Inducible Nitric Oxide Regulates Brush Border Membrane Na-Glucose Co-transport, but Not Na:H Exchange via p38 MAP Kinase in Intestinal Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Culture and Drug Treatment
2.2. Na-Glucose Cotransport in IEC-18 Cells
2.3. Na+/H+ Exchange in IEC-18 Cells
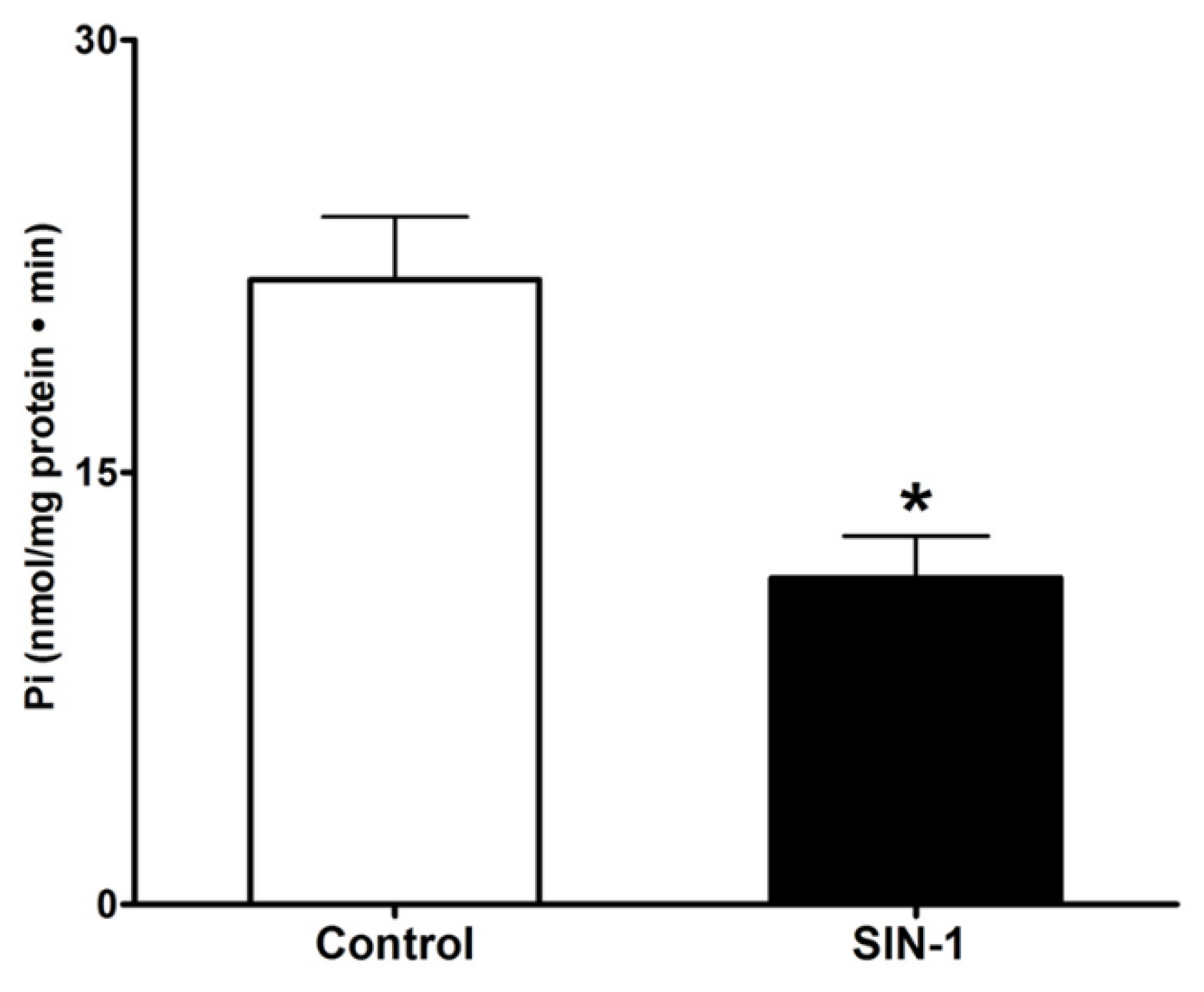
2.4. Na+/K+-ATPase Assay
2.5. Peroxynitrite Measurement
2.6. Western Blot
2.7. Immunocytochemistry
2.8. Immunoprecipitation
2.9. Data Presentation
3. Results
3.1. Effect of OONO on SGLT1 Activity in IEC-18 Cells
3.2. Effect of OONO on Na+/H+ Exchange IEC-18 Cells
3.3. Effect of OONO on Na+/K+-ATPase Activity in IEC-18 Cells
3.4. Uric Acid Prevents Effect of OONO on SGLT1 Activity
3.5. Measurement of OONO
3.6. Kinetic Studies for SGLT1 Activity
3.7. SGLT1 Protein Expression
3.8. p38 MAPK Signaling
3.9. 3-Nitrotyrosine Formation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, X.Z.; Coady, M.J.; Jackson, F.; Berteloot, A.; Lapointe, J.Y. Thermodynamic Determination of the Na+: Glucose Coupling Ratio for the Human Sglt1 Cotransporter. Biophys. J. 1995, 69, 2405–2414. [Google Scholar] [CrossRef]
- Loo, D.D.; Zeuthen, T.; Chandy, G.; Wright, E.M. Cotransport of Water by the Na+/Glucose Cotransporter. Proc. Natl. Acad. Sci. USA 1996, 93, 13367–13370. [Google Scholar] [CrossRef] [PubMed]
- Hirschhorn, N.; Kinzie, J.L.; Sachar, D.B.; Northrup, R.S.; Taylor, J.O.; Ahmad, S.Z.; Phillips, R.A. Decrease in Net Stool Output in Cholera During Intestinal Perfusion with Glucose-Containing Solutions. N. Engl. J. Med. 1968, 279, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Binder, H.J.; Ptak, T. Jejunal Absorption of Water and Electrolytes in Inflammatory Bowel Disease. J. Lab. Clin. Med. 1970, 76, 915–924. [Google Scholar] [PubMed]
- Field, M. Intestinal Ion Transport and the Pathophysiology of Diarrhea. J. Clin. Invest. 2003, 111, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Seidler, U.; Lenzen, H.; Cinar, A.; Tessema, T.; Bleich, A.; Riederer, B. Molecular Mechanisms of Disturbed Electrolyte Transport in Intestinal Inflammation. Ann. NY Acad. Sci. 2006, 1072, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, C.M. Mechanisms of Diarrhea. Curr. Gastroenterol. Rep. 2010, 12, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, U.; West, A.B. Effect of Chronic Inflammation on Electrolyte Transport in Rabbit Ileal Villus and Crypt Cells. Am. J. Physiol. 1997, 272, G732–G741. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, U.; Wisel, S.; Rajendren, V.M.; West, A.B. Mechanism of Inhibition of Na+-Glucose Cotransport in the Chronically Inflamed Rabbit Ileum. Am. J. Physiol. 1997, 273, G913–G919. [Google Scholar] [CrossRef] [PubMed]
