Importance of the Androgen Receptor Signaling in Gene Transactivation and Transrepression for Pubertal Maturation of the Testis
Abstract
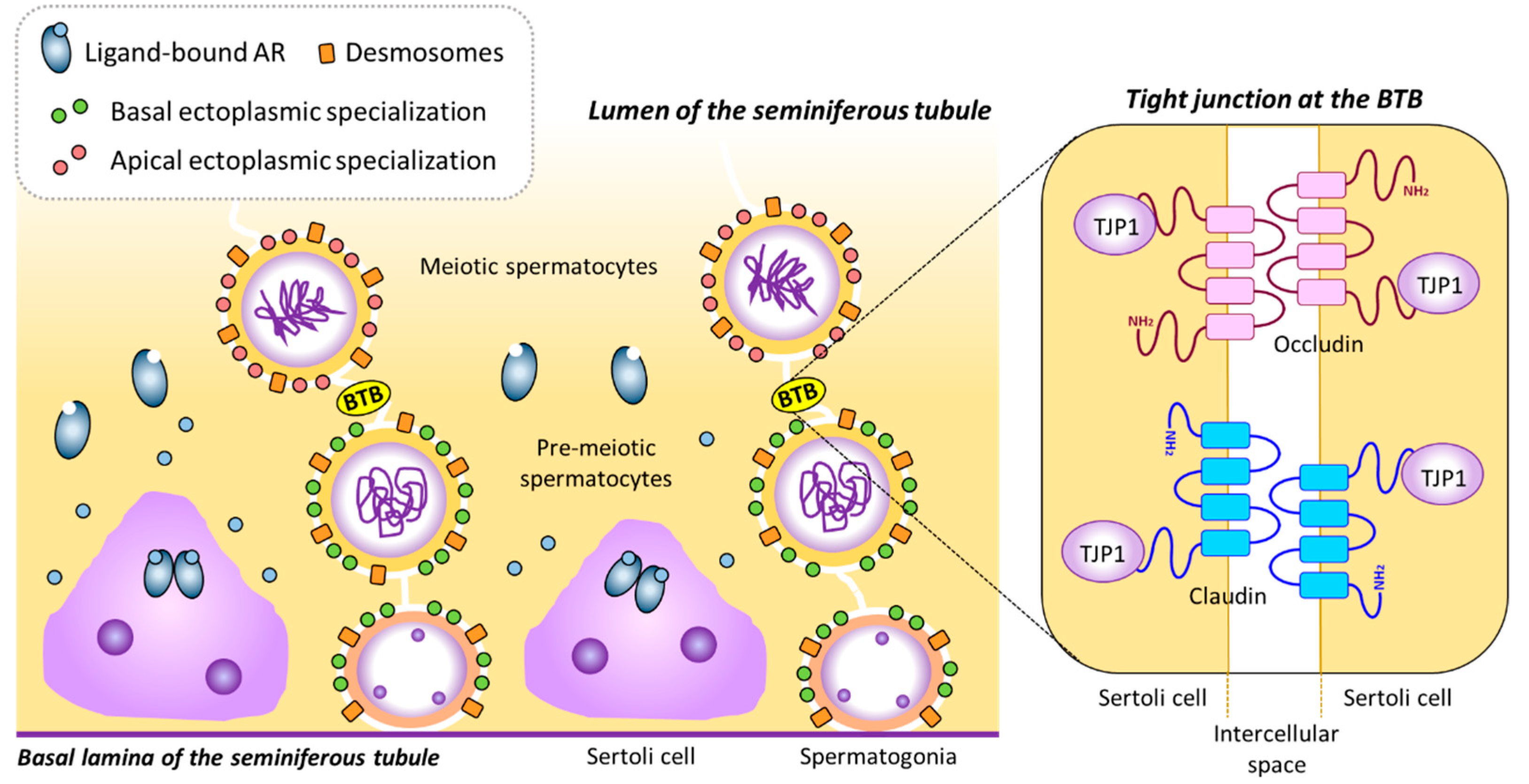
:1. Introduction
2. Sertoli Cell Maturation during Postnatal Development
Hormonal Regulation of Sertoli Cell Maturation in the Postnatal Testis
3. AR Signaling in Sertoli Cells
3.1. Classical Pathway of Androgen Action
3.2. Non-Classical Pathways of Androgen Action
3.3. Co-Repressors and Co-Activators of AR in Sertoli Cells
4. Androgens and the Sertoli Cell
4.1. Stimulatory Effects of Androgens on BTB-Related Gene Expression in Sertoli Cells and its Role on Meiotic Onset in the Testis
4.2. Inhibitory Effect of Androgens on AMH Gene Expression in Sertoli Cells
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Wang, R.S.; Yeh, S.; Tzeng, C.R.; Chang, C. Androgen receptor roles in spermatogenesis and fertility: Lessons from testicular cell-specific androgen receptor knockout mice. Endocr. Rev. 2009, 30, 119–132. [Google Scholar] [CrossRef]
- Ruwanpura, S.M.; McLachlan, R.I.; Meachem, S.J. Hormonal regulation of male germ cell development. J. Endocrinol. 2010, 205, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, G.; Willems, A.; Denolet, E.; Swinnen, J.V.; De Gendt, K. Androgens and spermatogenesis: Lessons from transgenic mouse models. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1537–1556. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L.; Nicholls, P.K.; O’Bryan, M.K.; McLachlan, R.I.; Stanton, P.G. Spermiation: The process of sperm release. Spermatogenesis 2011, 1, 14–35. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.B.; Walker, W.H. The regulation of spermatogenesis by androgens. Semin. Cell Dev. Biol. 2014, 30, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Shima, J.E.; Nie, R.; Friel, P.J.; Griswold, M.D. Androgen-regulated transcripts in the neonatal mouse testis as determined through microarray analysis. Biol. Reprod. 2005, 72, 1010–1019. [Google Scholar] [CrossRef]
- Orth, J.M. The role of follicle-stimulating hormone in controlling Sertoli cell proliferation in testes of fetal rats. Endocrinology 1984, 115, 1248–1255. [Google Scholar] [CrossRef]
- Al-Attar, L.; Noël, K.; Dutertre, M.; Belville, C.; Forest, M.G.; Burgoyne, P.S.; Josso, N.; Rey, R. Hormonal and cellular regulation of Sertoli cell anti-Müllerian hormone production in the postnatal mouse. J. Clin. Invest. 1997, 100, 1335–1343. [Google Scholar] [CrossRef]
- Migrenne, S.; Moreau, E.; Pakarinen, P.; Dierich, A.; Merlet, J.; Habert, R.; Racine, C. Mouse testis development and function are differently regulated by follicle-stimulating hormone receptors signaling during fetal and prepubertal life. PLoS ONE 2012, 7, e53257. [Google Scholar] [CrossRef]
- Lambert, A.S.; Bougnères, P. Growth and descent of the testes in infants with hypogonadotropic hypogonadism receiving subcutaneous gonadotropin infusion. Int. J. Pediatr. Endocrinol. 2016, 2016, 13. [Google Scholar] [CrossRef]
- Kumar, T.R. Fshb Knockout Mouse Model, Two Decades Later and Into the Future. Endocrinology 2018, 159, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, M.; Grinspon, R.P.; Rey, R.A. Comparing the role of anti-Mullerian hormone as a marker of FSH action in male and female fertility. Expert Rev. Endocrinol. Metab. 2019, 14, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Pitetti, J.L.; Calvel, P.; Romero, Y.; Conne, B.; Truong, V.; Papaioannou, M.D.; Schaad, O.; Docquier, M.; Herrera, P.L.; Wilhelm, D.; et al. Insulin and IGF1 Receptors Are Essential for XX and XY Gonadal Differentiation and Adrenal Development in Mice. PLoS Genet. 2013, 9, e1003160. [Google Scholar] [CrossRef] [PubMed]
- Meroni, S.B.; Galardo, M.N.; Rindone, G.; Gorga, A.; Riera, M.F.; Cigorraga, S.B. Molecular Mechanisms and Signaling Pathways Involved in Sertoli Cell Proliferation. Front. Endocrinol. (Lausanne) 2019, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Neirijnck, Y.; Kuhne, F.; Mayere, C.; Pavlova, E.; Sararols, P.; Foti, M.; Atanassova, N.; Nef, S. Tumor Suppressor PTEN Regulates Negatively Sertoli Cell Proliferation, Testis Size, and Sperm Production In Vivo. Endocrinology 2019, 160, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Orth, J.M. Proliferation of Sertoli cells in fetal and postnatal rats: A quantitative autoradiographic study. Anat. Rec. 1982, 203, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.D.; Ren, H.P.; Sinha Hikim, I.; Schulze, W.; Sinha Hikim, A.P. A comparative study in twelve mammalian species of volume densities, volumes, and numerical densities of selected testis components, emphasizing those related to the Sertoli cell. Am. J. Anat. 1990, 188, 21–30. [Google Scholar] [CrossRef]
- Cooke, P.S.; Hess, R.A.; Porcelli, J.; Meisami, E. Increased sperm production in adult rats after transient neonatal hypothyroidism. Endocrinology 1991, 129, 244–248. [Google Scholar] [CrossRef]
