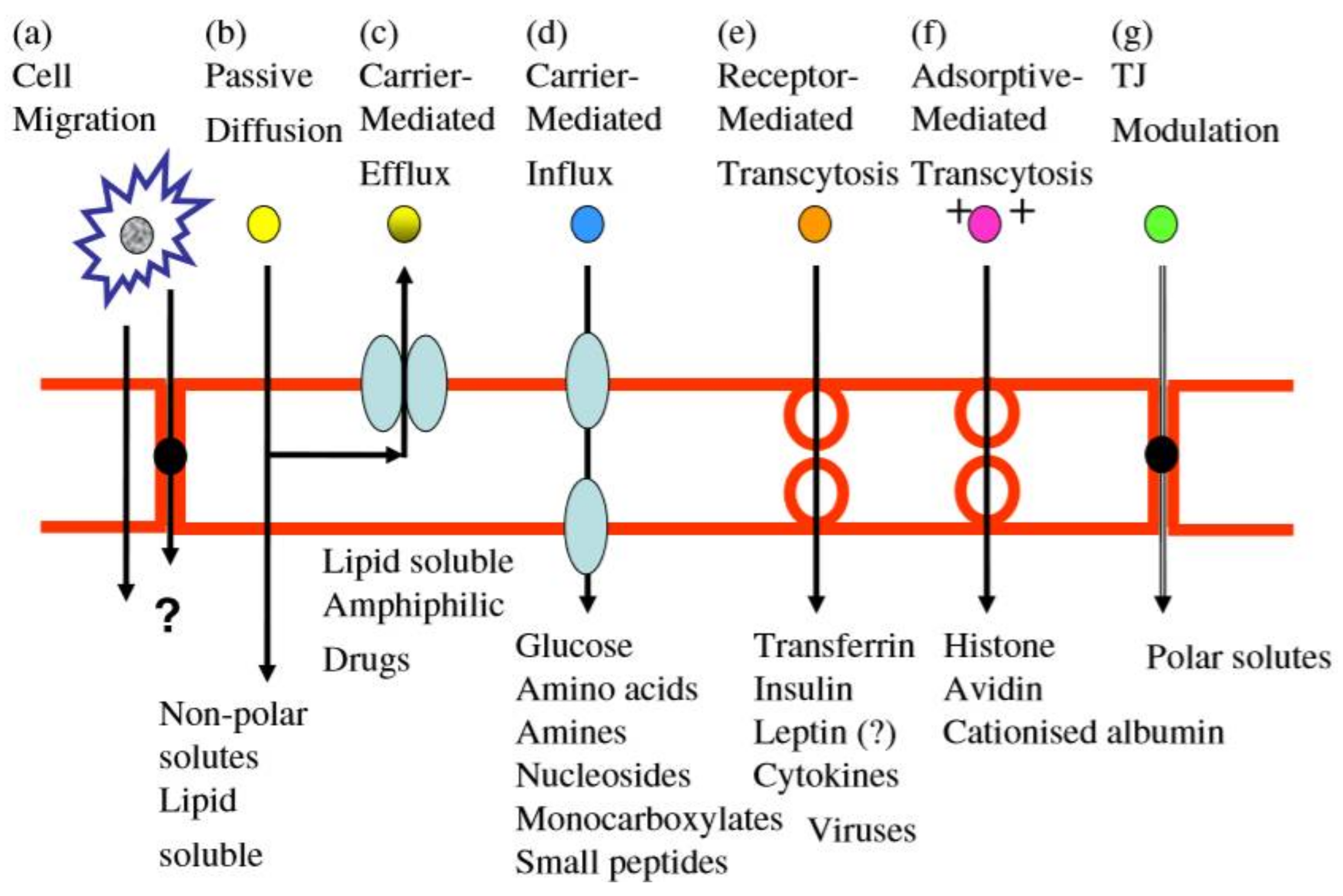
2. The Discovery and Structure of BBB
3. Research Progress in Methods for Blood–Brain Barrier Permeability and Circumvention
3.1. Physical Methods
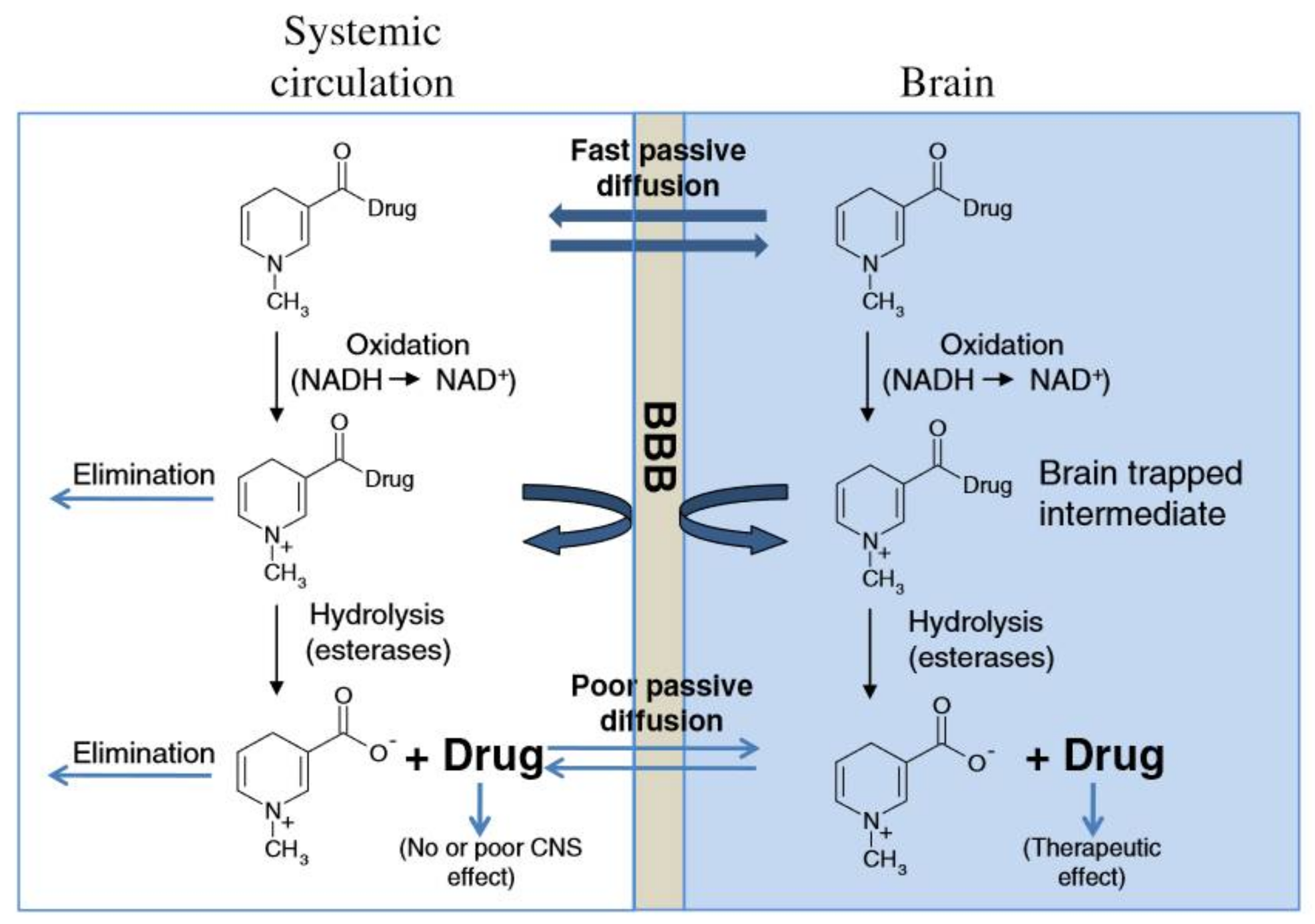
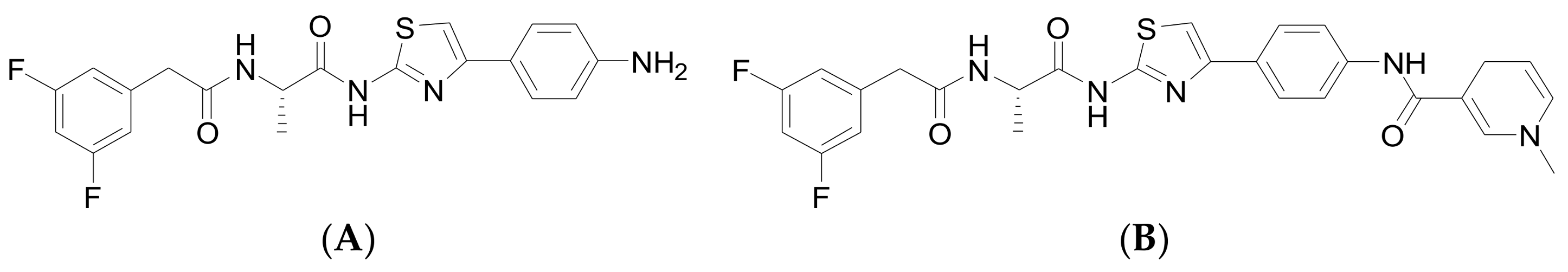
3.2. Chemical Methods
3.3. Biological Methods
3.4. Nanoparticles Drug-Delivery System Method
3.4.1. Liposome
3.4.2. Polymeric Nanoparticles
3.4.3. Solid Lipid Nanoparticles
3.4.4. Magnetic Nanoparticles
3.4.5. Novel Nanoscale Entities
4. Future Challenges of Delivery Drug across BBB to Brain
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dyrna, F.; Hanske, S.; Krueger, M.; Bechmann, I. The blood-brain barrier. J. Neuroimmune Pharm. 2013, 8, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, S.N.; Girouard, H.; Carret, A.S.; Martel, S. Remote control of the permeability of the blood-brain barrier by magnetic heating of nanoparticles: A proof of concept for brain drug delivery. J. Control. Release 2015, 206, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Booth, B.B.; Dunstone, N.J.; Halloran, P.R.; Andrews, T.; Bellouin, N. Aerosols implicated as a prime driver of twentieth-century North Atlantic climate variability. Nature 2012, 484, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, A.F.; Erickson, M.A.; Rhea, E.M.; Salameh, T.S.; Bankset, W.A. Gut reactions: How the blood–brain barrier connects the microbiome and the brain. Exp. Biol. Med. 2018, 243, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, F.; Schäfer, J.; Lyck, R.; Makrides, V.; Brunner, S.; Schaeren-Wiemers, N.; Deutsch, U.; Engelhardt, B. Claudin-1 induced sealing of blood–brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis. Acta Neuropathol. 2016, 122, 601. [Google Scholar] [CrossRef] [PubMed]
- Bicker, J.; Alves, G.; Fortuna, A.; Falcão, A. Blood-brain barrier models and their relevance for a successful development of CNS drug delivery systems: A review. Eur. J. Pharm. Biopharm. 2014, 87, 409–432. [Google Scholar] [CrossRef] [PubMed]
- Dyrna, F.; Hanske, S.; Krueger, M.; Bechmann, I. The blood-brain barrier. J. Neuroimmune Pharmacol. 2013, 8, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Sohet, F.; Lin, C.; Munji, R.N.; Lee, S.Y.; Ruderisch, N.; Soung, A.; Arnold, T.D.; Derugin, N.; Vexler, Z.S.; Yen, F.T. LSR/angulin-1 is a tricellular tight junction protein involved in blood-brain barrier formation. J. Cell Biol. 2015, 208, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, H.; Yilmaz, O.; Karaman, M.; Firinci, F.; Turkeli, A.; Kanik, E.T.; Inan, S. Vascular endothelial growth factor antagonism restores epithelial barrier dysfunction via affecting zonula occludens proteins. Exp. Ther. Med. 2015, 10, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.; Piquette-Miller, M. Blood-brain barrier: An impediment to neuropharmaceuticals. Clin. Pharmacol. Ther. 2015, 97, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M.; Arvanitis, C.D.; Alexander, P.M.; McDannold, N. Ultrasound-mediated blood-brain barrier disruption for targeted drug delivery in the central nervous system. Adv. Drug Deliv. Rev. 2014, 72, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Lundgaard, I.; Li, B.; Xie, L.; Kang, H.; Sanggaard, S.; Haswell, J.D.R.; Sun, W.; Goldman, S.; Blekot, S.; et al. Direct neuronal glucose uptake heralds activity-dependent increases in cerebral metabolism. Nat. Commun. 2015, 6, 6807. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J.; Zaruba, K.; Oravec, M.; Kunes, M.; Babula, P.; Ulbrich, P.; Brezaniova, I.; Opatrilova, R.; Triska, J.; Suchy, P. Preparation of silica nanoparticles loaded with nootropics and their in vivo permeation through blood-brain barrier. BioMed Res. Int. 2015, 2015, 812673. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. The Blood-Brain Barrier: Bottleneck in Brain Drug Development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.C.; Santos, A.O. Nanosystems in nose-to-brain drug delivery: A review of non-clinical brain targeting studies. J. Control. Release 2017, 270, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.B.; Patel, M.R.; Bhatt, K.K.; Patel, B.G.; Gaikwad, R.V. Microemulsion-based drug delivery system for transnasal delivery of Carbamazepine: Preliminary brain-targeting study. Drug Deliv. 2016, 23, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, Y.; Liu, X. Intranasal delivery: Circumventing the iron curtain to treat neurological disorders. Expert Opin. Drug Deliv. 2015, 12, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Da Fonseca, C.O.; Schönthal, A.H. Perillyl Alcohol and Its Drug-Conjugated Derivatives as Potential Novel Methods of Treating Brain Metastases. Int. J. Mol. Sci. 2016, 17, 1463. [Google Scholar] [CrossRef] [PubMed]
- Drapeau, A.I.; Poirier, M.B.; Madugundu, G.S.; Wagner, J.R.; Fortin, D. Intra-arterial Temozolomide, Osmotic Blood-brain Barrier Disruption and Radiotherapy in a Rat F98-Glioma Model. Clin. Cancer Drugs 2017, 4, 135–144. [Google Scholar] [CrossRef]
- Foley, C.P.; Rubin, D.G.; Santillan, A.; Sondhi, D.; Dyke, J.P.; Gobin, Y.P.; Crystal, R.G.; Ballon, D.J. Intra-arterial delivery of AAV vectors to the mouse brain after mannitol mediated blood brain barrier disruption. J. Control. Release 2014, 196, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Bellavance, M.A.; Blanchette, M.; Fortin, D. Recent advances in blood-brain barrier disruption as a CNS delivery strategy. AAPS J. 2008, 10, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Cherepanov, S.M.; Yokoyama, S.; Mizuno, A.; Ichinose, W.; Lopatina, O.; Shabalova, A.A.; Salmina, A.B.; Yamamoto, Y.; Okamoto, H.; Shuto, S.; et al. Structure-specific effects of lipidated oxytocin analogs on intracellular calcium levels, parental behavior, and oxytocin concentrations in the plasma and cerebrospinal fluid in mice. Pharmacol. Res. Perspect. 2017, 5, e00290. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, J.V.; Hoekstra, D.; Zuhorn, I.S. Smuggling drugs into the brain: An overview of ligands targeting transcytosis for drug delivery across the blood-brain barrier. Pharmaceutics 2014, 6, 557–583. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Chen, C.C.; Tung, Y.S.; Olumolade, O.O.; Konofagou, E.E. Effects of the microbubble shell physicochemical properties on ultrasound-mediated drug delivery to the brain. J. Control. Release 2015, 212, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M.; Park, J.; Vykhodtseva, N.; Zhang, Y.-Z.; McDannold, N. Enhancement in blood-tumor barrier permeability and delivery of liposomal doxorubicin using focused ultrasound and microbubbles: Evaluation during tumor progression in a rat glioma model. Phys. Med. Biol. 2015, 60, 2511. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M.; Martin, D.R.; Byrne, M.E. Recent advances in delivery through the blood-brain barrier. Curr. Top. Med. Chem. 2014, 14, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Polat, B.E.; Blankschtein, D.; Langer, R. Low-frequency sonophoresis: Application to the transdermal delivery of macromolecules and hydrophilic drugs. Expert Opin. Drug Deliv. 2010, 7, 1415–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgess, A.; Shah, K.; Hough, O.; Hynynen, K. Focused ultrasound-mediated drug delivery through the blood-brain barrier. Expert Rev. Neurother. 