Cannabidiol Regulates the Expression of Keratinocyte Proteins Involved in the Inflammation Process through Transcriptional Regulation
Abstract
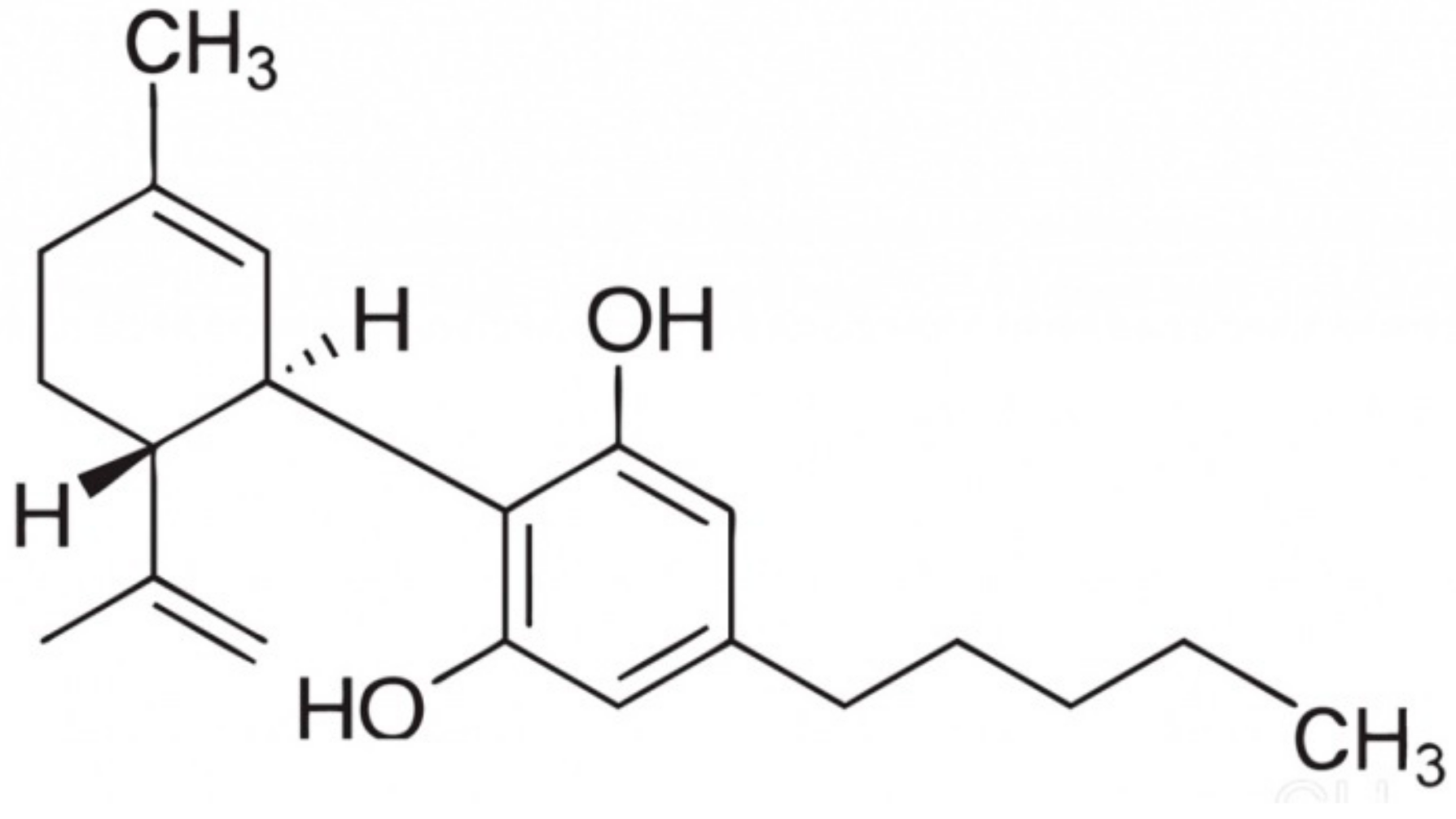
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Cell Viability
2.3. Determination of Superoxide Anion Generation
2.4. Determination of Antioxidant Enzyme Activity
2.5. Determination of Non-Enzymatic Antioxidant Level
2.6. Determination of Lipid Peroxidation Product
2.7. Determination Of Anti-Inflammatory Eicosanoid
2.8. Determination of Protein Expression
2.9. Determination of Keap1 Structure
2.10. Determination of Protein Localization
2.11. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mori, S.; Shiraishi, A.; Epplen, K.; Butcher, D.; Murase, D.; Yasuda, Y.; Murase, T. Characterization of skin function associated with obesity and specific correlation to local/systemic parameters in American women. Lipids Health Dis. 2017, 16, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Weng, Q.Y.; Fisher, D.E. UV signaling pathways within the skin. J. Investig. Dermatol. 2014, 134, 2080–2085. [Google Scholar] [CrossRef] [PubMed]
- Brash, D.E. UV signature mutations. Photochem. Photobiol. 2015, 91, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Skrzydlewska, E. The role of transcription factor Nrf2 in skin cells metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nita, M.; Grzybowski, A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Rojo De La Vega, M.; Krajisnik, A.; Zhang, D.; Wondrak, G. Targeting NRF2 for improved skin barrier function and photoprotection: Focus on the achiote-derived apocarotenoid bixin. Nutrients 2017, 9, 1371–1386. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Ma, Q. NRF2 cysteine residues are critical for oxidant/electrophile-sensing, Kelch-like ECH-associated protein-1-dependent ubiquitination-proteasomal degradation, and transcription activation. Mol. Pharmacol. 2009, 76, 1265–1278. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Nguyen, T.; Pickett, C.B. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc. Natl. Acad. Sci. USA 2000, 97, 12475–12480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Li, W.; Su, Z.Y.; Kong, A.T. The complexity of the Nrf2 pathway: Beyond the antioxidant response. J. Nutr. Biochem. 2015, 26, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Sova, M.; Saso, L. Design and development of Nrf2 modulators for cancer chemoprevention and therapy: A review. Drug Des. Devel. Ther. 2018, 12, 3181–3197. [Google Scholar] [CrossRef] [PubMed]
- Ligresti, A.; De Petrocellis, L.; Di Marzo, V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: Pleiotropic physiological and pathological roles through complex pharmacology. Physiol. Rev. 2016, 96, 1593–1659. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. 2018, 17, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Crippa, J.A.; Guimarães, F.S.; Campos, A.C.; Zuardi, A.W. Translational investigation of the therapeutic potential of cannabidiol (CBD): Toward a new age. Front. Immunol. 2018, 9, 2009–2024. [Google Scholar] [CrossRef] [PubMed]
