Cancer-Specific Telomerase Reverse Transcriptase (TERT) Promoter Mutations: Biological and Clinical Implications
Abstract
:1. Introduction
2. TERT Transcription: Aberrant Activation in Cancer
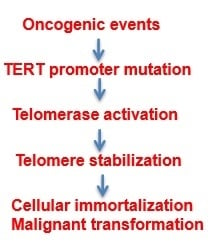
3. TERT Promoter Mutations: Novel Mechanism for Telomerase Activation in Malignant Transformation
4. TERT Promoter Mutations: Novel Biomarkers for Cancer Diagnostics/Screening
5. TERT Promoter Mutations: Novel Prognostic Factors in Cancer Patients
6. Perspectives
Acknowledgments
Conflicts of Interest
References
- Bernardes de Jesus, B.; Blasco, M.A. Telomerase at the intersection of cancer and aging. Trends Genet. 2013, 29, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Zheng, C.; Xu, D. Telomerase as a “stemness” enzyme. Sci. China Life Sci. 2014, 57, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, A.; Hanson, P.S.; Morris, C.M.; Saretzki, G. Telomerase activity is downregulated early during human brain development. Genes 2016. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Goldkorn, A. Telomere and telomerase therapeutics in cancer. Genes 2016. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.W.; Hodis, E.; Xu, M.J.; Kryukov, G.V.; Chin, L.; Garraway, L.A. Highly recurrent TERT promoter mutations in human melanoma. Science 2013, 339, 957–959. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Figl, A.; Rachakonda, P.S.; Fischer, C.; Sucker, A.; Gast, A.; Kadel, S.; Moll, I.; Nagore, E.; Hemminki, K.; et al. TERT promoter mutations in familial and sporadic melanoma. Science 2013, 339, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Killela, P.J.; Reitman, Z.J.; Jiao, Y.; Bettegowda, C.; Agrawal, N.; Diaz, L.A., Jr.; Friedman, A.H.; Friedman, H.; Gallia, G.L.; Giovanella, B.C.; et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc. Natl. Acad. Sci. USA 2013, 110, 6021–6026. [Google Scholar] [CrossRef] [PubMed]
- Aisner, D.L.; Wright, W.E.; Shay, J.W. Telomerase regulation: Not just flipping the switch. Curr. Opin. Genet. Dev. 2002, 12, 80–85. [Google Scholar] [CrossRef]
- Kyo, S.; Inoue, M. Complex regulatory mechanisms of telomerase activity in normal and cancer cells: How can we apply them for cancer therapy? Oncogene 2002, 21, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.S.; Wen, J.; Bacchetti, S. The human telomerase catalytic subunit hTERT: Organization of the gene and characterization of the promoter. Hum. Mol. Genet. 1999, 8, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, I.; Cable, P.L.; Afshari, C.; Barrett, J.C. Cloning and characterization of the promoter region of human telomerase reverse transcriptase gene. Cancer Res. 1999, 59, 826–830. [Google Scholar] [PubMed]
- Wick, M.; Zubov, D.; Hagen, G. Genomic organization and promoter characterization of the gene encoding the human telomerase reverse transcriptase (hTERT). Gene 1999, 232, 97–106. [Google Scholar] [CrossRef]
- Takakura, M.; Kyo, S.; Kanaya, T.; Hirano, H.; Takeda, J.; Yutsudo, M.; Inoue, M. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999, 59, 551–557. [Google Scholar] [PubMed]
- Liu, L.; Lai, S.; Andrews, L.G.; Tollefsbol, T.O. Genetic and epigenetic modulation of telomerase activity in development and disease. Gene 2004, 340, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Liu, C.; Bjorkholm, M.; Gruber, A.; Xu, D. Mitogen-activated protein kinase cascade-mediated histone H3 phosphorylation is critical for telomerase reverse transcriptase expression/telomerase activation induced by proliferation. Mol. Cell. Biol. 2006, 26, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Popov, N.; Hou, M.; Wang, Q.; Bjorkholm, M.; Gruber, A.; Menkel, A.R.; Henriksson, M. Switch from Myc/Max to Mad1/Max binding and decrease in histone acetylation at the telomerase reverse transcriptase promoter during differentiation of HL60 cells. Proc. Natl. Acad. Sci. USA 2001, 98, 3826–3831. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Li, W.; Wang, N.; Liu, C.; Zhu, Q.; Bjorkholm, M.; Gruber, A.; Xu, D. Chromatin remodeling: Recruitment of histone demethylase RBP2 by Mad1 for transcriptional repression of a Myc target gene, telomerase reverse transcriptase. FASEB J. 2010, 24, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Casillas, M.A.; Brotherton, S.L.; Andrews, L.G.; Ruppert, J.M.; Tollefsbol, T.O. Induction of endogenous telomerase (hTERT) by c-Myc in WI-38 fibroblasts transformed with specific genetic elements. Gene 2003, 316, 57–65. [Google Scholar] [CrossRef]
- Jagadeesh, S.; Kyo, S.; Banerjee, P.P. Genistein represses telomerase activity via both transcriptional and posttranslational mechanisms in human prostate cancer cells. Cancer Res. 2006, 66, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Cassar, L.; Li, H.; Pinto, A.R.; Nicholls, C.; Bayne, S.; Liu, J.P. Bone morphogenetic protein-7 inhibits telomerase activity, telomere maintenance, and cervical tumor growth. Cancer Res. 2008, 68, 9157–9166. [Google Scholar] [CrossRef] [PubMed]