2015, 15, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Rautio, J.; Laine, K.; Gynther, M.; Savolainen, J. Prodrug approaches for CNS delivery. AAPS J. 2008, 10, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Okuma, Y.; Wang, F.; Toyoshima, A.; MasahiroKameda, M.; TomohitoHishikawa, T.; Tokunaga, K.; Sugiu, K.; Liu, K.; Haruma, J.; Nishibori, M.; et al. Mannitol enhances therapeutic effects of intra-arterial transplantation of mesenchymal stem cells into the brain after traumatic brain injury. Neurosci. Lett. 2013, 554, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Wang, F.; Qu, L.; Li, Z.; Jin, L.; Liu, P.; Xu, X.; Cui, H. The distribution and expression of claudin-5 and occludin at the rat blood–optic nerve barrier after borneol treatment. Mol. Biol. Rep. 2011, 38, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Cao, L.; Ge, H.; Duanmua, W.; Tan, L.; Yuan, J.; Tunan, C.; Li, F.; Hu, R.; Gao, F.; et al. L-Borneol induces transient opening of the blood-brain barrier and enhances the therapeutic effect of cisplatin. Neuroreport 2017, 28, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Ruan, M.; Dong, X.; Yua, Y.; Cheng, H. The mechanism of the opening of the blood–brain barrier by borneol: A pharmacodynamics and pharmacokinetics combination study. J. Ethnopharmacol. 2013, 150, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, R.; Yang, L.; Huang, T.; Xu, Q.; Mi, S.; Wang, N. Pharmacokinetics of natural borneol after oral administration in mice brain and its effect on excitation ratio. Eur. J. Drug Metab. Pharmacokinet. 2012, 37, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, L.; Qin, J.; Lu, W.; Wang, J. The use of borneol as an enhancer for targeting aprotinin-conjugated PEG-PLGA nanoparticles to the Brain. Pharm. Res. 2013, 30, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Jogani, V.; Jinturkar, K.; Jogani, V. Recent patents review on intranasal administration for CNS drug delivery. Recent Pat. Drug Deliv. Formul. 2008, 2, 25–40. [Google Scholar] [CrossRef]
- Hitchcock, S.A.; Pennington, L.D. Structure-brain exposure relationships. J. Med. Chem. 2006, 49, 7559–7583. [Google Scholar] [CrossRef] [PubMed]
- Wood, I.S.; Trayhurn, P. Glucose transporters (GLUT and SGLT): Expanded families of sugar transport proteins. Br. J. Nutr. 2007, 89, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Graff, C.L.; Pollack, G.M. Nasal Drug Administration: Potential for Targeted Central Nervous System Delivery. J. Pharm. Sci. 2005, 94, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Widera, A.; Norouziyan, F.; Shen, W.C. Mechanisms of TfR-mediated transcytosis and sorting in epithelial cells and applications toward drug delivery. Adv. Drug Deliv. Rev. 2003, 55, 1439–1466. [Google Scholar] [CrossRef] [PubMed]
- Weaver, M.; Laske, D.W. Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas. J. Neuro-Oncol. 2003, 65, 3–14. [Google Scholar] [CrossRef]
- Li, M.; Luo, Z.; Xia, Z.; Shen, X.K.; Cai, K.Y. Time-sequenced drug delivery approaches towards effective chemotherapeutic treatment of glioma. Mater. Horiz. 2017, 4, 977–996. [Google Scholar] [CrossRef]
- Abbott, N.J. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J. Inherit. Metab. Dis. 2013, 36, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Srivalli, K.M.; Lakshmi, P.K. Overview of P-glycoprotein inhibitors: A rational look. Braz. J. Pharm. Sci. 2012, 48, 353–367. [Google Scholar] [CrossRef]
- Amin, L. P-glycoprotein inhibition for the optimal drug delivery. Drug Target Insights 2013, 7, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Karch, R.; Zeitlinger, M.; Philippe, C.; Römermann, K.; Stanek, J.; Salamon, A.M.; Wadsak, W.; Jäger, W.; Hacker, M.; et al. Approaching complete inhibition of P-glycoprotein at the human blood–brain barrier: An (R)-[11C] verapamil PET study. J. Cereb. Blood Flow Metab. 2015, 35, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Tournier, N.; Goutal, S.; Auvity, S.; Trax, A.; Mairinger, S.; Wanek, T.; Helal, O.B.; Buvat, I.; Soussan, M.; Caillé1, F.; et al. Strategies to inhibit ABCB1-and ABCG2-mediated efflux transport of erlotinib at the blood-brain barrier: A PET study on nonhuman primates. J. Nucl. Med. 2017, 58, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, L.A.; Markandaiah, S.; Bonanno, S.; Pasinelli, P.; Trotti1, D. Blood-Brain Barrier Driven Pharmacoresistance in Amyotrophic Lateral Sclerosis and Challenges for Effective Drug Therapies. AAPS J. 2017, 19, 1600–1614. [Google Scholar] [CrossRef] [PubMed]