- Hess, R.A.; Cooke, P.S.; Bunick, D.; Kirby, J.D. Adult testicular enlargement induced by neonatal hypothyroidism is accompanied by increased Sertoli and germ cell numbers. Endocrinology 1993, 132, 2607–2613. [Google Scholar] [CrossRef]
- Iczkowski, K.A.; Sun, E.L.; Gondos, B. Morphometric study of the prepubertal rabbit testis: Germ cell numbers and seminiferous tubule dimensions. Am. J. Anat. 1991, 190, 266–272. [Google Scholar] [CrossRef]
- Rey, R.A.; Campo, S.M.; Bedecarrás, P.; Nagle, C.A.; Chemes, H.E. Is infancy a quiescent period of testicular development? Histological, morphometric, and functional study of the seminiferous tubules of the cebus monkey from birth to the end of puberty. J. Clin. Endocrinol. Metab. 1993, 76, 1325–1331. [Google Scholar] [PubMed]
- Russell, L. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am. J. Anat. 1977, 148, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.D.; Bartke, A.; Goh, J.C. Postnatal development of the Sertoli cell barrier, tubular lumen, and cytoskeleton of Sertoli and myoid cells in the rat, and their relationship to tubular fluid secretion and flow. Am. J. Anat. 1989, 184, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Vogl, A.W.; Vaid, K.S.; Guttman, J.A. The Sertoli cell cytoskeleton. Adv. Exp. Med. Biol. 2008, 636, 186–211. [Google Scholar] [PubMed]
- Li, N.; Tang, E.I.; Cheng, C.Y. Regulation of blood-testis barrier by actin binding proteins and protein kinases. Reproduction 2016, 151, R29–R41. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Tang, E.I.; Li, N.; Mruk, D.D.; Lee, W.M.; Silvestrini, B.; Cheng, C.Y. Regulation of Blood-Testis Barrier (BTB) Dynamics, Role of Actin-, and Microtubule-Based Cytoskeletons. Methods Mol. Biol. 2018, 1748, 229–243. [Google Scholar] [Green Version]
- Dunleavy, J.E.M.; O’Bryan, M.; Stanton, P.G.; O’Donnell, L. The Cytoskeleton in Spermatogenesis. Reproduction 2019, 157, R53–R72. [Google Scholar] [CrossRef]
- Chemes, H.E.; Rey, R.A.; Nistal, M.; Regadera, J.; Musse, M.; Gonzalez-Peramato, P.; Serrano, A. Physiological androgen insensitivity of the fetal, neonatal, and early infantile testis is explained by the ontogeny of the androgen receptor expression in Sertoli cells. J. Clin. Endocrinol. Metab. 2008, 93, 4408–4412. [Google Scholar] [CrossRef]
- Rey, R.A.; Musse, M.; Venara, M.; Chemes, H.E. Ontogeny of the androgen receptor expression in the fetal and postnatal testis: Its relevance on Sertoli cell maturation and the onset of adult spermatogenesis. Microsc. Res. Tech. 2009, 72, 787–795. [Google Scholar] [CrossRef]
- Edelsztein, N.Y.; Racine, C.; di Clemente, N.; Schteingart, H.F.; Rey, R.A. Androgens downregulate anti-Mullerian hormone promoter activity in the Sertoli cell through the androgen receptor and intact SF1 sites. Biol. Reprod. 2018, 99, 1303–1312. [Google Scholar] [CrossRef]
- Smith, B.E.; Braun, R.E. Germ cell migration across Sertoli cell tight junctions. Science 2012, 338, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Mruk, D.D.; Cheng, C.Y. The Mammalian Blood-Testis Barrier: Its Biology and Regulation. Endocr. Rev. 2015, 36, 564–591. [Google Scholar] [CrossRef] [PubMed]
- Lukas-Croisier, C.; Lasala, C.; Nicaud, J.; Bedecarrás, P.; Kumar, T.R.; Dutertre, M.; Matzuk, M.M.; Picard, J.Y.; Josso, N.; Rey, R. Follicle-stimulating hormone increases testicular Anti-Müllerian hormone (AMH) production through sertoli cell proliferation and a nonclassical cyclic adenosine 5’-monophosphate-mediated activation of the AMH gene. Mol. Endocrinol. 2003, 17, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Josso, N.; Lamarre, I.; Picard, J.Y.; Berta, P.; Davies, N.; Morichon, N.; Peschanski, M.; Jeny, R. Anti-Müllerian hormone in early human development. Early Hum. Dev. 1993, 33, 91–99. [Google Scholar] [CrossRef]
- Lee, M.M.; Donahoe, P.K.; Silverman, B.L.; Hasegawa, T.; Hasegawa, Y.; Gustafson, M.L.; Chang, Y.C.; MacLaughlin, D.T. Measurements of serum Müllerian inhibiting substance in the evaluation of children with nonpalpable gonads. N. Engl. J. Med. 1997, 336, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Misra, M.; MacLaughlin, D.T.; Donahoe, P.K.; Lee, M.M. Measurement of Mullerian inhibiting substance facilitates management of boys with microphallus and cryptorchidism. J. Clin. Endocrinol. Metab. 2002, 87, 3598–3602. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Misra, M.; Donahoe, P.K.; MacLaughlin, D.T. MIS/AMH in the assessment of cryptorchidism and intersex conditions. Mol. Cell. Endocrinol. 2003, 211, 91–98. [Google Scholar] [CrossRef]
- Lindhardt Johansen, M.; Hagen, C.P.; Johannsen, T.H.; Main, K.M.; Picard, J.Y.; Jorgensen, A.; Rajpert-De Meyts, E.; Juul, A. Anti-mullerian hormone and its clinical use in pediatrics with special emphasis on disorders of sex development. Int. J. Endocrinol. 2013, 2013, 198698. [Google Scholar] [CrossRef]
- Grinspon, R.P.; Loreti, N.; Braslavsky, D.; Valeri, C.; Schteingart, H.; Ballerini, M.G.; Bedecarrás, P.; Ambao, V.; Gottlieb, S.; Ropelato, M.G.; et al. Spreading the Clinical Window for Diagnosing Fetal-Onset Hypogonadism in Boys. Front. Endocrinol. (Lausanne) 2014, 5, 51. [Google Scholar] [CrossRef]