- Fagherazzi, E.V.; Garcia, V.A.; Maurmann, N.; Bervanger, T.; Halmenschlager, L.H.; Busato, S.B.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Schröder, N. Memory-rescuing effects of cannabidiol in an animal model of cognitive impairment relevant to neurodegenerative disorders. Psychopharmacol. (Berl.) 2012, 219, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.S.; da Silva, A.B. Cannabidiol as an antioxidant. In Handbook of Cannabis and Related Pathologies, 1st ed.; Academic Press: San Diego, CA, USA, 2017; pp. 123–130. [Google Scholar]
- Blessing, E.M.; Steenkamp, M.M.; Manzanares, J.; Marmar, C.R. Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics 2015, 12, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.S.; Batista, J., Jr.; Viana, R.B.; Baetas, A.C.; Orestes, E.; Andrade, M.A.; Honório, K.M.; da Silva, A.B. Understanding the molecular aspects of tetrahydrocannabinol and cannabidiol as antioxidants. Molecules 2013, 18, 12663–12674. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Mukhopadhyay, P.; Rajesh, M.; Patel, V.; Mukhopadhyay, B.; Gao, B.; Haskó, G.; Pacher, P. Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J. Pharmacol. Exp. Ther. 2009, 328, 708–714. [Google Scholar] [CrossRef]
- Peres, F.F.; Lima, A.C.; Hallak, J.E.; Crippa, J.A.; Silva, R.H.; Abílio, V.C. Cannabidiol as a promising strategy to treat and prevent movement disorders? Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Liou, G.I.; Auchampach, J.A.; Hillard, C.J.; Zhu, G.; Yousufzai, B.; Mian, S.; Khan, S.; Khalifa, Y. Mediation of cannabidiol anti-inflammation in the retina by equilibrative nucleoside transporter and A2A adenosine receptor. Invest. Ophthalmol. Vis. Sci 2008, 49, 5526–5531. [Google Scholar] [CrossRef]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.; Koethe, D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2012, 2, e94. [Google Scholar] [CrossRef]
- Petrosino, S.; Verde, R.; Vaia, M.; Allarà, M.; Iuvone, T.; Marzo, V.D. Anti-inflammatory properties of cannabidiol, a non-psychotropic cannabinoid, in experimental allergic contact dermatitis. J. Pharmacol. Exp. Ther. 2018, 365, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Kumar, U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int. J. Mol. Sci. 2018, 19, 833–855. [Google Scholar]
- Laun, A.S.; Song, Z.H. GPR3 and GPR6, novel molecular targets for cannabidiol. Biochem. Biophys. Res. Commun. 2017, 490, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Mato, S.; Victoria Sánchez-Gómez, M.; Matute, C. Cannabidiol induces intracellular calcium elevation and cytotoxicity in oligodendrocytes. Glia 2010, 58, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Ferk, F.; Mišík, M.; Ropek, N.; Nersesyan, A.; Mejri, D.; Holzmann, K.; Lavorgna, M.; Isidori, M.; Knasmüller, S. Low doses of widely consumed cannabinoids (cannabidiol and cannabidivarin) cause DNA damage and chromosomal aberrations in human-derived cells. Arch. Toxicol. 2019, 93, 179–188. [Google Scholar] [CrossRef]
- Burstein, S. Cannabidiol (CBD) and its analogs: A review of their effects on inflammation. Bioorg. Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Pucci, M.; Rapino, C.; Di Francesco, A.; Dainese, E.; D’Addario, C.; Maccarrone, M. Epigenetic control of skin differentiation genes by phytocannabinoids. Br. J. Pharmacol. 2013, 170, 581–591. [Google Scholar] [CrossRef] [Green Version]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kuzkaya, N.; Weissmann, N.; Harrison, D.G.; Dikalov, S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: Implications for uncoupling endothelial nitricoxide synthase. J. Biol. Chem. 2003, 278, 22546–22554. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [PubMed]