- Schinkel, A.H. P-Glycoprotein, a gatekeeper in the blood-brain barrier. Adv. Drug Deliv. Rev. 1999, 36, 179–194. [Google Scholar] [CrossRef]
- Schuster, B.S.; Ensign, L.M.; Allan, D.B.; Suk, J.S.; Hanes, J. Particle tracking in drug and gene delivery research: State-of-the-art applications and methods. Adv. Drug Deliv. Rev. 2015, 91, 70–91. [Google Scholar] [CrossRef] [PubMed]
- Hynynen, K.; McDannold, N.; Vykhodtseva, N.; Raymond, S.; Weissleder, R.; Jolesz, F.A.; Sheikov, N. Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: A method for molecular imaging and targeted drug delivery. J. Neurosurg. 2006, 105, 445–454. [Google Scholar] [CrossRef] [PubMed]
- McDannold, N.; Vykhodtseva, N.; Hynynen, K. Blood-brain barrier disruption induced by focused ultrasound and circulating preformed microbubbles appears to be characterized by the mechanical index. Ultrasound Med. Biol. 2008, 34, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yang, J.Z.; Andersen, R.; Nielsen, L.H. Lactoferrin-modified procationic liposomes as a novel drug carrier for brain delivery. J. Pharm. Sci. 2010, 402, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Namanja, H.A.; Emmert, D.; Davis, D.A.; Campos, C.; Miller, D.S.; Hrycyna, C.A.; Chmielewski, J. Toward eradicating HIV reservoirs in the brain: Inhibiting P-glycoprotein at the blood-brain barrier with prodrug abacavir dimers. J. Am. Chem. Soc. 2011, 134, 2976–2980. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Bodor, N.; Wu, W.-M.; Prokai, L. Strategies to target kyotorphin analogues to the brain. J. Med. Chem. 1998, 41, 3773–3781. [Google Scholar] [CrossRef] [PubMed]
- Sheha, M.; Al-Tayeb, A.; El-Sherief, H.; Farag, H. New carrier for specific delivery of drugs to the brain. Bioorgan. Med. Chem. 2003, 11, 1865–1872. [Google Scholar] [CrossRef]
- Huwyler, J.; Wu, D.; Pardridge, W.M. Brain drug delivery of small molecules using immunoliposomes. Proc. Natl. Acad. Sci. USA 1996, 93, 14164–14169. [Google Scholar] [CrossRef] [PubMed]
- Malam, Y.; Loizidou, M.; Seifalian, A.M. Liposomes and nanoparticles: Nanosized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009, 30, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Lu, W.; Gao, H.; Hu, K.; Chen, J.; Zhang, C.; Gao, X.; Jiang, X.; Zhu, C. Preparation and brain delivery property of biodegradable polymersomes conjugated with OX26. J. Control. Release 2008, 128, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Fundarò, A.; Cavalli, R.; Bargoni, A.; Vighetto, D.; Zara, G.P.; Gasco, M.R. Non-stealth and stealth solid lipid nanoparticles (SLN) carrying doxorubicin: Pharmacokinetics and tissue distribution after iv administration to rats. Pharm. Res. 2000, 42, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Lu, L.F.; Cai, Y.; Zhu, J.B.; Liang, B.W.; Yang, C.Z. Body distribution in mice of intravenously injected camptothecin solid lipid nanoparticles and targeting effect on brain. J. Control. Release 1999, 59, 299–307. [Google Scholar] [CrossRef]