- Edelsztein, N.Y.; Grinspon, R.P.; Schteingart, H.F.; Rey, R.A. Anti-Müllerian hormone as a marker of steroid and gonadotropin action in the testis of children and adolescents with disorders of the gonadal axis. Int. J. Pediatr. Endocrinol. 2016, 2016, 20. [Google Scholar] [CrossRef]
- Condorelli, R.A.; Cannarella, R.; Calogero, A.E.; La Vignera, S. Evaluation of testicular function in prepubertal children. Endocrine 2018, 62, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Freire, A.V.; Grinspon, R.P.; Rey, R.A. Importance of Serum Testicular Protein Hormone Measurement in the Assessment of Disorders of Sex Development. Sex. Dev. 2018, 12, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Grinspon, R.P.; Gottlieb, S.; Bedecarras, P.; Rey, R.A. Anti-Müllerian Hormone and Testicular Function in Prepubertal Boys with Cryptorchidism. Front. Endocrinol. (Lausanne) 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Grinspon, R.P.; Urrutia, M.; Rey, R.A. Male Central Hypogonadism in Paediatrics – the Relevance of Follicle-stimulating Hormone and Sertoli Cell Markers. European Endocrinology 2018, 14, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Young, J.; Rey, R.; Couzinet, B.; Chanson, P.; Josso, N.; Schaison, G. Antimüllerian hormone in patients with hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 1999, 84, 2696–2699. [Google Scholar] [PubMed]
- Rey, R.A.; Belville, C.; Nihoul-Fékété, C.; Michel-Calemard, L.; Forest, M.G.; Lahlou, N.; Jaubert, F.; Mowszowicz, I.; David, M.; Saka, N.; et al. Evaluation of gonadal function in 107 intersex patients by means of serum antimüllerian hormone measurement. J. Clin. Endocrinol. Metab. 1999, 84, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Hasky, N.; Stemmer, S.M.; Shalgi, R.; Ben-Aharon, I. Anti-Mullerian Hormone Is a Marker for Chemotherapy-Induced Testicular Toxicity. Endocrinology 2015, 156, 3818–3827. [Google Scholar] [CrossRef]
- Grinspon, R.P.; Andreone, L.; Bedecarrás, P.; Ropelato, M.G.; Rey, R.A.; Campo, S.M.; Bergadá, I. Male Central Precocious Puberty: Serum Profile of Anti-Mullerian Hormone and Inhibin B before, during, and after Treatment with GnRH Analogue. Int. J. Endocrinol. 2013, 2013, 823064. [Google Scholar] [CrossRef]
- Milazzo, J.P.; Bironneau, A.; Vannier, J.P.; Liard-Zmuda, A.; Mace, B.; Nathalie, R. Precocious initiation of spermatogenesis in a 19-month-old boy with Hurler syndrome. Basic Clin Androl 2014, 24, 8. [Google Scholar] [CrossRef]
- Hunter, I.; Hay, C.W.; Esswein, B.; Watt, K.; McEwan, I.J. Tissue control of androgen action: The ups and downs of androgen receptor expression. Mol. Cell. Endocrinol. 2018, 465, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.K.; Chang, C. Endocrine mechanisms of disease: Expression and degradation of androgen receptor: Mechanism and clinical implication. J. Clin. Endocrinol. Metab. 2003, 88, 4043–4054. [Google Scholar] [CrossRef] [PubMed]
- Svechnikov, K.; Landreh, L.; Weisser, J.; Izzo, G.; Colon, E.; Svechnikova, I.; Söder, O. Origin, development and regulation of human Leydig cells. Horm. Res. Paediatr. 2010, 73, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.M.; Seieroe, K.; Pakkenberg, B. The total number of Leydig and Sertoli cells in the testes of men across various age groups - a stereological study. J. Anat. 2015, 226, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Cheng, C.Y.; Liu, Y.X. Development, function and fate of fetal Leydig cells. Semin. Cell Dev. Biol. 2016, 59, 89–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rey, R.; Lordereau-Richard, I.; Carel, J.C.; Barbet, P.; Cate, R.L.; Roger, M.; Chaussain, J.L.; Josso, N. Anti-müllerian hormone and testosterone serum levels are inversely related during normal and precocious pubertal development. J. Clin. Endocrinol. Metab. 1993, 77, 1220–1226. [Google Scholar] [PubMed]
- Grinspon, R.P.; Rey, R.A. Anti-mullerian hormone and Sertoli cell function in paediatric male hypogonadism. Horm. Res. Paediatr. 2010, 73, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Grinspon, R.; Chemes, H.; Rey, R.A. Decline in serum antimullerian hormone due to androgen action in early puberty in males. Fertil. Steril. 2012, 98, e23. [Google Scholar] [CrossRef] [PubMed]
- Hero, M.; Tommiska, J.; Vaaralahti, K.; Laitinen, E.M.; Sipila, I.; Puhakka, L.; Dunkel, L.; Raivio, T. Circulating antimullerian hormone levels in boys decline during early puberty and correlate with inhibin B. Fertil. Steril. 2012, 97, 1242–1247. [Google Scholar] [CrossRef]
- Tanaka, T.; Kanatsu-Shinohara, M.; Lei, Z.; Rao, C.V.; Shinohara, T. The Luteinizing Hormone-Testosterone Pathway Regulates Mouse Spermatogonial Stem Cell Self-Renewal by Suppressing WNT5A Expression in Sertoli Cells. Stem Cell Reports 2016, 7, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.S.; Kokontis, J.; Liao, S.T. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science 1988, 240, 324–326. [Google Scholar] [CrossRef]