- Coon, S.; Sundaram, U. Unique Regulation of Anion/Hco3- Exchangers by Constitutive Nitric Oxide in Rabbit Small Intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G1084–G1090. [Google Scholar] [CrossRef] [PubMed]
- Banan, A.; Fields, J.Z.; Zhang, Y.; Keshavarzian, A. Inos Upregulation Mediates Oxidant-Induced Disruption of F-Actin and Barrier of Intestinal Monolayers. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G1234–G1246. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, D.M. Peroxynitrite and Inflammatory Bowel Disease. Gut 2000, 46, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Rachmilewitz, D.; Stamler, J.S.; Bachwich, D.; Karmeli, F.; Ackerman, Z.; Podolsky, D.K. Enhanced Colonic Nitric Oxide Generation and Nitric Oxide Synthase Activity in Ulcerative Colitis and Crohn's Disease. Gut 1995, 36, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Koppenol, W.H. Nitric Oxide, Superoxide, and Peroxynitrite: The Good, the Bad, and Ugly. Am. J. Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, G.; Moshage, H.; van Dullemen, H.M.; de Jager-Krikken, A.; Tiebosch, A.T.; Kleibeuker, J.H.; Jansen, P.L.; van Goor, H. Expression of Nitric Oxide Synthases and Formation of Nitrotyrosine and Reactive Oxygen Species in Inflammatory Bowel Disease. J. Pathol. 1998, 186, 416–421. [Google Scholar] [CrossRef]
- Kimura, H.; Hokari, R.; Miura, S.; Shigematsu, T.; Hirokawa, M.; Akiba, Y.; Kurose, I.; Higuchi, H.; Fujimori, H.; Tsuzuki, Y.; et al. Increased Expression of an Inducible Isoform of Nitric Oxide Synthase and the Formation of Peroxynitrite in Colonic Mucosa of Patients with Active Ulcerative Colitis. Gut 1998, 42, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S.S.; Mastropaolo, L.A.; Murchie, R.; Griffiths, C.; Thoni, C.; Elkadri, A.; Xu, W.; Mack, A.; Walters, T.; Guo, C.; et al. Higher Activity of the Inducible Nitric Oxide Synthase Contributes to Very Early Onset Inflammatory Bowel Disease. Clin. Transl. Gastroenterol. 2014, 5, e46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.J.; Thompson, J.H.; Zhang, X.J.; Sadowska-Krowicka, H.; Kakkis, J.L.; Munshi, M.; Sandoval, U.K.; Rossi, J.L.; Eloby-Childress, S.; Beckman, J.S.; et al. Role of Inducible Nitric Oxide Synthase Expression and Peroxynitrite Formation in Guinea Pig Ileitis. Gastroenterology 1995, 5, 1475–1483. [Google Scholar] [CrossRef]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent Hydroxyl Radical Production by Peroxynitrite: Implications for Endothelial Injury from Nitric Oxide and Superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Crow, J.P. Dichlorodihydrofluorescein and Dihydrorhodamine 123 Are Sensitive Indicators of Peroxynitrite in Vitro: Implications for Intracellular Measurement of Reactive Nitrogen and Oxygen Species. Nitric Oxide 1997, 1, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Martin-Romero, F.J.; Gutierrez-Martin, Y.; Henao, F.; Gutierrez-Merino, C. Fluorescence Measurements of Steady State Peroxynitrite Production Upon Sin-1 Decomposition: Nadh Versus Dihydrodichlorofluorescein and Dihydrorhodamine 123. J. Fluoresc. 