- Neves-Coelho, S.; Eleutério, R.; Enguita, F.; Neves, V.; Castanho, M. A New Noncanonical Anionic Peptide That Translocates a Cellular Blood-Brain Barrier Model. Molecules 2017, 22, 1753. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Samanta, M.K.; Santhi, K.; Kumar, K.P.; Paramakrishnan, N.; Suresh, B. Poly (n-butylcyanoacrylate) nanoparticles coated with polysorbate 80 for the targeted delivery of rivastigmine into the brain to treat Alzheimer’s disease. Brain Res. 2008, 1200, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Szarmach, A.; Halena, G.; Kaszubowski, M.; Piskunowicz, M.; Studniarek, M.; Lass, P.; Szurowska, E.; Winklewski, P. Carotid Artery Stenting and Blood-Brain Barrier Permeability in Subjects with Chronic Carotid Artery Stenosis. Int. J. Mol. Sci. 2017, 18, 1008. [Google Scholar] [CrossRef] [PubMed]
- Sancini, G.; Gregori, M.; Salvati, E.; Cambianica, I.; Re, F.; Ornaghi, F.; Canovi, M.; Fracasso, C.; Cagnotto, A.; Colombo, M.; et al. Functionalization with TAT-peptide enhances blood-brain barrier crossing in vitro of nanoliposomes carrying a curcumin-derivative to bind amyloid-β peptide. J. Nanomed. Nanotechnol. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Al-Jamal, W.T.; Kostarelos, K. Liposomes: From a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Acc. Chem. Res. 2011, 44, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, P.J.; Appeldoorn, C.C.; Rip, J.; Dorland, R.; van der Pol, S.M.; Kooij, G.; de Vries, H.E.; Reijerkerk, A. Enhanced brain delivery of liposomal methylprednisolone improved therapeutic efficacy in a model of neuroinflammation. J. Control. Release 2012, 164, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, K.B.; Moos, T. Revisiting nanoparticle technology for blood-brain barrier transport: Unfolding at the endothelial gate improves the fate of transferrin receptor-targeted liposomes. J. Control. Release 2016, 222, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.D.; Ehlers, M.D. Breaching the blood-brain barrier for drug delivery. Neuron 2014, 81, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tang, L.; Qin, Y.; Yin, Y.; Tang, J.; Tang, W.; Sun, X.; Zhang, Z.; Liu, J.; He, Q. Lactoferrin-modified procationic liposomes as a novel drug carrier for brain delivery. Eur. J. Pharm. Sci. 2010, 40, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Norsted, E.; Gömüç, B.; Meister, B. Protein components of the blood–brain barrier (BBB) in the mediobasal hypothalamus. J. Chem. Neurosci. 2008, 36, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Negishi, Y.; Yamane, M.; Kurihara, N.; Endo-Takahashi, Y.; Sashida, S.; Takagi, N.; Suzuki, R.; Maruyama, K. Enhancement of Blood-Brain Barrier Permeability and Delivery of Antisense Oligonucleotides or Plasmid DNA to the Brain by the Combination of Bubble Liposomes and High-Intensity Focused Ultrasound. Pharmaceutics 2015, 7, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Fu, C.; Feng, Y.; Ji, Y.; Tao, L.; Huang, Q.; Li, X.; Wei, Y. PEGylation and polyPEGylation of nanodiamond. Polymer 2012, 53, 3178–3184. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Parveen, A.; Ibrahim, M. Antibody-drug conjugates as drug carrier systems for bioactive agents. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 1–10. [Google Scholar] [CrossRef]
- Kumari, A.; adav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloid Surf. 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Des, R.A.; Fievez, V.; Garinot, M.; Schneider, Y.J.; Préat, V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release 2006, 116, 1–27. [Google Scholar]
- Danhier, F.; Ansorena, E.; Silva, J.R.; Coco, R.; Breton, A.L.; Préat, V.V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Gao, X.; Gu, G. Penetratin-functionalized PEG–PLA nanoparticles for brain drug delivery. Int. J. Pharm. 2012, 436, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Karanth, H.; Murthy, R.S.R. Nanotechnology in Brain Targeting. Int. J. Pharm. Sci. Nanotechnol. 2008, 1, 10–24. [Google Scholar]