- Sykes, J.A.; McCormac, F.X.; O’Breien, T.J. Preliminary study of the superoxide dismutase content of some human tumors. Cancer Res. 1978, 38, 2759–2762. [Google Scholar] [PubMed]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Mize, C.E.; Longdon, R.G. Hepatic glutathione reductase. Purification and general kinetic properties. J. Biol. Chem. 1962, 237, 1589–1595. [Google Scholar] [PubMed]
- Holmgren, A. Thioredoxin and thioredoxin reductase. Methods Enzymol. 1995, 252, 199–208. [Google Scholar] [PubMed]
- Maeso, N.; Garcia-Martinez, D.; Ruperez, F.J.; Cifuentes, A.; Barbas, C. Capillary electrophoresis of glutathione to monitor oxidative stress and response to antioxidant treatments in an animal model. J. Chromatogr. B. 2005, 822, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Xie, C.; Gabbita, S.P.; Markesbery, W.R. Decreased thioredoxin and increased thioredoxin reductase levels in Alzheimer’s disease brain. Free Radic. Biol. Med. 2000, 28, 418–427. [Google Scholar] [CrossRef]
- Liu, G.H.; Qu, J.; Shen, X. NF-κB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2008, 1783, 713–727. [Google Scholar] [CrossRef]
- Morgenstern, J.; Fleming, T.; Kadiyska, I.; Brings, S.; Groener, J.B.; Nawroth, P.; Hecker, M.; Brune, M. Sensitive mass spectrometric assay for determination of 15-deoxy-Δ12,14-prostaglandin J2 and its application in human plasma samples of patients with diabetes. Anal. Bioanal. Chem. 2018, 410, 521–528. [Google Scholar] [CrossRef]
- Eissa, S.; Seada, L.S. Quantitation of bcl-2 protein in bladder cancer tissue by enzyme immunoassay: Comparison with Western blot and immunohistochemistry. Clin. Chem. 1998, 44, 1423–1429. [Google Scholar]
- Gęgotek, A.; Domingues, P.; Skrzydlewska, E. Proteins involved in the antioxidant and inflammatory response in rutin-treated human skin fibroblasts exposed to UVA or UVB irradiation. J. Dermatol. Sci. 2018, 90, 241–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łuczaj, W.; Gęgotek, A.; Skrzydlewska, E. Antioxidants and HNE in redox homeostasis. Free Radic. Biol. Med. 2017, 111, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Chanda, D.; Neumann, D.; Glatz, J.F.C. The endocannabinoid system: Overview of an emerging multi-faceted therapeutic target, Prostaglandins Leukot. Essent. Fatty Acids 2019, 140, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Lipina, C.; Harinder, S. Hundal Modulation of cellular redox homeostasis by the endocannabinoid system. Open Biol. 2016, 6, 150276. [Google Scholar] [CrossRef]
- Ambrożewicz, E.; Wójcik, P.; Wroński, A.; Łuczaj, W.; Jastrząb, A.; Žarković, N.; Skrzydlewska, E. Pathophysiological alterations of redox signaling and endocannabinoid system in granulocytes and plasma of psoriatic patients. Cells 2018, 7, 159–176. [Google Scholar] [CrossRef]
