The Influence of Competition Among C. elegans Small RNA Pathways on Development
Abstract
:1. Introduction
2. Results and Discussion
2.1. Rescue of let-7 Phenotypes by the eri-1 Mutant
2.2. eri-1 Mutant Worms Have Higher Expression of let-7 Regulated Genes at Mid-L4
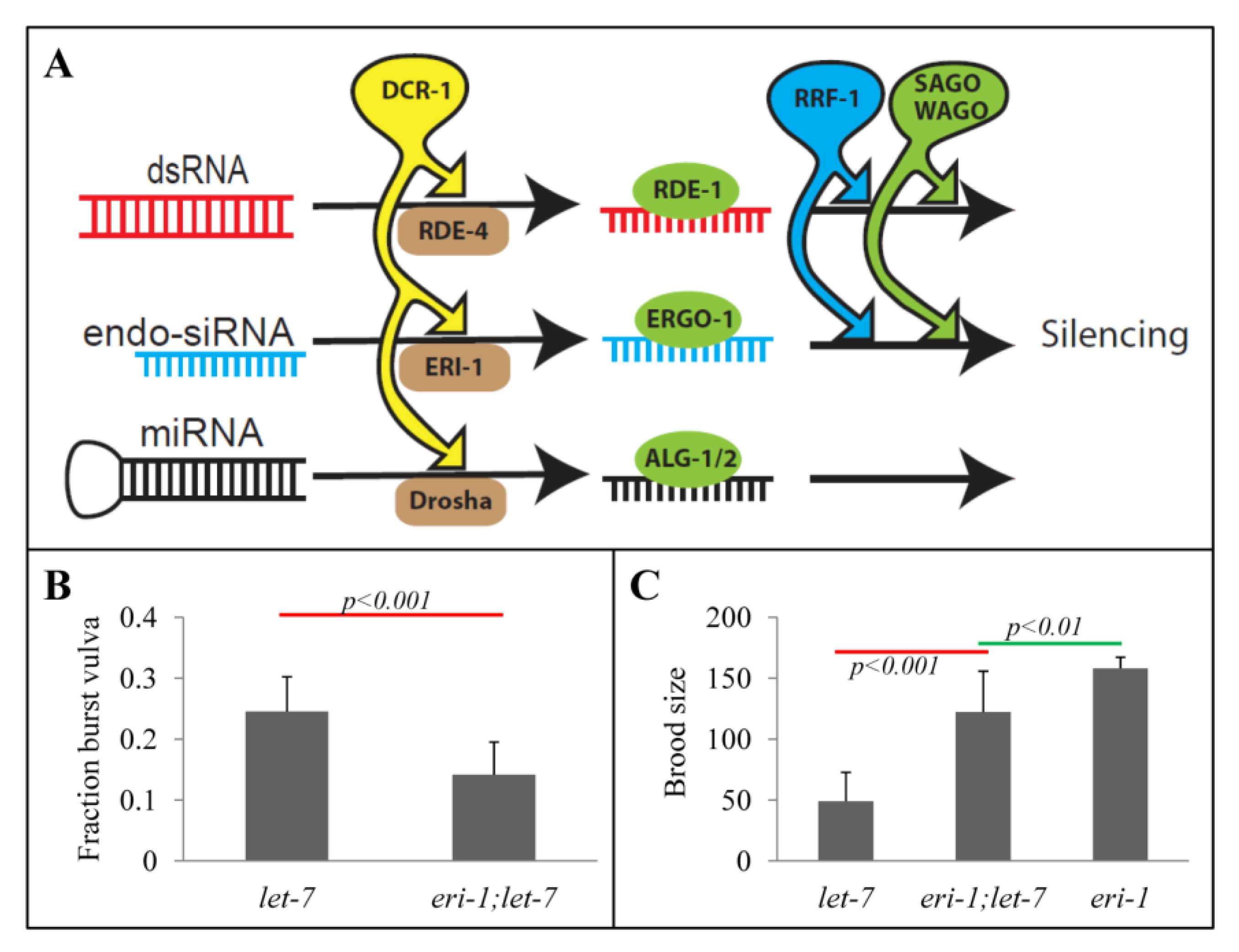
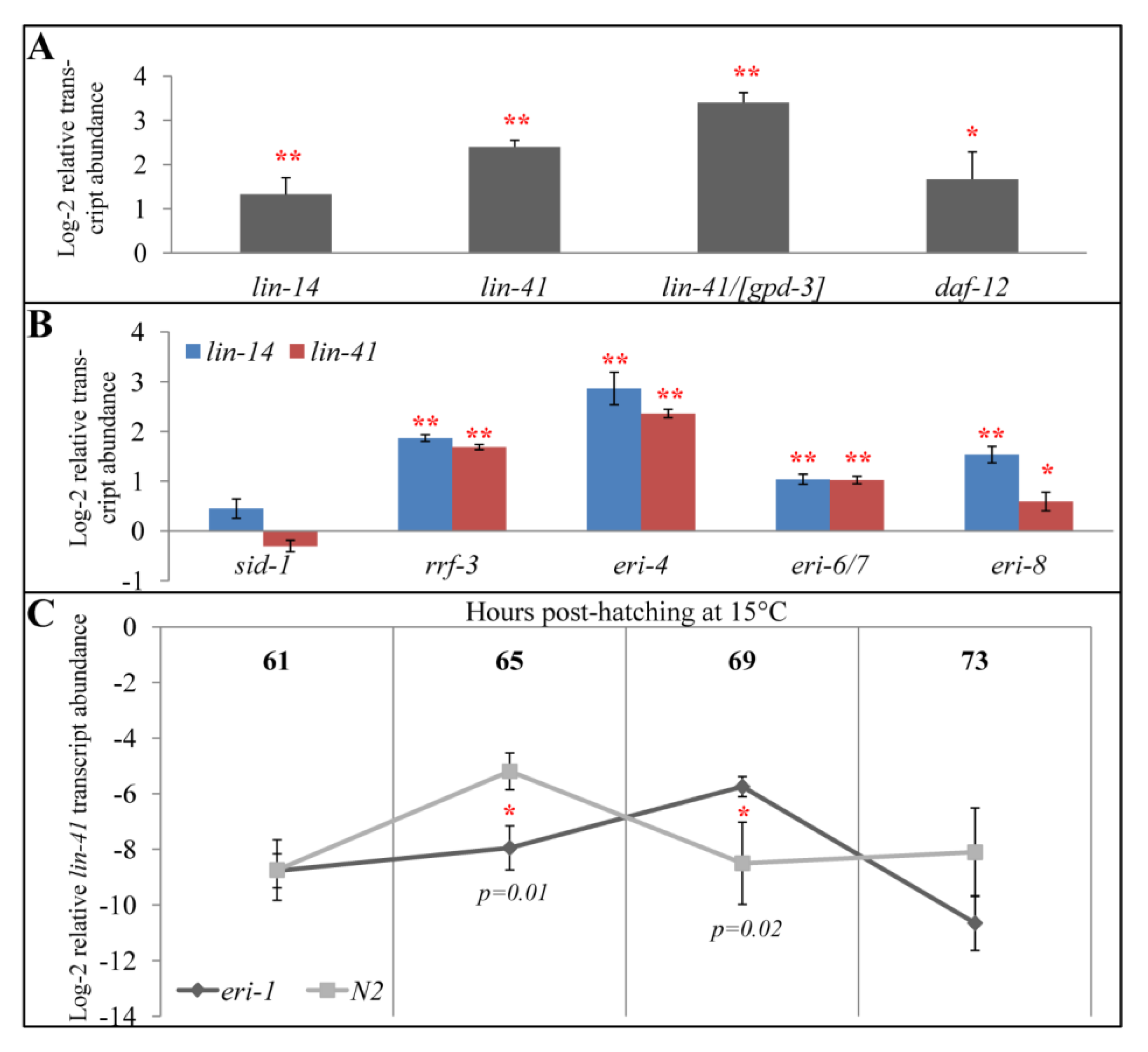
2.3. All Eri Mutants Have Higher Expression of let-7-regulated Genes at Mid-L4
2.4. eri-1 Mutants May Have Delayed Down-regulation of lin-41
2.5. Perturbations to Small RNA Pathways Affect Developmental Timing
2.6. Impact of Developmental Timing Disturbances on Expression Studies
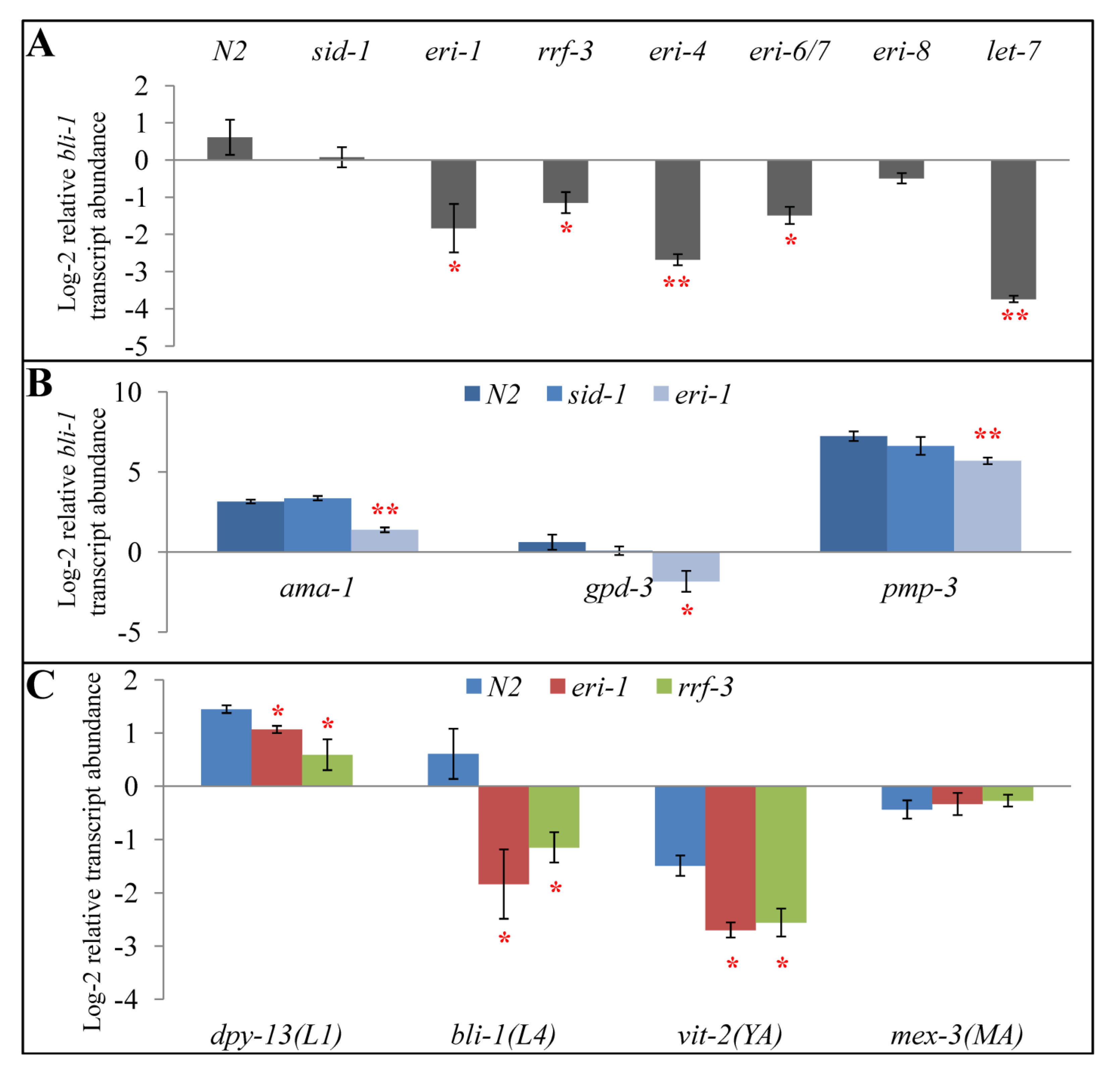
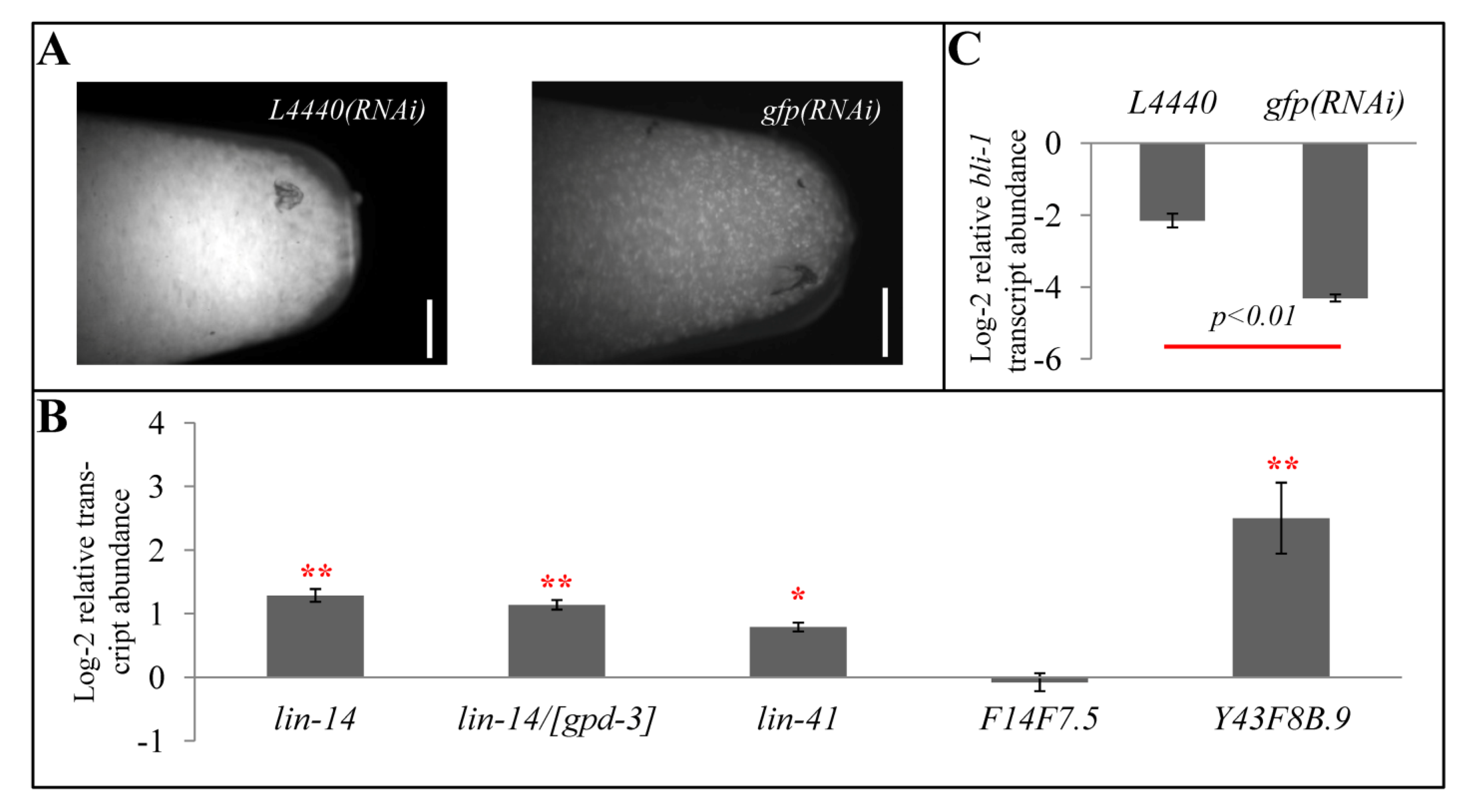
2.7. Exogenous RNAi Causes Higher Expression of Some let-7- and rrf-3- Regulated Genes
2.8. Chicken and Egg: Development and Small RNAs?
3. Materials and Methods
3.1. Strains
3.2. gfp(RNAi)
3.3. Reverse Transcription Real-time PCR
4. Conclusions and Future Scope
Acknowledgements
References
- Fischer, S.E. Small RNA-mediated gene silencing pathways in C. elegans. Int J. Biochem. Cell Biol. 2010, 42, 1306–1315. [Google Scholar] [CrossRef]
- Kaikkonen, M.U.; Lam, M.T.; Glass, C.K. Non-coding RNAs as regulators of gene expression and epigenetics. Cardiovasc. Res. 2011, 90, 430–440. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Reinhart, B.J.; Slack, F.J.; Basson, M.; Pasquinelli, A.E.; Bettinger, J.C.; Rougvie, A.E.; Horvitz, H.R.; Ruvkun, G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 2000, 403, 901–906. [Google Scholar] [CrossRef]