- Lubahn, D.B.; Joseph, D.R.; Sullivan, P.M.; Willard, H.F.; French, F.S.; Wilson, E.M. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science 1988, 240, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Al-Azzawi, F. Mechanism of androgen receptor action. Maturitas 2009, 63, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.H.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.L. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Heinlein, C.A.; Chang, C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol. Endocrinol. 2002, 16, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Foradori, C.D.; Weiser, M.J.; Handa, R.J. Non-genomic actions of androgens. Front. Neuroendocrinol. 2008, 29, 169–181. [Google Scholar] [CrossRef] [Green Version]
- Michels, G.; Hoppe, U.C. Rapid actions of androgens. Front. Neuroendocrinol. 2008, 29, 182–198. [Google Scholar] [CrossRef]
- McHenry, J.; Carrier, N.; Hull, E.; Kabbaj, M. Sex differences in anxiety and depression: role of testosterone. Front. Neuroendocrinol. 2014, 35, 42–57. [Google Scholar] [CrossRef]
- Quigley, C.A.; De Bellis, A.; Marschke, K.B.; el-Awady, M.K.; Wilson, E.M.; French, F.S. Androgen receptor defects: historical, clinical, and molecular perspectives [published erratum appears in Endocr Rev 1995 Aug;16(4):546]. Endocr. Rev. 1995, 16, 271–321. [Google Scholar] [CrossRef]
- Khorasanizadeh, S.; Rastinejad, F. Nuclear-receptor interactions on DNA-response elements. Trends Biochem. Sci. 2001, 26, 384–390. [Google Scholar] [CrossRef]
- Denayer, S.; Helsen, C.; Thorrez, L.; Haelens, A.; Claessens, F. The rules of DNA recognition by the androgen receptor. Mol. Endocrinol. 2010, 24, 898–913. [Google Scholar] [CrossRef]
- Barbulescu, K.; Geserick, C.; Schuttke, I.; Schleuning, W.D.; Haendler, B. New androgen response elements in the murine pem promoter mediate selective transactivation. Mol. Endocrinol. 2001, 15, 1803–1816. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Trinidad, M.; Youngblood, G.L.; Maroto, M.R.; Scheller, A.; Robins, D.M.; Payne, A.H. Repression of cAMP-induced expression of the mouse P450 17 alpha-hydroxylase/C17-20 lyase gene (Cyp17) by androgens. Mol. Endocrinol. 1997, 11, 87–96. [Google Scholar] [PubMed]
- Schauwaers, K.; De Gendt, K.; Saunders, P.T.K.; Atanassova, N.; Haelens, A.; Callewaert, L.; Moehren, U.; Swinnen, J.V.; Verhoeven, G.; Verrijdt, G.; et al. Loss of androgen receptor binding to selective androgen response elements causes a reproductive phenotype in a knockin mouse model. Proc. Natl. Acad. Sci. USA 2007, 104, 4961–4966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Gendt, K.; Denolet, E.; Willems, A.; Daniels, V.W.; Clinckemalie, L.; Denayer, S.; Wilkinson, M.F.; Claessens, F.; Swinnen, J.V.; Verhoeven, G. Expression of Tubb3, a beta-tubulin isotype, is regulated by androgens in mouse and rat Sertoli cells. Biol. Reprod. 2011, 85, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Myers, M.; Brown, M. Formation of the androgen receptor transcription complex. Mol. Cell 2002, 9, 601–610. [Google Scholar] [CrossRef]
- Gautam, M.; Bhattacharya, I.; Rai, U.; Majumdar, S.S. Hormone induced differential transcriptome analysis of Sertoli cells during postnatal maturation of rat testes. PLoS ONE 2018, 13, e0191201. [Google Scholar] [CrossRef] [PubMed]
- Fietz, D.; Markmann, M.; Lang, D.; Konrad, L.; Geyer, J.; Kliesch, S.; Chakraborty, T.; Hossain, H.; Bergmann, M. Transfection of Sertoli cells with androgen receptor alters gene expression without androgen stimulation. BMC Mol. Biol. 2015, 16, 23. [Google Scholar] [CrossRef]
- Sadate-Ngatchou, P.I.; Pouchnik, D.J.; Griswold, M.D. Identification of testosterone-regulated genes in testes of hypogonadal mice using oligonucleotide microarray. Mol. Endocrinol. 2004, 18, 422–433. [Google Scholar] [CrossRef]
- Shanker, S.; Hu, Z.; Wilkinson, M.F. Epigenetic regulation and downstream targets of the Rhox5 homeobox gene. Int. J. Androl. 2008, 31, 462–470. [Google Scholar] [CrossRef]
- Lindsey, J.S.; Wilkinson, M.F. Pem: a testosterone- and LH-regulated homeobox gene expressed in mouse Sertoli cells and epididymis. Dev. Biol. 1996, 179, 471–484. [Google Scholar] [CrossRef]
- Lee, S.E.; Lee, S.Y.; Lee, K.A. Rhox in mammalian reproduction and development. Clin Exp Reprod Med 2013, 40, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Heckert, L.L.; Wilson, E.M.; Nilson, J.H. Transcriptional Repression of the {alpha}-Subunit Gene by Androgen Receptor Occurs Independently of DNA Binding but Requires the DNA-Binding and Ligand-Binding Domains of the Receptor. Mol. Endocrinol. 1997, 11, 1497–1506. [Google Scholar] [PubMed]
- Keri, R.A.; Wolfe, M.W.; Saunders, T.L.; Anderson, I.; Kendall, S.K.; Wagner, T.; Yeung, J.; Gorski, J.; Nett, T.M.; Camper, S.A.; et al. The proximal promoter of the bovine luteinizing hormone beta-subunit gene confers gonadotrope-specific expression and regulation by gonadotropin-releasing hormone, testosterone, and 17 beta-estradiol in transgenic mice. Mol. Endocrinol. 1994, 8, 1807–1816. [Google Scholar] [PubMed]