- Crouzin, N.; de Jesus Ferreira, M.C.; Cohen-Solal, C.; M’Kadmi, C.; Bernad, N.; Martinez, J.; Guiramand, J. α-Tocopherol and α-tocopheryl phosphate interact with the cannabinoid system in the rodent hippocampus. Free Radic. Biol. Med. 2011, 51, 1643–1655. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Patel, V.; Saito, K.; Matsumoto, S.; Haskó, G. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 2115–2125. [Google Scholar] [CrossRef]
- Fouad, A.A.; Albuali, W.H.; Al-Mulhim, A.S.; Jresat, I. Cardioprotective effect of cannabidiol in rats exposed to doxorubicin toxicity. Environ. Toxicol. Pharmacol. 2013, 36, 347–357. [Google Scholar] [CrossRef]
- Romano, B.; Borrelli, F.; Pagano, E.; Cascio, M.G.; Pertwee, R.G.; Izzo, A.A. Inhibition of colon carcinogenesis by a standardized Cannabis sativa extract with high content of cannabidiol. Phytomedicine 2014, 21, 631–639. [Google Scholar] [CrossRef]
- Cebula, M.; Schmidt, E.E.; Arner, E.S. TrxR1 as a potent regulator of the Nrf2-Keap1 response system. Antioxid. Redox Signal. 2015, 23, 823–853. [Google Scholar] [CrossRef]
- Cassidy, P.B.; Edes, K.; Nelson, C.C.; Parsawar, K.; Fitzpatrick, F.A.; Moos, P.J. Thioredoxin reductase is required for the inactivation of tumor suppressor p53 and for apoptosis induced by endogenous electrophiles. Carcinogenesis 2006, 27, 2538–2549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasparovic, A.C.; Milkovic, L.; Sunjic, S.B.; Zarkovic, N. Cancer growth regulation by 4-hydroxynonenal. Free Radic. Biol. Med. 2017, 111, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Kostov, R.V.; Canning, P. Keap1, the cysteine-based mammalian intracellular sensor for electrophiles and oxidants. Arch. Biochem. Biophys. 2017, 617, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Telorack, M.; Meyer, M.; Ingold, I.; Conrad, M.; Bloch, W.; Werner, S.A. Glutathione-Nrf2-thioredoxin cross-talk ensures keratinocyte survival and efficient wound repair. PLoS Genet. 2016, 12, e1005800. [Google Scholar] [CrossRef] [PubMed]
- Alviz-Amador, A.; Galindo-Murillo, R.; Pineda-Alemán, R.; Pérez-González, H.; Rodríguez-Cavallo, E.; Vivas-Reyes, R.; Méndez-Cuadro, D. 4-HNE carbonylation induces local conformational changes on bovine serum albumin and thioredoxin. A molecular dynamics study. J. Mol. Graph. Model. 2019, 86, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Poet, G.J.; Oka, O.B.; Van Lith, M.; Cao, Z.; Robinson, P.J.; Pringle, M.A.; Bulleid, N.J. Cytosolic thioredoxin reductase 1 is required for correct disulfide formation in the ER. EMBO J. 2017, 36, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, A. Thioredoxin and redox signaling: Roles of the thioredoxin system in control of cell fate. Arch. Biochem. Biophys. 2017, 617, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Branco, V.; Carvalho, C. The thioredoxin system as a target for mercury compounds. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2018. S0304-4165. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, A.; Lu, J. Thioredoxin and thioredoxin reductase: Current research with special reference to human disease. Biochem. Biophys. Res. Commun. 2010, 396, 120–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Helwa, I.; Choudhary, V.; Chen, X.; Kaddour-Djebbar, I.; Bollag, W.B. Anti-psoriatic drug monomethylfumarate increases nuclear factor erythroid 2-related factor 2 levels and induces aquaporin-3 mRNA and protein expression. J. Pharmacol. Exp. Ther. 2017, 362, 243–253. [Google Scholar] [CrossRef] [PubMed]