- Pasquinelli, A.E.; Reinhart, B.J.; Slack, F.; Martindale, M.Q.; Kuroda, M.I.; Maller, B.; Hayward, D.C.; Ball, E.E.; Degnan, B.; Muller, P.; Spring, J.; Srinivasan, A.; Fishman, M.; Finnerty, J.; Corbo, J.; Levine, M.; Leahy, P.; Davidson, E.; Ruvkun, G. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature 2000, 408, 86–89. [Google Scholar]
- Kawaji, H.; Hayashizaki, Y. Exploration of small RNAs. PLoS Genet. 2008. [Google Scholar]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Lee, R.C.; Hammell, C.M.; Ambros, V. Interacting endogenous and exogenous RNAi pathways in Caenorhabditis elegans. RNA 2006, 12, 589–597. [Google Scholar] [CrossRef]
- Grishok, A. RNAi mechanisms in Caenorhabditis elegans. FEBS Lett. 2005, 579, 5932–5939. [Google Scholar] [CrossRef]
- Tabara, H.; Sarkissian, M.; Kelly, W.G.; Fleenor, J.; Grishok, A.; Timmons, L.; Fire, A.; Mello, C.C. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell 1999, 99, 123–132. [Google Scholar] [CrossRef]
- Gent, J.I.; Lamm, A.T.; Pavelec, D.M.; Maniar, J.M.; Parameswaran, P.; Tao, L.; Kennedy, S.; Fire, A.Z. Distinct phases of siRNA synthesis in an endogenous RNAi pathway in C. elegans soma. Mol. Cell 2010, 37, 679–689. [Google Scholar] [CrossRef]
- Sifuentes-Romero, I.; Milton, S.L.; Garcia-Gasca, A. Post-transcriptional gene silencing by RNA interference in non-mammalian vertebrate systems: where do we stand? Mutat. Res. 2011, 728, 158–171. [Google Scholar] [CrossRef]
- Das, P.P.; Bagijn, M.P.; Goldstein, L.D.; Woolford, J.R.; Lehrbach, N.J.; Sapetschnig, A.; Buhecha, H.R.; Gilchrist, M.J.; Howe, K.L.; Stark, R.; Matthews, N.; Berezikov, E.; Ketting, R.F.; Tavare, S.; Miska, E.A. Piwi and piRNAs act upstream of an endogenous siRNA pathway to suppress Tc3 transposon mobility in the Caenorhabditis elegans germline. Mol. Cell 2008, 31, 79–90. [Google Scholar] [CrossRef]