- Gewin, L.; Myers, H.; Kiyono, T.; Galloway, D.A. Identification of a novel telomerase repressor that interacts with the human papillomavirus type-16 E6/E6-AP complex. Genes Dev. 2004, 18, 2269–2282. [Google Scholar] [CrossRef] [PubMed]
- Koshiji, M.; Kageyama, Y.; Pete, E.A.; Horikawa, I.; Barrett, J.C.; Huang, L.E. HIF-1α induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004, 23, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Lafferty-Whyte, K.; Bilsland, A.; Hoare, S.F.; Burns, S.; Zaffaroni, N.; Cairney, C.J.; Keith, W.N. TCEAL7 inhibition of c-Myc activity in alternative lengthening of telomeres regulates hTERT expression. Neoplasia 2010, 12, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Wang, L.; Li, Q.; Li, W.; Bjorkholm, M.; Jia, J.; Xu, D. FoxM1 is up-regulated in gastric cancer and its inhibition leads to cellular senescence, partially dependent on p27 kip1. J. Pathol. 2009, 218, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Tollefsbol, T.O. Glucose restriction can extend normal cell lifespan and impair precancerous cell growth through epigenetic control of hTERT and p16 expression. FASEB J. 2010, 24, 1442–1453. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Dwyer, J.; Li, H.; Duan, W.; Liu, J.P. Ets2 maintains hTERT gene expression and breast cancer cell proliferation by interacting with c-Myc. J. Biol. Chem. 2008, 283, 23567–23580. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.C.; Hawkins, A.L.; Barrett, J.F.; Griffin, C.A.; Dang, C.V. Arsenic inhibition of telomerase transcription leads to genetic instability. J. Clin. Invest. 2001, 108, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Dimri, G.P.; Martinez, J.L.; Jacobs, J.J.; Keblusek, P.; Itahana, K.; Van Lohuizen, M.; Campisi, J.; Wazer, D.E.; Band, V. The Bmi-1 oncogene induces telomerase activity and immortalizes human mammary epithelial cells. Cancer Res. 2002, 62, 4736–4745. [Google Scholar] [PubMed]
- Yang, H.; Ou, C.C.; Feldman, R.I.; Nicosia, S.V.; Kruk, P.A.; Cheng, J.Q. Aurora-A kinase regulates telomerase activity through c-Myc in human ovarian and breast epithelial cells. Cancer Res. 2004, 64, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Sitaram, R.T.; Cairney, C.J.; Grabowski, P.; Keith, W.N.; Hallberg, B.; Ljungberg, B.; Roos, G. The PTEN regulator DJ-1 is associated with hTERT expression in clear cell renal cell carcinoma. Int. J. Cancer 2009, 125, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Toh, L.; Lau, P.; Wang, X. Human telomerase reverse transcriptase (hTERT) is a novel target of the Wnt/β-catenin pathway in human cancer. J. Biol. Chem. 2012, 287, 32494–32511. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.J.; Hoare, S.F.; Ashcroft, M.; Bilsland, A.E.; Keith, W.N. Hypoxic regulation of telomerase gene expression by transcriptional and post-transcriptional mechanisms. Oncogene 2006, 25, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Maida, Y.; Kyo, S.; Kanaya, T.; Wang, Z.; Yatabe, N.; Tanaka, M.; Nakamura, M.; Ohmichi, M.; Gotoh, N.; Murakami, S.; et al. Direct activation of telomerase by EGF through Ets-mediated transactivation of TERT via MAP kinase signaling pathway. Oncogene 2002, 21, 4071–4079. [Google Scholar] [CrossRef] [PubMed]
- Won, J.; Yim, J.; Kim, T.K. Opposing regulatory roles of E2F in human telomerase reverse transcriptase (hTERT) gene expression in human tumor and normal somatic cells. FASEB J. 2002, 16, 1943–1945. [Google Scholar] [CrossRef] [PubMed]
- Lou, F.; Chen, X.; Jalink, M.; Zhu, Q.; Ge, N.; Zhao, S.; Fang, X.; Fan, Y.; Bjorkholm, M.; Liu, Z.; et al. The opposing effect of hypoxia-inducible factor-2alpha on expression of telomerase reverse transcriptase. Mol. Cancer Res. 2007, 5, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeyer, K.; Raggioli, A.; Rudloff, S.; Anton, R.; Hierholzer, A.; del Valle, I.; Hein, K.; Vogt, R.; Kemler, R. Wnt/β-catenin signaling regulates telomerase in stem cells and cancer cells. Science 2012, 336, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Wang, X.; Popov, N.; Zhang, A.; Zhao, X.; Zhou, R.; Zetterberg, A.; Bjorkholm, M.; Henriksson, M.; Gruber, A.; et al. The histone deacetylase inhibitor trichostatin A derepresses the telomerase reverse transcriptase (hTERT) gene in human cells. Exp. Cell Res. 2002, 274, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Takakura, M.; Kyo, S.; Sowa, Y.; Wang, Z.; Yatabe, N.; Maida, Y.; Tanaka, M.; Inoue, M. Telomerase activation by histone deacetylase inhibitor in normal cells. Nucleic Acids Res. 2001, 29, 3006–3011. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.S.; Bacchetti, S. Histone deacetylation is involved in the transcriptional repression of hTERT in normal human cells. J. Biol. Chem. 2000, 275, 35665–35668. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fang, X.; Ge, Z.; Jalink, M.; Kyo, S.; Bjorkholm, M.; Gruber, A.; Sjoberg, J.; Xu, D. The telomerase reverse transcriptase (hTERT) gene is a direct target of the histone methyltransferase SMYD3. Cancer Res. 2007, 67, 2626–2631. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhao, Y.; Wang, S. Chromatin and epigenetic regulation of the telomerase reverse transcriptase gene. Protein Cell 2010, 1, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Bellon, M.; Nicot, C. Regulation of telomerase and telomeres: Human tumor viruses take control. J. Natl. Cancer Inst. 2008, 100, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Bellon, M.; Nicot, C. Central role of PI3K in transcriptional activation of hTERT in HTLV-I-infected cells. Blood 2008, 112, 2946–2955. [Google Scholar] [CrossRef] [PubMed]
- Straat, K.; Liu, C.; Rahbar, A.; Zhu, Q.; Liu, L.; Wolmer-Solberg, N.; Lou, F.; Liu, Z.; Shen, J.; Jia, J.; et al. Activation of telomerase by human cytomegalovirus. J. Natl. Cancer Inst. 2009, 101, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Roberts, J.; Dakic, A.; Zhang, Y.; Schlegel, R. HPV E7 contributes to the telomerase activity of immortalized and tumorigenic cells and augments E6-induced hTERT promoter function. Virology 2008, 375, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yuan, H.; Fu, B.; Disbrow, G.L.; Apolinario, T.; Tomaic, V.; Kelley, M.L.; Baker, C.C.; Huibregtse, J.; Schlegel, R. The E6AP ubiquitin ligase is required for transactivation of the hTERT promoter by the human papillomavirus E6 oncoprotein. J. Biol. Chem. 2005, 280, 10807–10816. [Google Scholar] [CrossRef] [PubMed]
- Hosler, G.A.; Davoli, T.; Mender, I.; Litzner, B.; Choi, J.; Kapur, P.; Shay, J.W.; Wang, R.C. A primary melanoma and its asynchronous metastasis highlight the role of BRAF, CDKN2A, and TERT. J. Cutan. Pathol. 2015, 42, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Egberts, F.; Bohne, A.S.; Kruger, S.; Hedderich, J.; Rompel, R.; Haag, J.; Rocken, C.; Hauschild, A. Varying mutational alterations in multiple primary melanomas. J. Mol. Diagn. 2016, 18, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Egberts, F.; Kruger, S.; Behrens, H.M.; Bergner, I.; Papaspyrou, G.; Werner, J.A.; Alkatout, I.; Haag, J.; Hauschild, A.; Rocken, C. Melanomas of unknown primary frequently harbor TERT-promoter mutations. Melanoma Res. 2014, 24, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Griewank, K.G.; Murali, R.; Puig-Butille, J.A.; Schilling, B.; Livingstone, E.; Potrony, M.; Carrera, C.; Schimming, T.; Moller, I.; Schwamborn, M.; et al. TERT promoter mutation status as an independent prognostic factor in cutaneous melanoma. J. Natl. Cancer Inst. 2014. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, B.; Nagore, E.; Rachakonda, P.S.; Garcia-Casado, Z.; Requena, C.; Traves, V.; Becker, J.; Soufir, N.; Hemminki, K.; Kumar, R. Telomerase reverse transcriptase promoter mutations in primary cutaneous melanoma. Nat. Commun. 2014. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, A.E.; Ober, K.; Dubbink, H.J.; Paridaens, D.; Naus, N.C.; Belunek, S.; Krist, B.; Post, E.; Zwarthoff, E.C.; de Klein, A.; et al. Prevalence and implications of TERT promoter mutation in uveal and conjunctival melanoma and in benign and premalignant conjunctival melanocytic lesions. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6024–6030. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Barnhill, R.L.; Dummer, R.; Dalton, J.; Wu, J.; Pappo, A.; Bahrami, A. TERT promoter mutations are predictive of aggressive clinical behavior in patients with spitzoid melanocytic neoplasms. Sci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed]
- Macerola, E.; Loggini, B.; Giannini, R.; Garavello, G.; Giordano, M.; Proietti, A.; Niccoli, C.; Basolo, F.; Fontanini, G. Coexistence of TERT promoter and BRAF mutations in cutaneous melanoma is associated with more clinicopathological features of aggressiveness. Virchows Arch. 2015, 467, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Nagore, E.; Heidenreich, B.; Requena, C.; Casado, Z.G.; Martorell-Calatayud, A.; Pont-Sanjuan, V.; Ana, J.I.; Kumar, R. TERT promoter mutations associate with fast growing melanoma. Pigment Cell Melanoma Res. 2015, 29, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Shain, A.H.; Yeh, I.; Kovalyshyn, I.; Sriharan, A.; Talevich, E.; Gagnon, A.; Dummer, R.; North, J.; Pincus, L.; Ruben, B.; et al. The genetic evolution of melanoma from precursor lesions. N. Engl. J. Med. 2015, 373, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, J.; Almeida, A.; Populo, H.; Batista, R.; Lyra, J.; Pinto, V.; Coelho, R.; Celestino, R.; Prazeres, H.; Lima, L.; et al. Frequency of TERT promoter mutations in human cancers. Nat. Commun. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arita, H.; Narita, Y.; Fukushima, S.; Tateishi, K.; Matsushita, Y.; Yoshida, A.; Miyakita, Y.; Ohno, M.; Collins, V.P.; Kawahara, N.; et al. Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 2013, 126, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Arita, H.; Narita, Y.; Takami, H.; Fukushima, S.; Matsushita, Y.; Yoshida, A.; Miyakita, Y.; Ohno, M.; Shibui, S.; Ichimura, K. TERT promoter mutations rather than methylation are the main mechanism for TERT upregulation in adult gliomas. Acta Neuropathol. 2013, 126, 939–941. [Google Scholar] [CrossRef] [PubMed]