- Fix, C.; Jordan, C.; Cano, P.; Walker, W.H. Testosterone activates mitogen-activated protein kinase and the cAMP response element binding protein transcription factor in Sertoli cells. Proc. Natl. Acad. Sci. USA 2004, 101, 10919–10924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shupe, J.; Cheng, J.; Puri, P.; Kostereva, N.; Walker, W.H. Regulation of Sertoli-germ cell adhesion and sperm release by FSH and nonclassical testosterone signaling. Mol. Endocrinol. 2011, 25, 238–252. [Google Scholar] [CrossRef]
- Toocheck, C.; Clister, T.; Shupe, J.; Crum, C.; Ravindranathan, P.; Lee, T.K.; Ahn, J.M.; Raj, G.V.; Sukhwani, M.; Orwig, K.E.; et al. Mouse Spermatogenesis Requires Classical and Nonclassical Testosterone Signaling. Biol. Reprod. 2016, 94, 11. [Google Scholar] [CrossRef] [PubMed]
- Konoplya, E.F.; Popoff, E.H. Identification of the classical androgen receptor in male rat liver and prostate cell plasma membranes. Int. J. Biochem. 1992, 24, 1979–1983. [Google Scholar] [CrossRef]
- Benten, W.P.; Lieberherr, M.; Giese, G.; Wrehlke, C.; Stamm, O.; Sekeris, C.E.; Mossmann, H.; Wunderlich, F. Functional testosterone receptors in plasma membranes of T cells. FASEB J. 1999, 13, 123–133. [Google Scholar] [CrossRef]
- Berg, A.H.; Rice, C.D.; Rahman, M.S.; Dong, J.; Thomas, P. Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: I. Discovery in female atlantic croaker and evidence ZIP9 mediates testosterone-induced apoptosis of ovarian follicle cells. Endocrinology 2014, 155, 4237–4249. [Google Scholar] [CrossRef]
- Bulldan, A.; Dietze, R.; Shihan, M.; Scheiner-Bobis, G. Non-classical testosterone signaling mediated through ZIP9 stimulates claudin expression and tight junction formation in Sertoli cells. Cell. Signal. 2016, 28, 1075–1085. [Google Scholar] [CrossRef]
- Vija, L.; Meduri, G.; Comperat, E.; Vasiliu, V.; Izard, V.; Ferlicot, S.; Boukari, K.; Camparo, P.; Viengchareun, S.; Constancis, E.; et al. Expression and characterization of androgen receptor coregulators, SRC-2 and HBO1, during human testis ontogenesis and in androgen signaling deficient patients. Mol.Cell Endocrinol 2013, 375, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Heinlein, C.A.; Chang, C. Androgen Receptor (AR) Coregulators: An Overview. Endocr. Rev. 2002, 23, 175–200. [Google Scholar] [CrossRef] [PubMed]
- Gehin, M.; Mark, M.; Dennefeld, C.; Dierich, A.; Gronemeyer, H.; Chambon, P. The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Mol. Cell. Biol. 2002, 22, 5923–5937. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Han, S.J.; Tsai, S.Y.; DeMayo, F.J.; Xu, J.; Tsai, M.J.; O’Malley, B.W. Roles of steroid receptor coactivator (SRC)-1 and transcriptional intermediary factor (TIF) 2 in androgen receptor activity in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 9487–9492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.C.; Luo, M.L.; Guo, H.; Wang, T.T.; Lin, S.R.; Chen, J.B.; Ma, Q.; Gu, Y.L.; Jiang, Z.M.; Gui, Y.T. Identification of NR0B1 as a novel androgen receptor co-repressor in mouse Sertoli cells. Int. J. Mol. Med. 2016, 38, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Rey, R.; Mebarki, F.; Forest, M.G.; Mowszowicz, I.; Cate, R.L.; Morel, Y.; Chaussain, J.L.; Josso, N. Anti-müllerian hormone in children with androgen insensitivity. J. Clin. Endocrinol. Metab. 1994, 79, 960–964. [Google Scholar] [PubMed]
- Hellmann, P.; Christiansen, P.; Johannsen, T.H.; Main, K.M.; Duno, M.; Juul, A. Male patients with partial androgen insensitivity syndrome: A longitudinal follow-up of growth, reproductive hormones and the development of gynaecomastia. Arch. Dis. Child. 2012, 97, 403–409. [Google Scholar] [CrossRef]
- Hughes, I.A.; Davies, J.D.; Bunch, T.I.; Pasterski, V.; Mastroyannopoulou, K.; MacDougall, J. Androgen insensitivity syndrome. Lancet 2012, 380, 1419–1428. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; O’Neill, C.; Handelsman, D.J. Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology 1995, 136, 5311–5321. [Google Scholar] [CrossRef]
- Meachem, S.J.; Wreford, N.G.; Robertson, D.M.; McLachlan, R.I. Androgen action on the restoration of spermatogenesis in adult rats: Effects of human chorionic gonadotrophin, testosterone and flutamide administration on germ cell number. Int. J. Androl. 1997, 20, 70–79. [Google Scholar] [CrossRef]