2004, 14, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Shacka, J.J.; Sahawneh, M.A.; Gonzalez, J.D.; Ye, Y.Z.; D'Alessandro, T.L.; Estevez, A.G. Two Distinct Signaling Pathways Regulate Peroxynitrite-Induced Apoptosis in Pc12 Cells. Cell Death Differ. 2006, 13, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Van Den Blink, B.; Ten Hove, T.; Van Den Brink, G.R.; Peppelenbosch, M.; Van Deventer, S.J. From Extracellular to Intracellular Targets, Inhibiting Map Kinases in Treatment of Crohn’s Disease. Ann. NY Acad. Sci. 2002, 973, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Hommes, D.; van den Blink, B.; Plasse, T.; Bartelsman, J.; Xu, C.; Macpherson, B.; Tytgat, G.; Peppelenbosch, M.; Van Deventer, S. Inhibition of Stress-Activated Map Kinases Induces Clinical Improvement in Moderate to Severe Crohn's Disease. Gastroenterology 2002, 122, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Forbush, B. Assay of Na,K-Atpase in Plasma Membrane Preparations: Increasing the Permeability of Membrane Vesicles Using Sodium Dodecyl Sulfate Buffered with Bovine Serum Albumin. Anal. Biochem. 1983, 128, 159–163. [Google Scholar] [CrossRef]
- Coon, S.; Kim, J.; Shao, G.; Sundaram, U. Na-Glucose and Na-Neutral Amino Acid Cotransport Are Uniquely Regulated by Constitutive Nitric Oxide in Rabbit Small Intestinal Villus Cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G1030–G1035. [Google Scholar] [CrossRef] [PubMed]
- Knickelbein, R.; Aronson, P.S.; Schron, C.M.; Seifter, J.; Dobbins, J.W. Sodium and Chloride Transport across Rabbit Ileal Brush Border. Ii. Evidence for Cl-Hco3 Exchange and Mechanism of Coupling. Am. J. Physiol. 1985, 249, G236–G245. [Google Scholar] [CrossRef] [PubMed]
- Coon, S.; Kekuda, R.; Saha, P.; Sundaram, U. Reciprocal Regulation of the Primary Sodium Absorptive Pathways in Rat Intestinal Epithelial Cells. Am. J. Physiol. Cell Physiol. 2011, 300, C496–C505. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, P.; Coon, S.; Baseler, W.; Sundaram, S.; Kekuda, R.; Sundaram, U. Prostaglandins, Not the Leukotrienes, Regulate Cl(-)/HCO(3)(-) Exchange (Dra, Slc26a3) in Villus Cells in the Chronically Inflamed Rabbit Ileum. Biochim. Biophys. Acta 2013, 1828, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Manoharan, P.; Arthur, S.; Sundaram, S.; Kekuda, R.; Sundaram, U. Molecular Mechanism of Regulation of Villus Cell Na-K-Atpase in the Chronically Inflamed Mammalian Small Intestine. Biochim. Biophys. Acta 2015, 