- Brat, D.J.; Verhaak, R.G.; Aldape, K.D.; Yung, W.K.; Salama, S.R.; Cooper, L.A.; Rheinbay, E.; Miller, C.R.; Vitucci, M.; Morozova, O.; et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N. Engl. J. Med. 2015, 372, 2481–2498. [Google Scholar] [PubMed]
- Chen, Y.L.; Jeng, Y.M.; Chang, C.N.; Lee, H.J.; Hsu, H.C.; Lai, P.L.; Yuan, R.H. TERT promoter mutation in resectable hepatocellular carcinomas: A strong association with hepatitis C infection and absence of hepatitis B infection. Int. J. Surg. 2014, 12, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Han, S.; Meng, L.; Li, Z.; Zhang, X.; Wu, A. TERT promoter mutations lead to high transcriptional activity under hypoxia and temozolomide treatment and predict poor prognosis in gliomas. PLoS ONE 2014, 9, e100297. [Google Scholar] [CrossRef] [PubMed]
- Eckel-Passow, J.E.; Lachance, D.H.; Molinaro, A.M.; Walsh, K.M.; Decker, P.A.; Sicotte, H.; Pekmezci, M.; Rice, T.; Kosel, M.L.; Smirnov, I.V.; et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N. Engl. J. Med. 2015, 372, 2499–2508. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Li, G.; Qu, Y.; Wang, M.; Cui, B.; Ji, M.; Shi, B.; Hou, P. TERT promoter mutations and long telomere length predict poor survival and radiotherapy resistance in gliomas. Oncotarget 2016, 7, 8712–8725. [Google Scholar] [PubMed]
- Killela, P.J.; Pirozzi, C.J.; Healy, P.; Reitman, Z.J.; Lipp, E.; Rasheed, B.A.; Yang, R.; Diplas, B.H.; Wang, Z.; Greer, P.K.; et al. Mutations in IDH1, IDH2, and in the TERT promoter define clinically distinct subgroups of adult malignant gliomas. Oncotarget 2014, 5, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Koelsche, C.; Sahm, F.; Capper, D.; Reuss, D.; Sturm, D.; Jones, D.T.; Kool, M.; Northcott, P.A.; Wiestler, B.; Bohmer, K.; et al. Distribution of TERT promoter mutations in pediatric and adult tumors of the nervous system. Acta Neuropathol. 2013, 126, 907–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labussiere, M.; Di Stefano, A.L.; Gleize, V.; Boisselier, B.; Giry, M.; Mangesius, S.; Bruno, A.; Paterra, R.; Marie, Y.; Rahimian, A.; et al. TERT promoter mutations in gliomas, genetic associations and clinico-pathological correlations. Br. J. Cancer 2014, 111, 2024–2032. [Google Scholar] [CrossRef] [PubMed]
- Lotsch, D.; Ghanim, B.; Laaber, M.; Wurm, G.; Weis, S.; Lenz, S.; Webersinke, G.; Pichler, J.; Berger, W.; Spiegl-Kreinecker, S. Prognostic significance of telomerase-associated parameters in glioblastoma: Effect of patient age. Neuro Oncol. 2015, 15, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Nencha, U.; Rahimian, A.; Giry, M.; Sechi, A.; Mokhtari, K.; Polivka, M.; Schmitt, Y.; Di Stefano, A.L.; Alentorn, A.; Labussiere, M.; et al. TERT promoter mutations and rs2853669 polymorphism: Prognostic impact and interactions with common alterations in glioblastomas. J. Neurooncol. 2016, 126, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Nonoguchi, N.; Ohta, T.; Oh, J.E.; Kim, Y.H.; Kleihues, P.; Ohgaki, H. TERT promoter mutations in primary and secondary glioblastomas. Acta Neuropathol. 2013, 126, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Hosen, I.; Gousias, K.; Rachakonda, S.; Heidenreich, B.; Gessi, M.; Schramm, J.; Hemminki, K.; Waha, A.; Kumar, R. TERT promoter mutations: A novel independent prognostic factor in primary glioblastomas. Neuro Oncol. 2015, 17, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Spiegl-Kreinecker, S.; Lotsch, D.; Ghanim, B.; Pirker, C.; Mohr, T.; Laaber, M.; Weis, S.; Olschowski, A.; Webersinke, G.; Pichler, J.; et al. Prognostic quality of activating TERT promoter mutations in glioblastoma: Interaction with the rs2853669 polymorphism and patient age at diagnosis. Neuro Oncol. 2015, 17, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.L.; Chan, A.K.; Chen, L.C.; Tang, C.; Zhang, Z.Y.; Ding, X.J.; Wang, Y.; Sun, C.R.; Ng, H.K.; Yao, Y.; et al. TERT promoter mutated WHO grades II and III gliomas are located preferentially in the frontal lobe and avoid the midline. Int. J. Clin. Exp. Pathol. 2015, 8, 11485–11494. [Google Scholar] [PubMed]
- Abedalthagafi, M.S.; Bi, W.L.; Merrill, P.H.; Gibson, W.J.; Rose, M.F.; Du, Z.; Francis, J.M.; Du, R.; Dunn, I.F.; Ligon, A.H.; et al. ARID1A and TERT promoter mutations in dedifferentiated meningioma. Cancer Genet. 2015, 208, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Goutagny, S.; Nault, J.C.; Mallet, M.; Henin, D.; Rossi, J.Z.; Kalamarides, M. High incidence of activating TERT promoter mutations in meningiomas undergoing malignant progression. Brain Pathol. 2014, 24, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Sahm, F.; Schrimpf, D.; Olar, A.; Koelsche, C.; Reuss, D.; Bissel, J.; Kratz, A.; Capper, D.; Schefzyk, S.; Hielscher, T.; et al. TERT Promoter Mutations and Risk of Recurrence in Meningioma. J. Natl. Cancer Inst. 2015. [Google Scholar] [CrossRef] [PubMed]