- Yeh, S.; Tsai, M.Y.; Xu, Q.; Mu, X.M.; Lardy, H.; Huang, K.E.; Lin, H.; Yeh, S.D.; Altuwaijri, S.; Zhou, X.; et al. Generation and characterization of androgen receptor knockout (ARKO) mice: An in vivo model for the study of androgen functions in selective tissues. Proc. Natl. Acad. Sci. USA 2002, 99, 13498–13503. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Chen, Y.T.; Yeh, S.D.; Xu, Q.; Wang, R.S.; Guillou, F.; Lardy, H.; Yeh, S. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc. Natl. Acad. Sci. USA 2004, 101, 6876–6881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Gendt, K.; Swinnen, J.V.; Saunders, P.T.K.; Schoonjans, L.; Dewerchin, M.; Devos, A.; Tan, K.; Atanassova, N.; Claessens, F.; Lecureuil, C.; et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc. Natl. Acad. Sci. USA 2004, 101, 1327–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitale, R.; Fawcett, D.W.; Dym, M. The normal development of the blood-testis barrier and the effects of clomiphene and estrogen treatment. Anat. Rec. 1973, 176, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.D.; Peterson, R.N. Sertoli cell junctions: Morphological and functional correlates. Int. Rev. Cytol. 1985, 94, 177–211. [Google Scholar] [PubMed]
- Dym, M.; Fawcett, D.W. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol. Reprod. 1970, 3, 308–326. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Mruk, D.D. The Blood-Testis Barrier and Its Implications for Male Contraception. Pharmacol. Rev. 2012, 64, 16–64. [Google Scholar] [CrossRef]
- Flickinger, C.; Fawcett, D.W. The junctional specializations of Sertoli cells in the seminiferous epithelium. Anat. Rec. 1967, 158, 207–221. [Google Scholar] [CrossRef]
- Gilula, N.B.; Fawcett, D.W.; Aoki, A. The Sertoli cell occluding junctions and gap junctions in mature and developing mammalian testis. Dev. Biol. 1976, 50, 142–168. [Google Scholar] [CrossRef]
- Pelletier, G.; Dupont, E.; Simard, J.; Luu-The, V.; Belanger, A.; Labrie, F. Ontogeny and subcellular localization of 3 beta-hydroxysteroid dehydrogenase (3 beta-HSD) in the human and rat adrenal, ovary and testis. J. Steroid Biochem. Mol. Biol. 1992, 43, 451–467. [Google Scholar] [CrossRef]
- Pelletier, R.M. The blood-testis barrier: The junctional permeability, the proteins and the lipids. Prog. Histochem. Cytochem. 2011, 46, 49–127. [Google Scholar] [CrossRef] [PubMed]
- Gow, A.; Southwood, C.M.; Li, J.S.; Pariali, M.; Riordan, G.P.; Brodie, S.E.; Danias, J.; Bronstein, J.M.; Kachar, B.; Lazzarini, R.A. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell 1999, 99, 649–659. [Google Scholar] [CrossRef]
- Chakraborty, P.; Buaas, F.W.; Sharma, M.; Snyder, E.; de Rooij, D.G.; Braun, R.E. LIN28A marks the spermatogonial progenitor population and regulates its cyclic expansion. Stem Cells 2014, 32, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Chihara, M.; Ikebuchi, R.; Otsuka, S.; Ichii, O.; Hashimoto, Y.; Suzuki, A.; Saga, Y.; Kon, Y. Mice stage-specific claudin 3 expression regulates progression of meiosis in early stage spermatocytes. Biol. Reprod. 2013, 89, 3. [Google Scholar] [CrossRef] [PubMed]
- Moroi, S.; Saitou, M.; Fujimoto, K.; Sakakibara, A.; Furuse, M.; Yoshida, O.; Tsukita, S. Occludin is concentrated at tight junctions of mouse/rat but not human/guinea pig Sertoli cells in testes. Am. J. Physiol. 1998, 274, C1708–C1717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellani, A.; Ji, J.; Mauduit, C.; Deschildre, C.; Tabone, E.; Benahmed, M. Developmental and hormonal regulation of the expression of oligodendrocyte-specific protein/claudin 11 in mouse testis. Endocrinology 2000, 141, 3012–3019. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Holdcraft, R.W.; Shima, J.E.; Griswold, M.D.; Braun, R.E. Androgens regulate the permeability of the blood-testis barrier. Proc. Natl. Acad. Sci. USA 2005, 102, 16696–16700. [Google Scholar] [CrossRef]
- Willems, A.; Batlouni, S.R.; Esnal, A.; Swinnen, J.V.; Saunders, P.T.K.; Sharpe, R.M.; Fran+ºa, L.R.; De Gendt, K.; Verhoeven, G. Selective Ablation of the Androgen Receptor in Mouse Sertoli Cells Affects Sertoli Cell Maturation, Barrier Formation and Cytoskeletal Development. PLoS ONE 2010, 5, e14168. [Google Scholar] [CrossRef]
- Byers, S.; Graham, R.; Dai, H.N.; Hoxter, B. Development of Sertoli cell junctional specializations and the distribution of the tight-junction-associated protein ZO-1 in the mouse testis. Am. J. Anat. 1991, 191, 35–47. [Google Scholar] [CrossRef]
- Mazaud-Guittot, S.; Meugnier, E.; Pesenti, S.; Wu, X.; Vidal, H.; Gow, A.; Le Magueresse-Battistoni, B. Claudin 11 deficiency in mice results in loss of the Sertoli cell epithelial phenotype in the testis. Biol. Reprod. 2010, 82, 202–213. [Google Scholar] [CrossRef]
- Johnston, H.; Baker, P.J.; Abel, M.; Charlton, H.M.; Jackson, G.; Fleming, L.; Kumar, T.R.; O’Shaughnessy, P.J. Regulation of Sertoli cell number and activity by Follicle-Stimulating Hormone and androgen during postnatal development in the mouse. Endocrinology 2004, 145, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Haywood, M.; Spaliviero, J.; Jimemez, M.; King, N.J.; Handelsman, D.J.; Allan, C.M. Sertoli and germ cell development in hypogonadal (hpg) mice expressing transgenic follicle-stimulating hormone alone or in combination with testosterone. Endocrinology 2003, 144, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.; Ebling, F.J.; Nwagwu, M.; Boulton, R.; Wadhwa, K.; Stewart, J.; Kerr, J.B. Atypical development of Sertoli cells and impairment of spermatogenesis in the hypogonadal (hpg) mouse. J. Anat. 2005, 207, 797–811. [Google Scholar] [CrossRef] [PubMed]