- Wilusz, J.E.; Sunwoo, H.; Spector, D.L. Long noncoding RNAs: Functional surprises from the RNA world. Genes Dev. 2009, 23, 1494–1504. [Google Scholar] [CrossRef]
- Claycomb, J.M.; Batista, P.J.; Pang, K.M.; Gu, W.; Vasale, J.J.; van Wolfswinkel, J.C.; Chaves, D.A.; Shirayama, M.; Mitani, S.; Ketting, R.F.; Conte, D., Jr.; Mello, C.C. The Argonaute CSR-1 and its 22G-RNA cofactors are required for holocentric chromosome segregation. Cell 2009, 139, 123–134. [Google Scholar] [CrossRef]
- Ohta, H.; Fujiwara, M.; Ohshima, Y.; Ishihara, T. ADBP-1 regulates an ADAR RNA-editing enzyme to antagonize RNA-interference-mediated gene silencing in Caenorhabditis elegans. Genetics 2008, 180, 785–796. [Google Scholar] [CrossRef]
- Gu, S.G.; Pak, J.; Guang, S.; Maniar, J.M.; Kennedy, S.; Fire, A. Amplification of siRNA in Caenorhabditis elegans generates a transgenerational sequence-targeted histone H3 lysine 9 methylation footprint. Nat. Genet. 2012, 44, 157–164. [Google Scholar] [CrossRef]
- Azimzadeh Jamalkandi, S.; Masoudi-Nejad, A. RNAi pathway integration in Caenorhabditis elegans development. Funct. Integr. Genomics 2011, 11, 389–405. [Google Scholar] [CrossRef]
- Simmer, F.; Tijsterman, M.; Parrish, S.; Koushika, S.P.; Nonet, M.L.; Fire, A.; Ahringer, J.; Plasterk, R.H. Loss of the putative RNA-directed RNA polymerase RRF-3 makes C. elegans hypersensitive to RNAi. Curr. Biol. 2002, 12, 1317–1319. [Google Scholar] [CrossRef]
- Kennedy, S.; Wang, D.; Ruvkun, G. A conserved siRNA-degrading RNase negatively regulates RNA interference in C. elegans. Nature 2004, 427, 645–649. [Google Scholar] [CrossRef]