- O’Mealey, G.B.; Plafker, K.S.; Berry, W.L.; Janknecht, R.; Chan, J.Y.; Plafker, S.M. A PGAM5–KEAP1–Nrf2 complex is required for stress-induced mitochondrial retrograde trafficking. J. Cell Sci. 2017, 130, 3467–3480. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Warabi, E.; Yanagawa, T.; Ma, D.; Itoh, K.; Ishii, Y.; Ishii, T. Essential role of Nrf2 in keratinocyte protection from UVA by quercetin. Biochem. Biophys. Res. Commun. 2009, 387, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Pae, H.O.; Lee, Y.C.; Chung, H.T. Heme oxygenase-1 and carbon monoxide: Emerging therapeutic targets in inflammation and allergy. Recent Pat. Inflamm. Allergy Drug Discov. 2008, 2, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, T.; Godlewski, G.; Cinar, R.; Bertola, A.; Szanda, G.; Liu, J.; Ju, C. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat. Med. 2013, 19, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Min, D.S.; Park, H.; Kim, H.P. Flavonoids interfere with NLRP3 inflammasome activation. Toxicol. Appl. Pharmacol. 2018, 355, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, D.W. Eicosanoids and the endogenous control of acute inflammatory resolution. Int. J. Biochem. Cell Biol. 2010, 42, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Kozela, E.; Pietr, M.; Juknat, A.; Rimmerman, N.; Levy, R.; Vogel, Z. Cannabinoids Δ9-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-κB and interferon-β/STAT proinflammatory pathways in BV-2 microglial cells. J. Biol. Chem. 2010, 285, 1616–1626. [Google Scholar] [CrossRef]
- Oh, J.Y.; Giles, N.; Landar, A.; Darley-Usmar, V. Accumulation of 15-deoxy-D12,14- prostaglandin J2 adductformation with Keap1 over time: Effects on potency for intracellular antioxidant defence induction. Biochem. J. 2008, 411, 297–306. [Google Scholar] [CrossRef]
- Pajaud, J.; Kumar, S.; Rauch, C.; Morel, F.; Aninat, C. Regulation of signal transduction by glutathione transferases. Int. J. Hepatol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Yamamoto, M. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Communi. 2016, 7, 11624–11637. [Google Scholar] [CrossRef] [PubMed]
- Stefanson, A.; Bakovic, M. Dietary regulation of Keap1/Nrf2/ARE pathway: Focus on plant-derived compounds and trace minerals. Nutrients 2014, 6, 3777–3801. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.D. Integrating cell-signalling pathways with NF-κB and IKK function. Nat. Rev. Mol. Cell Biol. 2007, 8, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; You, D.J.; Lee, C.; Ahn, C.; Seong, J.Y.; Hwang, J.I. Suppression of NF-κB signaling by KEAP1 regulation of IKKβ activity through autophagic degradation and inhibition of phosphorylation. Cell. Signal. 2010, 22, 1645–1654. [Google Scholar] [CrossRef]
- Chen, K.; Li, J.; Li, S.; Feng, J.; Wu, L.; Liu, T.; Zhou, S. 15d-PGJ2 alleviates ConA-induced acute liver injury in mice by up-regulating HO-1 and reducing hepatic cell autophagy. Biomed. Pharmacother. 2016, 80, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [Green Version]