- McCabe, M.J.; Allan, C.M.; Foo, C.F.; Nicholls, P.K.; McTavish, K.J.; Stanton, P.G. Androgen initiates Sertoli cell tight junction formation in the hypogonadal (hpg) mouse. Biol. Reprod. 2012, 87, 38. [Google Scholar] [CrossRef] [PubMed]
- Fritz, I.B.; Lyon, M.F.; Setchell, B.P. Evidence for a defective seminiferous tubule barrier in testes of Tfm and Sxr mice. J. Reprod. Fertil. 1983, 67, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Denolet, E.; De Gendt, K.; Allemeersch, J.; Engelen, K.; Marchal, K.; Van Hummelen, P.; Tan, K.A.; Sharpe, R.M.; Saunders, P.T.; Swinnen, J.V.; et al. The effect of a sertoli cell-selective knockout of the androgen receptor on testicular gene expression in prepubertal mice. Mol. Endocrinol. 2006, 20, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Soffientini, U.; Rebourcet, D.; Abel, M.H.; Lee, S.; Hamilton, G.; Fowler, P.A.; Smith, L.B.; O’Shaughnessy, P.J. Identification of Sertoli cell-specific transcripts in the mouse testis and the role of FSH and androgen in the control of Sertoli cell activity. BMC Genomics 2017, 18, 972. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.A.; De Gendt, K.; Atanassova, N.; Walker, M.; Sharpe, R.M.; Saunders, P.T.; Denolet, E.; Verhoeven, G. The role of androgens in sertoli cell proliferation and functional maturation: Studies in mice with total or Sertoli cell-selective ablation of the androgen receptor. Endocrinology 2005, 146, 2674–2683. [Google Scholar] [CrossRef]
- Wang, R.S.; Yeh, S.; Chen, L.M.; Lin, H.Y.; Zhang, C.; Ni, J.; Wu, C.C.; di Sant’Agnese, P.A.; deMesy-Bentley, K.L.; Tzeng, C.R.; et al. Androgen receptor in sertoli cell is essential for germ cell nursery and junctional complex formation in mouse testes. Endocrinology 2006, 147, 5624–5633. [Google Scholar] [CrossRef]
- Willems, A.; De Gendt, K.; Allemeersch, J.; Smith, L.B.; Welsh, M.; Swinnen, J.V.; Verhoeven, G. Early effects of Sertoli cell-selective androgen receptor ablation on testicular gene expression. Int. J. Androl. 2010, 33, 507–517. [Google Scholar] [CrossRef]
- Abel, M.H.; Baker, P.J.; Charlton, H.M.; Monteiro, A.; Verhoeven, G.; De Gendt, K.; Guillou, F.; O’Shaughnessy, P.J. Spermatogenesis and sertoli cell activity in mice lacking sertoli cell receptors for follicle-stimulating hormone and androgen. Endocrinology 2008, 149, 3279–3285. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; William Buaas, F.; Sharma, M.; Smith, B.E.; Greenlee, A.R.; Eacker, S.M.; Braun, R.E. Androgen-dependent sertoli cell tight junction remodeling is mediated by multiple tight junction components. Mol. Endocrinol. 2014, 28, 1055–1072. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, D.; Dietze, R.; Shihan, M.; Kirch, U.; Scheiner-Bobis, G. Dehydroepiandrosterone Sulfate Stimulates Expression of Blood-Testis-Barrier Proteins Claudin-3 and -5 and Tight Junction Formation via a Gnalpha11-Coupled Receptor in Sertoli Cells. PLoS ONE 2016, 11, e0150143. [Google Scholar] [CrossRef] [PubMed]
- Hazra, R.; Corcoran, L.; Robson, M.; McTavish, K.J.; Upton, D.; Handelsman, D.J.; Allan, C.M. Temporal role of Sertoli cell androgen receptor expression in spermatogenic development. Mol. Endocrinol. 2013, 27, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Greenlee, A.R.; Taub, C.J.; Braun, R.E. Sertoli cell-specific deletion of the androgen receptor compromises testicular immune privilege in mice. Biol. Reprod. 2011, 85, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Magit, D.; Sager, R. Expression of maspin in prostate cells is regulated by a positive ets element and a negative hormonal responsive element site recognized by androgen receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 5673–5678. [Google Scholar] [CrossRef] [PubMed]
- He, M.L.; Jiang, A.L.; Zhang, P.J.; Hu, X.Y.; Liu, Z.F.; Yuan, H.Q.; Zhang, J.Y. Identification of androgen-responsive element ARE and Sp1 element in the maspin promoter. Chin. J. Physiol. 2005, 48, 160–166. [Google Scholar] [PubMed]
- Lanzino, M.; Sisci, D.; Morelli, C.; Garofalo, C.; Catalano, S.; Casaburi, I.; Capparelli, C.; Giordano, C.; Giordano, F.; Maggiolini, M.; et al. Inhibition of cyclin D1 expression by androgen receptor in breast cancer cells--identification of a novel androgen response element. Nucleic Acids Res. 2010, 38, 5351–5365. [Google Scholar] [CrossRef]
- Kallio, P.J.; Poukka, H.; Moilanen, A.; Jänne, O.A.; Palvimo, J.J. Androgen receptor-mediated transcriptional regulation in the absence of direct interaction with a specific DNA element. Mol. Endocrinol. 1995, 9, 1017–1028. [Google Scholar] [PubMed]
- Jorgensen, J.S.; Nilson, J.H. AR suppresses transcription of the alpha glycoprotein hormone subunit gene through protein-protein interactions with cJun and activation transcription factor 2. Mol. Endocrinol. 2001, 15, 1496–1504. [Google Scholar]