- Duchaine, T.F.; Wohlschlegel, J.A.; Kennedy, S.; Bei, Y.; Conte, D., Jr.; Pang, K.; Brownell, D.R.; Harding, S.; Mitani, S.; Ruvkun, G.; Yates, J.R., 3rd; Mello, C.C. Functional proteomics reveals the biochemical niche of C. elegans DCR-1 in multiple small-RNA-mediated pathways. Cell 2006, 124, 343–354. [Google Scholar] [CrossRef]
- Fischer, S.E.; Butler, M.D.; Pan, Q.; Ruvkun, G. Trans-splicing in C. elegans generates the negative RNAi regulator ERI-6/7. Nature 2008, 455, 491–496. [Google Scholar] [CrossRef]
- Pavelec, D.M.; Lachowiec, J.; Duchaine, T.F.; Smith, H.E.; Kennedy, S. Requirement for the ERI/DICER complex in endogenous RNA interference and sperm development in Caenorhabditis elegans. Genetics 2009, 183, 1283–1295. [Google Scholar] [CrossRef]
- Gent, J.I.; Schvarzstein, M.; Villeneuve, A.M.; Gu, S.G.; Jantsch, V.; Fire, A.Z.; Baudrimont, A. A Caenorhabditis elegans RNA-directed RNA polymerase in sperm development and endogenous RNA interference. Genetics 2009, 183, 1297–1314. [Google Scholar] [CrossRef]
- Fischer, S.E.; Montgomery, T.A.; Zhang, C.; Fahlgren, N.; Breen, P.C.; Hwang, A.; Sullivan, C.M.; Carrington, J.C.; Ruvkun, G. The ERI-6/7 Helicase Acts at the First Stage of an siRNA Amplification Pathway That Targets Recent Gene Duplications. PLoS Genet. 2011. [Google Scholar]
- Yigit, E.; Batista, P.J.; Bei, Y.; Pang, K.M.; Chen, C.C.; Tolia, N.H.; Joshua-Tor, L.; Mitani, S.; Simard, M.J.; Mello, C.C. Analysis of the C. elegans Argonaute family reveals that distinct Argonautes act sequentially during RNAi. Cell 2006, 127, 747–757. [Google Scholar] [CrossRef]
- Knight, S.W.; Bass, B.L. A role for the RNase III enzyme DCR-1 in RNA interference and germ line development in Caenorhabditis elegans. Science 2001, 293, 2269–2271. [Google Scholar] [CrossRef]