1848, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Arthur, S.; Sundaram, U. Unique Regulation of Na-Glutamine Cotransporter Sn2/Snat5 in Rabbit Intestinal Crypt Cells During Chronic Enteritis. J. Cell Mol. Med. 2018, 22, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Arthur, S.; Saha, P.; Sundaram, S.; Kekuda, R.; Sundaram, U. Regulation of Sodium-Glutamine Cotransport in Villus and Crypt Cells by Glucocorticoids During Chronic Enteritis. Inflamm. Bowel Dis. 2012, 18, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Singer, I.I.; Kawka, D.W.; Scott, S.; Weidner, J.R.; Mumford, R.A.; Riehl, T.E.; Stenson, W.F. Expression of Inducible Nitric Oxide Synthase and Nitrotyrosine in Colonic Epithelium in Inflammatory Bowel Disease. Gastroenterology 1996, 111, 871–885. [Google Scholar] [CrossRef]
- Zingarelli, B.; Szabo, C.; Salzman, A.L. Reduced Oxidative and Nitrosative Damage in Murine Experimental Colitis in the Absence of Inducible Nitric Oxide Synthase. Gut 1999, 45, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Reifenberger, M.S.; Arnett, K.L.; Gatto, C.; Milanick, M.A. The Reactive Nitrogen Species Peroxynitrite Is a Potent Inhibitor of Renal Na-K-Atpase Activity. Am. J. Physiol. Renal. Physiol. 2008, 295, F1191–F1198. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C.; Scott, G.S.; Zborek, A.; Mikheeva, T.; Kean, R.B.; Koprowski, H.; Spitsin, S.V. Uric Acid, a Peroxynitrite Scavenger, Inhibits Cns Inflammation, Blood-Cns Barrier Permeability Changes, and Tissue Damage in a Mouse Model of Multiple Sclerosis. FASEB J. 2000, 14, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C.; Spitsin, S.; Kean, R.B.; Champion, J.M.; Dickson, G.M.; Chaudhry, I.; Koprowski, H. Uric Acid, a Natural Scavenger of Peroxynitrite, in Experimental Allergic Encephalomyelitis and Multiple Sclerosis. Proc. Natl. Acad. Sci. USA 1998, 95, 675–680. [Google Scholar] [CrossRef] [PubMed]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manoharan, P.; Sundaram, S.; Singh, S.; Sundaram, U. Inducible Nitric Oxide Regulates Brush Border Membrane Na-Glucose Co-transport, but Not Na:H Exchange via p38 MAP Kinase in Intestinal Epithelial Cells. Cells 2018, 7, 111. https://doi.org/10.3390/cells7080111
Manoharan P, Sundaram S, Singh S, Sundaram U. Inducible Nitric Oxide Regulates Brush Border Membrane Na-Glucose Co-transport, but Not Na:H Exchange via p38 MAP Kinase in Intestinal Epithelial Cells. Cells. 2018; 7(8):111. https://doi.org/10.3390/cells7080111
Chicago/Turabian StyleManoharan, Palanikumar, Shanmuga Sundaram, Soudamani Singh, and Uma Sundaram. 2018. "Inducible Nitric Oxide Regulates Brush Border Membrane Na-Glucose Co-transport, but Not Na:H Exchange via p38 MAP Kinase in Intestinal Epithelial Cells" Cells 7, no. 8: 111. https://doi.org/10.3390/cells7080111