- Jorgensen, J.S.; Nilson, J.H. AR suppresses transcription of the LHbeta subunit by interacting with steroidogenic factor-1. Mol. Endocrinol. 2001, 15, 1505–1516. [Google Scholar] [PubMed]
- Lan, K.C.; Chen, Y.T.; Chang, C.; Chang, Y.C.; Lin, H.J.; Huang, K.E.; Kang, H.Y. Up-regulation of SOX9 in sertoli cells from testiculopathic patients accounts for increasing anti-mullerian hormone expression via impaired androgen receptor signaling. PLoS ONE 2013, 8, e76303. [Google Scholar] [CrossRef] [PubMed]
- Lasala, C.; Carré-Eusèbe, D.; Picard, J.Y.; Rey, R. Subcellular and molecular mechanisms regulating anti-Müllerian hormone gene expression in mammalian and nonmammalian species. DNA Cell Biol. 2004, 23, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Rojas-García, P.P.; Recabarren, M.P.; Sarabia, L.; Schon, J.; Gabler, C.; Einspanier, R.; Maliqueo, M.; Sir-Petermann, T.; Rey, R.; Recabarren, S.E. Prenatal testosterone excess alters Sertoli and germ cell number and testicular FSH receptor expression in rams. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E998–E1005. [Google Scholar] [CrossRef]
- Rojas-García, P.P.; Recabarren, M.P.; Sir-Petermann, T.; Rey, R.; Palma, S.; Carrasco, A.; Pérez-Marin, C.C.; Padmanabhan, V.; Recabarren, S.E. Altered testicular development as a consequence of increase number of sertoli cell in male lambs exposed prenatally to excess testosterone. Endocrine 2013, 43, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.; Meusy-Dessolle, N.; Josso, N. Waning of anti-Müllerian activity: An early sign of sertoli cell maturation in the developing pig. Biol. Reprod. 1981, 24, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Song, H.; Guan, K.; Zhou, J.; Xia, X.; Li, F. Characterization of swine testicular cell line as immature porcine Sertoli cell line. In Vitro Cell. Dev. Biol. Anim. 2016, 52, 427–433. [Google Scholar] [CrossRef]
- Almeida, J.; Conley, A.J.; Mathewson, L.; Ball, B.A. Expression of anti-Mullerian hormone, cyclin-dependent kinase inhibitor (CDKN1B), androgen receptor, and connexin 43 in equine testes during puberty. Theriogenology 2012, 77, 847–857. [Google Scholar] [CrossRef]
- Vigier, B.; Tran, D.; du Mesnil du Buisson, F.; Heyman, Y.; Josso, N. Use of monoclonal antibody techniques to study the ontogeny of bovine anti-Müllerian hormone. J. Reprod. Fertil. 1983, 69, 207–214. [Google Scholar] [CrossRef]
- Rota, A.; Ballarin, C.; Vigier, B.; Cozzi, B.; Rey, R. Age dependent changes in plasma anti-Müllerian hormone concentrations in the bovine male, female, and freemartin from birth to puberty: Relationship between testosterone production and influence on sex differentiation. Gen. Comp. Endocrinol. 2002, 129, 39–44. [Google Scholar] [CrossRef]
- Racine, C.; Pask, A.J.; Wijayanti, G.E.; di Clemente, N.; Picard, J.Y.; Shaw, G.; Renfree, M.B.; Josso, N. Early expression of the androgen receptor in the sertoli cells of a marsupial coincides with downregulation of anti-Mullerian hormone at the time of urogenital virilization. Sex. Dev. 2009, 3, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Münsterberg, A.; Lovell-Badge, R. Expression of the mouse anti-Müllerian hormone gene suggests a role in both male and female sexual differentiation. Development 1991, 113, 613–624. [Google Scholar] [PubMed]
- Watanabe, K.; Clarke, T.R.; Lane, A.H.; Wang, X.; Donahoe, P.K. Endogenous expression of Mullerian inhibiting substance in early postnatal rat sertoli cells requires multiple steroidogenic factor-1 and GATA-4-binding sites. Proc. Natl. Acad Sci. USA 2000, 97, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Nachtigal, M.W.; Hirokawa, Y.; Enyeart-VanHouten, D.L.; Flanagan, J.N.; Hammer, G.D.; Ingraham, H.A. Wilms’ tumor 1 and Dax-1 modulate the orphan nuclear receptor SF-1 in sex-specific gene expression. Cell 1998, 93, 445–454. [Google Scholar] [CrossRef]
- Tremblay, J.J.; Viger, R.S. Nuclear receptor Dax-1 represses the transcriptional cooperation between GATA-4 and SF-1 in Sertoli cells. Biol. Reprod. 2001, 64, 1191–1199. [Google Scholar] [CrossRef]
- Dutertre, M.; Rey, R.; Porteu, A.; Josso, N.; Picard, J.Y. A mouse Sertoli cell line expressing anti-Müllerian hormone and its type II receptor. Mol. Cell. Endocrinol. 1997, 136, 57–65. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edelsztein, N.Y.; Rey, R.A. Importance of the Androgen Receptor Signaling in Gene Transactivation and Transrepression for Pubertal Maturation of the Testis. Cells 2019, 8, 861. https://doi.org/10.3390/cells8080861
Edelsztein NY, Rey RA. Importance of the Androgen Receptor Signaling in Gene Transactivation and Transrepression for Pubertal Maturation of the Testis. Cells. 2019; 8(8):861. https://doi.org/10.3390/cells8080861
Chicago/Turabian StyleEdelsztein, Nadia Y., and Rodolfo A. Rey. 2019. "Importance of the Androgen Receptor Signaling in Gene Transactivation and Transrepression for Pubertal Maturation of the Testis" Cells 8, no. 8: 861. https://doi.org/10.3390/cells8080861