- Ambrozova, N.; Ulrichova, J.; Galandakova, A. Models for the study of skin wound healing. The role of Nrf2 and NF-κB. Biomed. Pap. Med. Fac. Palacky. Olomouc. Czech. Repub. 2017, 161, 1–13. [Google Scholar] [CrossRef]






| Determined Parameter | Groups | |||||
|---|---|---|---|---|---|---|
| Control | CBD | UVA | UVA + CBD | UVB | UVB + CBD | |
| Superoxide anion (nmol/min/mg protein) | 90 ± 7 | 58 ± 5 x | 240 ± 12 x | 87 ± 9 a | 216 ± 11 x | 105 ± 9 x,a |
| Cu.Zn-SOD [mU/mg protein] | 28.0 ± 1.5 | 48.4 ± 3.1 x | 23.1 ± 1.4 x | 51.4 ± 2.9 x,a | 21.5 ± 1.3 x | 53.6 ± 3.1 x,a |
| GSH-Px [U/mg protein] | 14.6 ± 0.9 | 13.5 ± 1.2 | 34.4 ± 1.9 x | 22.8 ± 1.7 x,a | 37.5 ± 2.2 x | 26.5 ± 1.5 x,a |
| GSSG-R [mU/mg protein] | 14.3 ± 1.2 | 7.2 ± 0.4 x | 18.6 ± 0.9 x | 26.5 ± 1.8 x,a | 20.2 ± 1.0 | 14.7 ± 0.9 |
| GSH [nmol/mg protein] | 17.1 ± 0.8 | 11.7 ± 0.7 x | 11.5 ± 0.6 x | 10.3 ± 0.6 x,a | 10.9 ± 0.5 x | 9.3 ± 0.5 x,a |
| TxrR [U/mg protein] | 14.0 ± 0.7 | 17.2 ± 0.8 x | 10.2 ± 0.6 x | 16.1 ± 0.7 x,a | 8.2 ± 0.3 x | 15.4 ± 0.6 x,a |
| Trx [g/mg protein] | 1.63 ± 0.09 | 2.74 ± 0.17 x | 1.05 ± 0.05 x | 1.79 ± 0.11 x,a | 0.84 ± 0.04 x | 1.16 ± 0.06 x,a |
| Ref-1 [vs. control] | 1.00 ± 0.06 | 0.96 ± 0.04 | 3.10 ± 0.07 x | 1.60 ± 0.06 x,a | 3.10 ± 0.09 x | 1.70 ± 0.06 x,a |
| phospho-ASK-1 [vs. control] | 1.00 ± 0.04 | 1.10 ± 0.04 | 2.10 ± 0.07 x | 1.70 ± 0.06 x,a | 2.30 ± 0.09 x | 1.60 ± 0.06 x,a |
| 4-HNE [nmol/mg protein] | 26.1 ± 1.3 | 23.5 ± 1.4 | 52.4 ± 2.7 x | 35.6 ± 1.9 x,a | 73.4 ± 3.7 x | 64.7 ± 3.5 x,a |
| Determined Parameter | Groups | |||||
|---|---|---|---|---|---|---|
| Control | CBD | UVA | UVA + CBD | UVB | UVB + CBD | |
| Keap1 [vs. control] | 1.00 ± 0.05 | 0.79 ± 0.04 x | 0.81 ± 0.04 x | 0.63 ± 0.03 x,a | 0.78 ± 0.04 x | 0.47 ± 0.02 x,a |
| Inhibitors | ||||||
| 4-HNE [nmol/mg protein] | 26.1 ± 1.3 | 23.5 ± 1.4 | 52.4 ± 2.7 x | 35.6 ± 1.9 x,a | 73.4 ± 3.7 x | 64.7 ± 3.5 x,a |
| 15d-PGJ2 [pg/mg protein] | 6.2 ± 0.36 | 11.8 ± 0.86 x | 13.8 ± 0.81 x | 10.1 ± 0.60 x,a | 15.4 ± 0.91 x | 11.2 ± 0.72 x,a |
| PGAM5 [vs. control] | 1.00 ± 0.04 | 0.93 ± 0.05 | 0.88 ± 0.04 x | 0.81 ± 0.04 | 0.39 ± 0.02 x | 0.30 ± 0.02 x,a |
| Activators | ||||||
| WTX [vs. control] | 1.00 ± 0.04 | 0.92 ± 0.05 | 0.82 ± 0.04 x | 0.75 ± 0.04 x | 0.75 ± 0.04 x | 0.45 ± 0.02 x,a |
| DPP3 [vs. control] | 1.00 ± 0.05 | 1.81 ± 0.09 x | 0.66 ± 0.03 x | 1.47 ± 0.07 x,a | 0.44 ± 0.02 x | 1.83 ± 0.11 x,a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jastrząb, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Regulates the Expression of Keratinocyte Proteins Involved in the Inflammation Process through Transcriptional Regulation. Cells 2019, 8, 827. https://doi.org/10.3390/cells8080827
Jastrząb A, Gęgotek A, Skrzydlewska E. Cannabidiol Regulates the Expression of Keratinocyte Proteins Involved in the Inflammation Process through Transcriptional Regulation. Cells. 2019; 8(8):827. https://doi.org/10.3390/cells8080827
Chicago/Turabian StyleJastrząb, Anna, Agnieszka Gęgotek, and Elżbieta Skrzydlewska. 2019. "Cannabidiol Regulates the Expression of Keratinocyte Proteins Involved in the Inflammation Process through Transcriptional Regulation" Cells 8, no. 8: 827. https://doi.org/10.3390/cells8080827