- Allory, Y.; Beukers, W.; Sagrera, A.; Flandez, M.; Marques, M.; Marquez, M.; van der Keur, K.A.; Dyrskjot, L.; Lurkin, I.; Vermeij, M.; et al. Telomerase teverse transcriptase promoter mutations in bladder cancer: High frequency across stages, detection in urine, and lack of association with outcome. Eur. Urol. 2014, 65, 360–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurst, C.D.; Platt, F.M.; Knowles, M.A. Comprehensive mutation analysis of the TERT promoter in bladder cancer and detection of mutations in voided urine. Eur. Urol. 2014, 65, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, G.; Hartmann, C.; Xing, M. Highly prevalent TERT promoter mutations in bladder cancer and brain gliobastoma. Cell Cycle 2013, 12, 1637–1638. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, T.; Liu, C.; Meng, Y.; Yuan, X.; Liu, L.; Ge, N.; Liu, J.; Wang, C.; Ren, H.; et al. TERT promoter mutations and TERT mRNA but not FGFR3 mutations are urinary biomarkers in Han Chinese patients with urothelial bladder cancer. Oncologist 2015, 20, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Hosen, I.; Rachakonda, P.S.; Heidenreich, B.; de Verdier, P.J.; Ryk, C.; Steineck, G.; Hemminki, K.; Kumar, R. Mutations in TERT promoter and FGFR3 and telomere length in bladder cancer. Int. J. Cancer 2015, 137, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Rachakonda, P.S.; Hosen, I.; de Verdier, P.J.; Fallah, M.; Heidenreich, B.; Ryk, C.; Wiklund, N.P.; Steineck, G.; Schadendorf, D.; Hemminki, K.; et al. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc. Natl. Acad. Sci. USA 2013, 110, 17426–17431. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Kim, Y.; Jeon, S.; Kim, S.H.; Kim, T.J.; Lee, S.; Kim, M.H.; Lim, D.J.; Lee, Y.S.; Jung, C.K. Clinical utility of TERT promoter mutations and ALK rearrangement in thyroid cancer patients with a high prevalence of the BRAF V600E mutation. Diagn. Pathol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bullock, M.; Ren, Y.; O’Neill, C.; Gill, A.; Aniss, A.; Sywak, M.; Sidhu, S.; Delbridge, L.; Learoyd, D.; de Vathaire, F.; et al. TERT Promoter Mutations Are a Major Indicator of Recurrence and Death due to Papillary Thyroid Carcinomas. Clin. Endocrinol. 2015. [Google Scholar] [CrossRef]
- Chindris, A.M.; Casler, J.D.; Bernet, V.J.; Rivera, M.; Thomas, C.; Kachergus, J.M.; Necela, B.M.; Hay, I.D.; Westphal, S.A.; Grant, C.S.; et al. Clinical and molecular features of Hurthle cell carcinoma of the thyroid. J. Clin. Endocrinol. Metab. 2015, 100, 55–62. [Google Scholar] [CrossRef] [PubMed]
- De Biase, D.; Gandolfi, G.; Ragazzi, M.; Eszlinger, M.; Sancisi, V.; Gugnoni, M.; Visani, M.; Pession, A.; Casadei, G.; Durante, C.; et al. TERT Promoter Mutations in Papillary Thyroid Microcarcinomas. Thyroid 2015, 25, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.T.; Yu, K.; Lu, R.Q.; Li, X.; Xu, J.; Lei, M.; Li, H.; Wang, Y.; Liu, Z. Clinicopathological significance of TERT promoter mutation in papillary thyroid carcinomas: A systematic review and meta-analysis. Clin. Endocrinol. 2016. [Google Scholar] [CrossRef]
- Dettmer, M.S.; Schmitt, A.; Steinert, H.; Capper, D.; Moch, H.; Komminoth, P.; Perren, A. Tall cell papillary thyroid carcinoma. new diagnostic criteria and mutations in BRAF and TERT. Endocr. Relat. Cancer 2015, 22, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, G.; Ragazzi, M.; Frasoldati, A.; Piana, S.; Ciarrocchi, A.; Sancisi, V. TERT promoter mutations are associated with distant metastases in papillary thyroid carcinoma. Eur. J. Endocrinol. 2015, 172, 403–413. [Google Scholar] [CrossRef] [PubMed]
- George, J.R.; Henderson, Y.C.; Williams, M.D.; Roberts, D.B.; Hei, H.; Lai, S.Y.; Clayman, G.L. Association of TERT Promoter Mutation, But Not BRAF Mutation, With Increased Mortality in PTC. J. Clin. Endocrinol. Metab. 2015, 100, E1550–E1559. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, Y.; Jeon, S.; Bae, J.S.; Jung, S.L.; Jung, C.K. Cytologic, clinicopathologic, and molecular features of papillary thyroid carcinoma with prominent hobnail features: 10 case reports and systematic literature review. Int. J. Clin. Exp. Pathol. 2015, 8, 7988–7997. [Google Scholar] [PubMed]