- Wohlfart, S.; Khalansky, A.S.; Gelperina, S. Efficient Chemotherapy of Rat Glioblastoma Using Doxorubicin-Loaded PLGA Nanoparticles with Different Stabilizers. PLoS ONE 2011, 6, 19121. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Sakurai, Y.; Kajimoto, K.; Nakamura, T.; Yamada, Y.; Akita, H.; Harashima, H. Innovative Technologies in Nanomedicines: From Passive Targeting to Active Targeting/From Controlled Pharmacokinetics to Controlled Intracellular Pharmacokinetics. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ng, W.K.; Kanaujia, P.; Kim, S.; Tan, R.B. Formulation design, preparation and physicochemical characterizations of solid lipid nanoparticles containing a hydrophobic drug: Effects of process variables. Colloid Surf. B 2011, 88, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Wissing, S.; Kayser, O.; Müller, R. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhu, J.; Lu, Y.; Liang, B.; Yang, C. Body Distribution of Camptothecin Solid Lipid Nanoparticles after Oral Administration. Pharm Res. 1999, 16, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Koziara, J.M.; Lockman, P.R.; Allen, D.D.; Mumper, R.J. Paclitaxel nanoparticles for the potential treatment of brain tumors. J. Control. Release 2004, 99, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Shevtsov, M.A.; Nikolaev, B.P.; Yakovleva, L.Y. Superparamagnetic iron oxide nanoparticles conjugated with epidermal growth factor (SPION-EGF) for targeting brain tumors. Int. J. Nanomed. 2014, 9, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, G.G.; Rizvi, S.A.; Souza, M.J.; Do, D.P. Formulation and evaluation of drug-loaded targeted magnetic microspheres for cancer therapy. Int. J. Nanomed. 2013, 8, 1393–1402. [Google Scholar]
- Kong, S.D.; Lee, J.; Ramachandran, S.; Eliceiri, B.P.; Shubayev, V.I.; Lal, R.; Jin, S. Magnetic targeting of nanoparticles across the intact blood-brain barrier. J. Control. Release 2012, 164, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, S.; Liao, Z.; Wang, C.; Liu, Y.; Feng, S.; Jiang, X.; Chang, J. PEG lated magnetic polymeric liposome anchored with TAT for delivery of drugs across the blood-spinal cord barrier. Biomaterials 2010, 31, 6589–6596. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Jia, Q.; Huwel, S.; Xia, R.; Liu, T.; Gao, F.; Galla, H.J.; Gao, M. Receptor-Mediated Delivery of Magnetic Nanoparticles across the Blood-Brain Barrier. ACS Nano 2012, 6, 3304–3310. [Google Scholar] [CrossRef] [PubMed]
- Kim, H. Effects of Biophysical Parameters in Radiosensitizing Prostate Tumours with Ultrasound-stimulated Microbubbles. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 2013. [Google Scholar]
- Loureiro, J.A.; Andrade, S.; Duarte, A.; Neves, A.R.; Queiroz, J.F.; Nunes, C.; Sevin, E.; Fenart, L.; Gosselet, F.; Coelho, M.A.; et al. Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer’s disease. Molecules 2017, 22, 277. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yang, J.Z.; Andersen, R.; Nielsen, L.H.; Borchardt, R.T. Evaluation of the Permeation Characteristics of a Model Opioid Peptide, H-Tyr-d-Ala-Gly-Phe-d-Leu-OH (DADLE), and Its Cyclic Prodrugs across the Blood-Brain Barrier Using an In Situ Perfused Rat Brain Model. J. Pharmacol. Exp. Ther. 2002, 303, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Kooti, M.; Sedeh, A.N.; Motamedi, H.; Rezatofighi, S.E. Magnetic graphene oxide inlaid with silver nanoparticles as antibacterial and drug delivery composite. Appl. Microbiol. Biothnol. 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liang, J.; Liu, X.; Tuo, D.; Li, W. Chemically Surface Tunable Solubility Parameter for Controllable Drug Delivery—An Example and Perspective from Hollow PAA-Coated Magnetite Nanoparticles with R6G Model Drug. Materials 2018, 11, 247. [Google Scholar] [CrossRef] [PubMed]