- Ketting, R.F.; Fischer, S.E.J.; Bernstein, E.; Sijen, T.; Hannon, G.J.; Plasterk, R.H.A. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C-elegans. Gene Dev. 2001, 15, 2654–2659. [Google Scholar] [CrossRef]
- Page, A.P.; Johnstone, I.L. The cuticle. Available online: http://www.wormbook.org/chapters/www_cuticle/cuticle.html (accessed on 27 September 2012).
- Welker, N.C.; Pavelec, D.M.; Nix, D.A.; Duchaine, T.F.; Kennedy, S.; Bass, B.L. Dicer's helicase domain is required for accumulation of some, but not all, C. elegans endogenous siRNAs. RNA 2010, 16, 893–903. [Google Scholar] [CrossRef]
- Zhang, H.; Fire, A.Z. Cell autonomous specification of temporal identity by Caenorhabditis elegans microRNA lin-4. Dev. Biol. 2010, 344, 603–610. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, D.; Smith, M.A.; Zhang, B.; Pan, X. Selection of reliable reference genes in Caenorhabditis elegans for analysis of nanotoxicity. PLoS One 2012. [Google Scholar]
- Zhuang, J.J.; Hunter, C.P. Tissue Specificity of Caenorhabditis elegans Enhanced RNA Interference Mutants. Genetics 2011, 188, 235–237. [Google Scholar] [CrossRef]
- Johnstone, I.L.; Barry, J.D. Temporal reiteration of a precise gene expression pattern during nematode development. EMBO J. 1996, 15, 3633–3639. [Google Scholar]
- Schedin, P.; Hunter, C.P.; Wood, W.B. Autonomy and nonautonomy of sex determination in triploid intersex mosaics of C. elegans. Development 1991, 112, 863–879. [Google Scholar]
- Yanai, I.; Baugh, L.R.; Smith, J.J.; Roehrig, C.; Shen-Orr, S.S.; Claggett, J.M.; Hill, A.A.; Slonim, D.K.; Hunter, C.P. Pairing of competitive and topologically distinct regulatory modules enhances patterned gene expression. Mol. Syst. Biol. 2008, 4, 163. [Google Scholar]