- Liu, T.; Brown, T.C.; Juhlin, C.C.; Andreasson, A.; Wang, N.; Backdahl, M.; Healy, J.M.; Prasad, M.L.; Korah, R.; Carling, T.; et al. The activating TERT promoter mutation C228T is recurrent in subsets of adrenal tumors. Endocr. Relat. Cancer 2014, 21, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, N.; Cao, J.; Dinets, A.; Sofiadis, A.; Zedenius, J.; Larsson, C.; Xu, D. The age- and shorter telomere-dependent TERT promoter mutation in follicular thyroid cell-derived carcinomas. Oncogene 2014, 33, 4978–4984. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bishop, J.; Shan, Y.; Pai, S.; Liu, D.; Murugan, A.K.; Sun, H.; El-Naggar, A.; Xing, M. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr. Relat. Cancer 2013, 20, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qu, S.; Liu, R.; Sheng, C.; Shi, X.; Zhu, G.; Murugan, A.K.; Guan, H.; Yu, H.; Wang, Y.; et al. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. J. Clin. Endocrinol. Metab. 2014, 99, E1130–E1136. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.; da Rocha, A.G.; Vinagre, J.; Batista, R.; Peixoto, J.; Tavares, C.; Celestino, R.; Almeida, A.; Salgado, C.; Eloy, C.; et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab. 2014, 99, E754–E765. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.K.; Kwak, B.K.; Lim, J.A.; Lee, M.C.; Kim, M.J. Promoter Mutations and Tumor Persistence/Recurrence in Papillary Thyroid Cancer. Cancer Res. Treat. 2015. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Liu, R.; Qu, S.; Zhu, G.; Bishop, J.; Liu, X.; Sun, H.; Shan, Z.; Wang, E.; Luo, Y.; et al. Association of TERT promoter mutation 1,295,228 C>T with BRAF V600E mutation, older patient age, and distant metastasis in anaplastic thyroid cancer. J. Clin. Endocrinol. Metab. 2015, 100, E632–E637. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, T.; Sofiadis, A.; Juhlin, C.C.; Zedenius, J.; Hoog, A.; Larsson, C.; Xu, D. TERT promoter mutation as an early genetic event activating telomerase in follicular thyroid adenoma (FTA) and atypical FTA. Cancer 2014, 120, 2965–2979. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Liu, R.; Liu, X.; Murugan, A.K.; Zhu, G.; Zeiger, M.A.; Pai, S.; Bishop, J. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J. Clin. Oncol. 2015, 32, 2718–2726. [Google Scholar] [CrossRef] [PubMed]
- Cevik, D.; Yildiz, G.; Ozturk, M. Common telomerase reverse transcriptase promoter mutations in hepatocellular carcinomas from different geographical locations. World J. Gastroenterol. 2015, 21, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Zheng, S.; Dong, K. TERT promoter mutation during development of hepatoblastoma to hepatocellular carcinoma. J. Hepatol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.C.; Mallet, M.; Pilati, C.; Calderaro, J.; Bioulac-Sage, P.; Laurent, C.; Laurent, A.; Cherqui, D.; Balabaud, C.; Zucman-Rossi, J. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat. Commun. 2013. [Google Scholar] [CrossRef] [PubMed]
- Tallet, A.; Nault, J.C.; Renier, A.; Hysi, I.; Galateau-Salle, F.; Cazes, A.; Copin, M.C.; Hofman, P.; Andujar, P.; le Pimpec-Barthes, F.; et al. Overexpression and promoter mutation of the TERT gene in malignant pleural mesothelioma. Oncogene 2014, 33, 3748–3752. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Ogawa, R.; Yoshida, H.; Maeshima, A.; Kanai, Y.; Kinoshita, T.; Hiraoka, N.; Sekine, S. TERT promoter mutations are frequent and show association with MED12 mutations in phyllodes tumors of the breast. Br. J. Cancer 2015, 113, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Panero, J.; Alves-Paiva, R.M.; Roisman, A.; Santana-Lemos, B.A.; Falcao, R.P.; Oliveira, G.; Martins, D.; Stanganelli, C.; Slavutsky, I.; Calado, R.T. Acquired TERT promoter mutations stimulate TERT transcription in mantle cell lymphoma. Am. J. Hematol. 2016, 91, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Koelsche, C.; Renner, M.; Hartmann, W.; Brandt, R.; Lehner, B.; Waldburger, N.; Alldinger, I.; Schmitt, T.; Egerer, G.; Penzel, R.; et al. TERT promoter hotspot mutations are recurrent in myxoid liposarcomas but rare in other soft tissue sarcoma entities. J. Exp. Clin. Cancer Res. 2014. [Google Scholar] [CrossRef] [PubMed]
- Griewank, K.G.; Schilling, B.; Murali, R.; Bielefeld, N.; Schwamborn, M.; Sucker, A.; Zimmer, L.; Hillen, U.; Schaller, J.; Brenn, T.; et al. TERT promoter mutations are frequent in atypical fibroxanthomas and pleomorphic dermal sarcomas. Mod. Pathol. 2014, 27, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Campanella, N.C.; Celestino, R.; Pestana, A.; Scapulatempo-Neto, C.; de Oliveira, A.T.; Brito, M.J.; Gouveia, A.; Lopes, J.M.; Guimaraes, D.P.; Soares, P.; et al. Low frequency of TERT promoter mutations in gastrointestinal stromal tumors (GISTs). Eur. J. Hum. Genet. 2015, 23, 877–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J. Clin. Investig. 2016, 126, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, X.; Chen, Y.; Chen, G.; Ma, Y.; Huang, K.; Zhang, Y.; Zhao, Q.; Winkler, C.A.; An, P.; et al. Telomerase reverse transcriptase promoter mutations in hepatitis B virus-associated hepatocellular carcinoma. Oncotarget 2016, 7, 27838–27847. [Google Scholar] [CrossRef] [PubMed]