- D’Agata, F.; Ruffinatti, F.; Boschi, S.; Stura, I.; Rainero, I.; Abollino, O.; Cavalli, R.; Guiot, C. Magnetic Nanoparticles in the Central Nervous System: Targeting Principles, Applications and Safety Issues. Molecules 2018, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Lembo, D.; Donalisio, M.; Civra, A.; Argenziano, M.; Cavalli, R. Nanomedicine formulations for the delivery of antiviral drugs: A promising solution for the treatment of viral infections. Expert Opin. Drug Deliv. 2018, 15, 93–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Hua, M.Y.; Yang, H.W.; Huang, C.Y.; Chu, P.C.; Wu, J.S.; Tseng, I.C.; Wang, J.J.; Yen, T.C.; Chen, P.Y. Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc. Natl. Acad. Sci. USA 2010, 10, 15205–15210. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, V.N.; Nguyen, D.T.; Kodibagkar, V.D.; Stabenfeldt, S.E. Nanoparticle-Based Therapeutics for Brain Injury. Adv. Health Mater. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liang, J.; Liu, X.; Ding, Z.; Tuo, D.; Li, W. Sodium Acetate Orientated Hollow/Mesoporous Magnetite Nanoparticles: Facile Synthesis, Characterization and Formation Mechanism. Appl. Sci. 2018, 8, 292. [Google Scholar] [CrossRef]
- Zou, L.; Ma, J.; Wang, T.; Yang, T.; Liu, C. Cell-Penetrating Peptide-Mediated Therapeutic Molecule Delivery into the Central Nervous System. Curr. Neuropharmacol. 2013, 11, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Hanada, S.; Fujioka, K.; Inoue, Y.; Kanaya, F.; Manome, Y.; Yamamoto, K. Cell-Based in Vitro Blood-Brain Barrier Model Can Rapidly Evaluate Nanoparticles’ Brain Permeability in Association with Particle Size and Surface Modification. Int. J. Mol. Sci. 2014, 15, 1812–1825. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R. Drug Delivery Systems, CNS Protection, and the Blood Brain Barrier. BioMed Res. Int. 2014, 2014, 869269. [Google Scholar] [CrossRef] [PubMed]
- Ambikanandan, M.; Ganesh, S.; Aliasgar, S. Drug delivery to the central nervous system: A review. J. Pharm. Pharm. Sci. 2003, 6, 252–273. [Google Scholar]
- Alyautdin, R.; Khalin, I.; Nafeeza, M.; Haron, M.; Kuznetsov, D. Nanoscale drug delivery systems and the blood-brain barrier. In. J. Nanomed. 2014, 9, 795–811. [Google Scholar]
- Vlieghe, P.; Khrestchatisky, M. Peptide-based vectors for blood-brain barrier targeting and delivery of drugs to the central nervous system. Ther. Deliv. 2010, 1, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Zankel, T.; Starr, C.M.; Gabathuler, R. Methods of Increasing Delivery of Active Agents to Brain Comprising Administering Receptor Associated Protein (RAP) Fragments Conjugated to Active Agents. U.S. Patent 7,560,431, 4 August 2009. [Google Scholar]
- Drappatz, J.; Brenner, A.J.; Rosenfeld, S.; Groves, M.; Mikkelsen, T.; Schiff, D.; Sarantopoulos, J.; Wong, E.; Wen, P.Y.; Castaigne, J. ANG1005: Results of a phase I study in patients with recurrent malignant glioma. J. Clin. Oncol. 2010, 28, 2009. [Google Scholar] [CrossRef]
- Gaillard, P.J.; Visser, C.C.; Appeldoorn, C.C.M.; Rip, J. Enhanced brain drug delivery: Safely crossing the blood-brain barrier. Drug Discov. Today Technol. 2012, 9, e155–e160. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.H. Core Concept: Circumventing the blood-brain barrier. Proc. Natl. Acad. Sci. USA 2017, 114, 11261–11263. [Google Scholar] [CrossRef] [PubMed]