- Karlen, Y.; McNair, A.; Perseguers, S.; Mazza, C.; Mermod, N. Statistical significance of quantitative PCR. BMC Bioinf. 2007, 8, 131. [Google Scholar] [CrossRef]
- Shen, J.; Xie, K.; Xiong, L. Global expression profiling of rice microRNAs by one-tube stem-loop reverse transcription quantitative PCR revealed important roles of microRNAs in abiotic stress responses. Mol. Genet. Genomics 2010, 284, 477–488. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar]
- Timmons, L.; Fire, A. Specific interference by ingested dsRNA. Nature 1998, 395, 854. [Google Scholar] [CrossRef]
- Winston, W.M.; Molodowitch, C.; Hunter, C.P. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science 2002, 295, 2456–2459. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Khvorova, A.; Wolfson, A. New competition in RNA regulation. Nat. Biotechnol 2012, 30, 58–59. [Google Scholar] [CrossRef]
- Karreth, F.A.; Tay, Y.; Perna, D.; Ala, U.; Tan, S.M.; Rust, A.G.; DeNicola, G.; Webster, K.A.; Weiss, D.; Perez-Mancera, P.A.; Krauthammer, M.; Halaban, R.; Provero, P.; Adams, D.J.; Tuveson, D.A.; Pandolfi, P.P. In vivo identification of tumor- suppressive PTEN ceRNAs in an oncogenic BRAF-induced mouse model of melanoma. Cell 2011, 147, 382–395. [Google Scholar] [CrossRef]
- Tay, Y.; Kats, L.; Salmena, L.; Weiss, D.; Tan, S.M.; Ala, U.; Karreth, F.; Poliseno, L.; Provero, P.; Di Cunto, F.; Lieberman, J.; Rigoutsos, I.; Pandolfi, P.P. Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. Cell 2011, 147, 344–357. [Google Scholar] [CrossRef]