- Griewank, K.G.; Murali, R.; Schilling, B.; Schimming, T.; Moller, I.; Moll, I.; Schwamborn, M.; Sucker, A.; Zimmer, L.; Schadendorf, D.; et al. TERT promoter mutations are frequent in cutaneous basal cell carcinoma and squamous cell carcinoma. PLoS ONE 2014, 11, e80354. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.A.; Laughlin, T.S.; Rothberg, P.G. Mutations of the TERT promoter are common in basal cell carcinoma and squamous cell carcinoma. Mod. Pathol. 2014, 27, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Liao, S.L.; Hong, J.B.; Chu, C.Y.; Sheen, Y.S.; Jhuang, J.Y.; Tsai, J.H.; Liau, J.Y. TERT promoter mutations in periocular carcinomas: Implications of ultraviolet light in pathogenesis. Br. J. Ophthalmol. 2016, 100, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Liu, C.; Wang, K.; Liu, L.; Liu, T.; Ge, N.; Kong, F.; Yang, L.; Bjorkholm, M.; Fan, Y.; et al. The genetic difference between Western and Chinese urothelial cell carcinomas: Infrequent FGFR3 mutation in Han Chinese patients. Oncotarget 2016, 7, 25826–25835. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Meng, Y.; Li, P.; Ge, N.; Kong, F.; Yang, L.; Bjorkholm, M.; Zhao, S.; Xu, D. The association between the TERT rs2736100 AC genotype and reduced risk of upper tract urothelial carcinomas in a Han Chinese population. Oncotarget 2016, 7, 31972–31979. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, B.; Rachakonda, P.S.; Hemminki, K.; Kumar, R. TERT promoter mutations in cancer development. Curr. Opin. Genet. Dev. 2014, 24, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Davidson, D.D.; Wang, M.; Lopez-Beltran, A.; Montironi, R.; Wang, L.; Tan, P.H.; MacLennan, G.T.; Williamson, S.R.; Zhang, S. Telomerase reverse transcriptase (TERT) promoter mutation analysis of benign, malignant and reactive urothelial lesions reveals a subpopulation of inverted papilloma with immortalizing genetic change. Histopathology 2016, 69, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, A.; Trimboli, P.; Modica, D.C.; Taffon, C.; Guidobaldi, L.; Taccogna, S.; Rainer, A.; Trombetta, M.; Papini, E.; Zelano, G. Preoperative assessment of TERT promoter mutation on thyroid core needle biopsies supports diagnosis of malignancy and addresses surgical strategy. Horm. Metab. Res. 2016, 48, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Nagore, E.; Heidenreich, B.; Rachakonda, S.; Garcia-Casado, Z.; Requena, C.; Soriano, V.; Frank, C.; Traves, V.; Quecedo, E.; Sanjuan-Gimenez, J.; et al. TERT promoter mutations in melanoma survival. Int. J. Cancer 2016, 139, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Campanella, N.C.; Penna, V.; Abrahao-Machado, L.F.; Cruvinel-Carloni, A.; Ribeiro, G.; Soares, P.; Scapulatempo-Neto, C.; Reis, R.M. TERT promoter mutations in soft tissue sarcomas. Int. J. Biol. Markers 2016, 31, e62–e67. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, T.; Liu, L.; Liu, J.; Liu, C.; Wang, C.; Ge, N.; Ren, H.; Yan, K.; Hu, S.; et al. TERT promoter mutations in renal cell carcinomas and upper tract urothelial carcinomas. Oncotarget 2014, 5, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.J.; Rube, H.T.; Kreig, A.; Mancini, A.; Fouse, S.D.; Nagarajan, R.P.; Choi, S.; Hong, C.; He, D.; Pekmezci, M.; et al. The transcription factor GABP selectively binds and activates the mutant TERT promoter in cancer. Science 2015, 348, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Chiba, K.; Johnson, J.Z.; Vogan, J.M.; Wagner, T.; Boyle, J.M.; Hockemeyer, D. Cancer-associated TERT promoter mutations abrogate telomerase silencing. eLife 2015, 4, e07918. [Google Scholar] [CrossRef] [PubMed]
- Makowski, M.M.; Willems, E.; Fang, J.; Choi, J.; Zhang, T.; Jansen, P.W.; Brown, K.M.; Vermeulen, M. An interaction proteomics survey of transcription factor binding at recurrent TERT promoter mutations. Proteomics 2015, 16, 417–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stern, J.L.; Theodorescu, D.; Vogelstein, B.; Papadopoulos, N.; Cech, T.R. Mutation of the TERT promoter, switch to active chromatin, and monoallelic TERT expression in multiple cancers. Genes Dev. 2015, 29, 2219–2224. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Liu, T.; Wang, N.; Björnhagen, V.; Höög, A.; Larsson, C.; Lui, W.-O.; Xu, D. TERT promoter mutations and gene amplification: Promoting TERT expression in Merkel cell carcinoma. Oncotarget 2014, 5, 10048–10057. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liang, X.; Bjorkholm, M.; Jia, J.; Xu, D. The absence of TERT promoter mutations in primary gastric cancer. Gene 2014, 540, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Stoehr, R.; Taubert, H.; Zinnall, U.; Giedl, J.; Gaisa, N.T.; Burger, M.; Ruemmele, P.; Hurst, C.D.; Knowles, M.A.; Wullich, B.; et al. Frequency of TERT promoter mutations in prostate cancer. Pathobiology 2015, 82, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Xu, D.; Sofiadis, A.; Hoog, A.; Vukojevic, V.; Backdahl, M.; Zedenius, J.; Larsson, C. Telomerase-dependent and independent telomere maintenance and its clinical implications in medullary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2014, 99, E1571–E1579. [Google Scholar] [CrossRef] [PubMed]