- Azad, T.D.; Pan, J.; Connolly, I.D.; Remington, A.; Wilson, C.M.; Grant, G.A. Therapeutic strategies to improve drug delivery across the blood-brain barrier. Neurosurg. Focus 2015, 38, E9. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Salvador, E.; Pastorin, G.; Forster, C. Blood-brain barrier transport studies, aggregation, and molecular dynamics simulation of multiwalled carbon nanotube functionalized with fluorescein isothiocyanate. Int. J. Nanomed. 2015, 10, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Forster, C. In silico predictive model to determine vector-mediated transport properties for the blood-brain barrier choline transporter. Adv. Appl. Bioinform. Chem. 2014, 7, 23–36. [Google Scholar] [CrossRef] [PubMed]
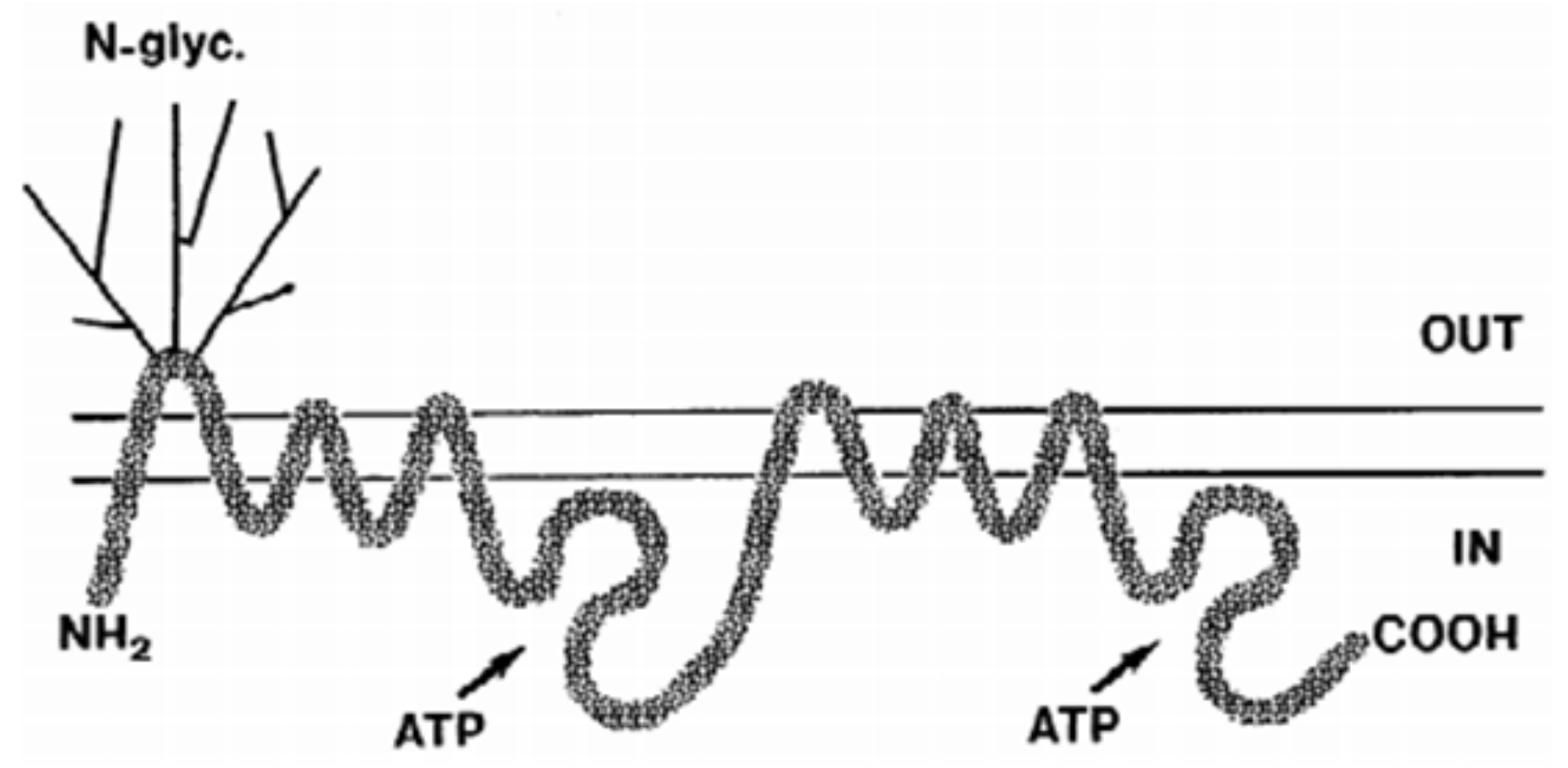
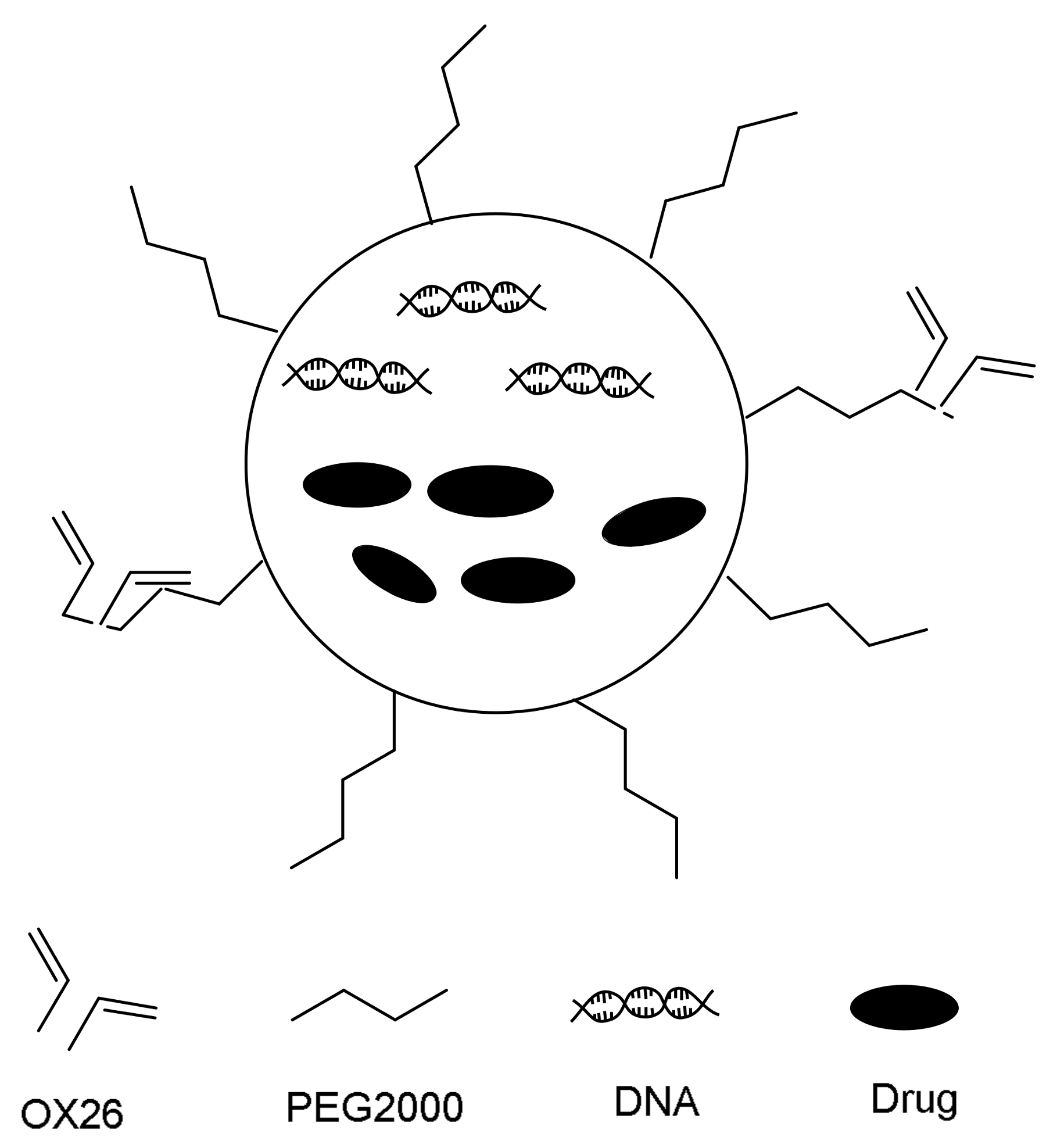
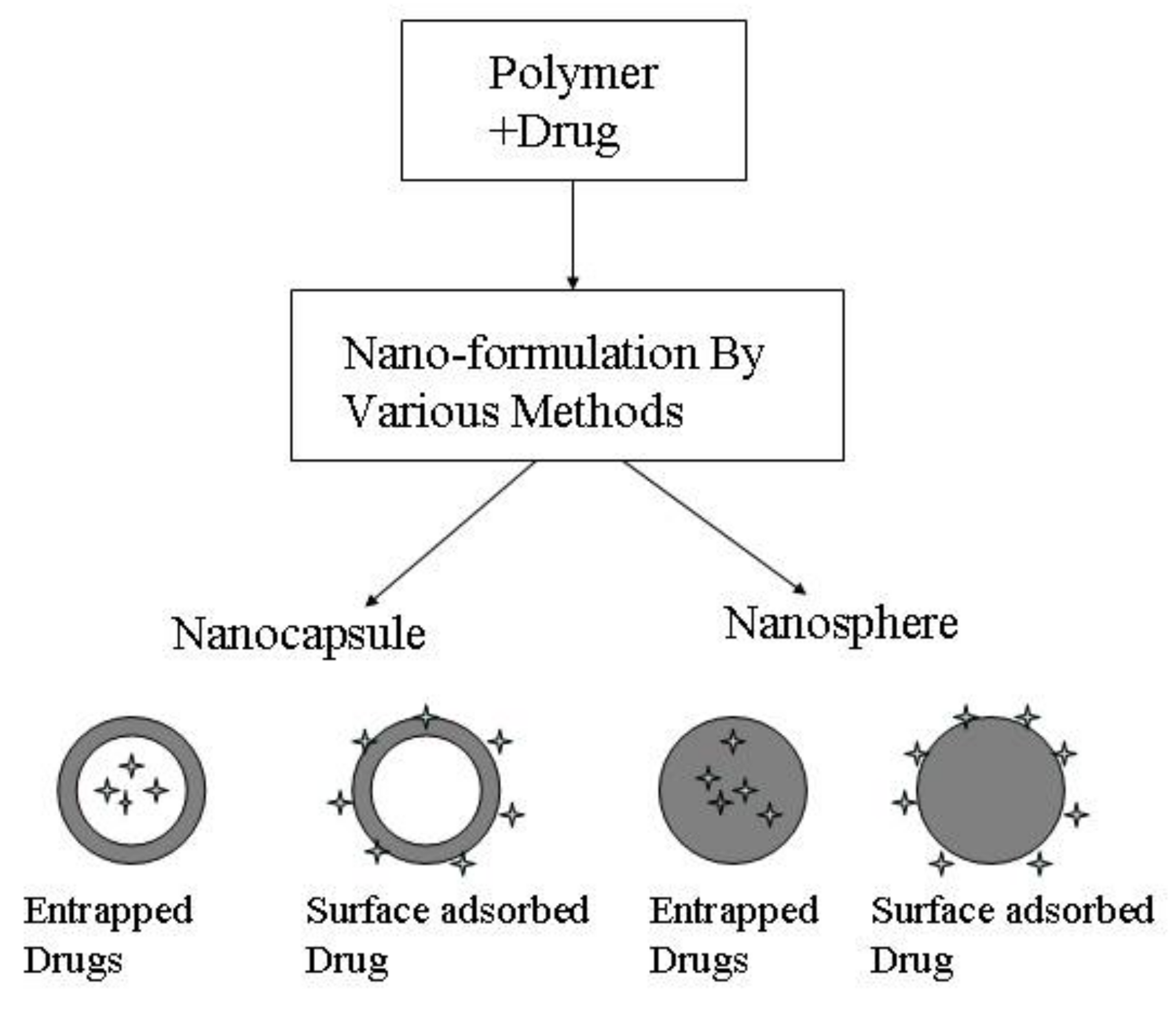
| Method | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Direct injection | High local drug concentrations can be achieved; systemic administration avoided | Side effects; hard to control; and repeat | [14,15,16] |
| Transnasal delivery | Noninvasive; easy to operate and repeat; low risk | Smaller drug delivery volume; interindividual difference | [17,18,19,20] |
| Arterial injection of osmotic solution | High drug concentrations achieved; large clinical experience | Requires general anesthesia; side effects; hard to repeat | [21,22] |
| Lipidation of small molecule drug | Easy to operate; delivered to the whole brain | Applies to easily etherified drugs | [23,24,25] |
| Parameter | Effect on BBB Disruption |
|---|---|
| Pressure amplitude | Increase in BBB disruption magnitude as pressure amplitude increases; saturation at some point |
| Ultrasound frequency | Decrease in BBB disruption threshold as frequency decreases; some evidence of improved safety for lower frequencies |
| Burst length | For burst lengths, less than 10 ms, BBB disruption threshold increases and BBB disruption magnitude decreases as burst length is reduced |
| Pulse repetition frequency | BBB disruption magnitude increases as repetition frequency increases up to a point. |
| Ultrasound contrast agent dose | Magnitude of BBB disruption increases with dose |
| Sonication duration | Longer durations or repeated sonication increase magnitude of BBB disruption |
| Microbubble diameter | Disruption magnitude increased with larger microbubbles |
| Drug | Structure | The Method of Across the BBB | Function | Reference |
|---|---|---|---|---|
| cis-Dichlorodiamineplatinum |  | Ultrasound | alkylating agent, Antitumor Effect | [53,54] |
| Barbital |  | Lipophilic prodrug | Sedative-hypnotics, sedative-hypnotic, antiepileptic and anticonvulsant effect | [55,56] |
| 5-fluorouracil |  | Chemical delivery systems | Antineoplastic | [57,58] |
| Adriamycin |  | Liposomes | Antitumor antibiotics | [59,60,61] |
| Idebenone |  | Sold liposome nanoparticles | Anti-senile dementia drug and mental symptoms drug | [62,63] |
| Saquinavir |  | Nanocarriers | Antivirals. For selective HIV protease inhibitors. | [64] |
| Rivastigmine |  | Nanocarriers | Acetylcholinesterase inhibitor | [65] |
| Idebenone, IDBN |  | Nanocarriers | Alzheimer’s disease and cognitive defects | [66] |
| Curcumin |  | Liposomes | Antineoplastic | [67] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Liu, J.; Liang, J.; Liu, X.; Li, W.; Liu, Z.; Ding, Z.; Tuo, D. Towards Improvements for Penetrating the Blood–Brain Barrier—Recent Progress from a Material and Pharmaceutical Perspective. Cells 2018, 7, 24. https://doi.org/10.3390/cells7040024
He Q, Liu J, Liang J, Liu X, Li W, Liu Z, Ding Z, Tuo D. Towards Improvements for Penetrating the Blood–Brain Barrier—Recent Progress from a Material and Pharmaceutical Perspective. Cells. 2018; 7(4):24. https://doi.org/10.3390/cells7040024
Chicago/Turabian StyleHe, Quanguo, Jun Liu, Jing Liang, Xiaopeng Liu, Wen Li, Zhi Liu, Ziyu Ding, and Du Tuo. 2018. "Towards Improvements for Penetrating the Blood–Brain Barrier—Recent Progress from a Material and Pharmaceutical Perspective" Cells 7, no. 4: 24. https://doi.org/10.3390/cells7040024