- Cesana, M.; Cacchiarelli, D.; Legnini, I.; Santini, T.; Sthandier, O.; Chinappi, M.; Tramontano, A.; Bozzoni, I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 2011, 147, 358–369. [Google Scholar] [CrossRef]
- Sumazin, P.; Yang, X.; Chiu, H.S.; Chung, W.J.; Iyer, A.; Llobet-Navas, D.; Rajbhandari, P.; Bansal, M.; Guarnieri, P.; Silva, J.; Califano, A. An extensive microRNA-mediated network of RNA-RNA interactions regulates established oncogenic pathways in glioblastoma. Cell 2011, 147, 370–381. [Google Scholar] [CrossRef]
- Leuschner, F.; Dutta, P.; Gorbatov, R.; Novobrantseva, T.I.; Donahoe, J.S.; Courties, G.; Lee, K.M.; Kim, J.I.; Markmann, J.F.; Marinelli, B.; Panizzi, P.; Lee, W.W.; Iwamoto, Y.; Milstein, S.; Epstein-Barash, H.; Cantley, W.; Wong, J.; Cortez-Retamozo, V.; Newton, A.; Love, K.; Libby, P.; Pittet, M.J.; Swirski, F.K.; Koteliansky, V.; Langer, R.; Weissleder, R.; Anderson, D.G.; Nahrendorf, M. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat. Biotechnol. 2011, 29, 1005–1010. [Google Scholar]
- Stein, P.; Svoboda, P.; Anger, M.; Schultz, R.M. RNAi: mammalian oocytes do it without RNA-dependent RNA polymerase. RNA 2003, 9, 187–192. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhuang, J.J.; Hunter, C.P. The Influence of Competition Among C. elegans Small RNA Pathways on Development. Genes 2012, 3, 671-685. https://doi.org/10.3390/genes3040671
Zhuang JJ, Hunter CP. The Influence of Competition Among C. elegans Small RNA Pathways on Development. Genes. 2012; 3(4):671-685. https://doi.org/10.3390/genes3040671
Chicago/Turabian StyleZhuang, Jimmy J., and Craig P. Hunter. 2012. "The Influence of Competition Among C. elegans Small RNA Pathways on Development" Genes 3, no. 4: 671-685. https://doi.org/10.3390/genes3040671