- Vinothkumar, V.; Arunkumar, G.; Revathidevi, S.; Arun, K.; Manikandan, M.; Rao, A.K.; Rajkumar, K.S.; Ajay, C.; Rajaraman, R.; Ramani, R.; et al. TERT promoter hot spot mutations are frequent in Indian cervical and oral squamous cell carcinomas. Tumour Biol. 2016, 37, 7907–7913. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Huang, P.; Li, C.; Huang, Y.; Li, X.; Wang, Y.; Chen, C.; Lv, Z.; Tang, A.; Sun, X.; et al. Telomerase reverse transcriptase gene promoter mutations help discern the origin of urogenital tumors: A genomic and molecular study. Eur. Urol. 2014, 65, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.J.; Kim, W.G.; Sim, S.; Lim, S.; Kwon, H.; Kim, T.Y.; Shong, Y.K.; Kim, W.B. Low prevalence of somatic TERT promoter mutations in classic papillary thyroid carcinoma. Endocrinol. Metab. 2016, 31, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.; da Rocha, A.G.; Vinagre, J.; Sobrinho-Simoes, M.; Soares, P. Coexistence of TERT promoter and BRAF mutations in papillary thyroid carcinoma: Added value in patient prognosis? J. Clin. Oncol. 2015, 33, 667–668. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.A.; Kurtis, B.; Babayeva, S.; Zhuge, J.; Tantchou, I.; Lafaro, R.J.; Fallon, J.T.; Zhong, M. Heterogeneity of TERT promoter mutations status in squamous cell carcinomas of different anatomical sites. Ann. Diagn. Pathol. 2015, 19, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, T.; Ge, N.; Liu, L.; Yuan, X.; Liu, J.; Kong, F.; Wang, C.; Ren, H.; Yan, K.; et al. TERT promoter mutations are associated with distant metastases in upper tract urothelial carcinomas and serve as urinary biomarkers detected by a sensitive castPCR. Oncotarget 2014, 5, 12428–12439. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, Z.; Sun, M.; Chen, K.; Yuan, W.; Jiang, G. The sensitive detection of telomerase reverse transcriptase promoter mutation by amplification refractory mutation system-PCR. Genet. Test Mol. Biomarkers 2016, 20, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xing, M. Diagnostic and prognostic TERT promoter mutations in thyroid fine-needle aspiration biopsy. Endocr. Relat. Cancer 2014, 21, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Xu, F.; Zhang, Y.; Liu, H.; Tao, Y. Association of telomerase reverse transcriptase promoter mutations with the prognosis of glioma patients: A Meta-Analysis. Mol. Neurobiol. 2016, 53, 2726–2732. [Google Scholar] [CrossRef] [PubMed]
- Labussiere, M.; Boisselier, B.; Mokhtari, K.; Di Stefano, A.L.; Rahimian, A.; Rossetto, M.; Ciccarino, P.; Saulnier, O.; Paterra, R.; Marie, Y.; et al. Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology 2014, 83, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, B.; Rachakonda, P.S.; Hosen, I.; Volz, F.; Hemminki, K.; Weyerbrock, A.; Kumar, R. TERT promoter mutations and telomere length in adult malignant gliomas and recurrences. Oncotarget 2015, 6, 10617–10633. [Google Scholar] [CrossRef] [PubMed]
- Vail, E.; Zheng, X.; Zhou, M.; Yang, X.; Fallon, J.T.; Epstein, J.I.; Zhong, M. Telomerase reverse transcriptase promoter mutations in glandular lesions of the urinary bladder. Ann. Diagn. Pathol. 2015, 19, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.N.; Chiang, Y.C.; Cheng, W.F.; Chen, C.A.; Lin, M.C.; Kuo, K.T. Molecular alterations in endometrial and ovarian clear cell carcinomas: Clinical impacts of telomerase reverse transcriptase promoter mutation. Mod. Pathol. 2014, 28, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.C.; Ayhan, A.; Maeda, D.; Kim, K.R.; Clarke, B.A.; Shaw, P.; Chui, M.H.; Rosen, B.; Shih Ie, M.; Wang, T.L. Frequent somatic mutations of the telomerase reverse transcriptase promoter in ovarian clear cell carcinoma but not in other major types of gynaecological malignancy. J. Pathol. 2014, 232, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Dang, S.; Wu, K.; Shao, Y.; Yang, Q.; Ji, M.; Shi, B.; Hou, P. TERT promoter mutations predict worse survival in laryngeal cancer patients. Int. J. Cancer 2014, 135, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Sanchini, M.A.; Gunelli, R.; Nanni, O.; Bravaccini, S.; Fabbri, C.; Sermasi, A.; Bercovich, E.; Ravaioli, A.; Amadori, D.; Calistri, D. Relevance of urine telomerase in the diagnosis of bladder cancer. JAMA 2005, 294, 2052–2056. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, P.; Choufani, S.; Mack, S.; Gallagher, D.; Zhang, C.; Lipman, T.; Zhukova, N.; Walker, E.J.; Martin, D.; Merino, D.; et al. Methylation of the TERT promoter and risk stratification of childhood brain tumours: An integrative genomic and molecular study. Lancet Oncol. 2013, 14, 534–542. [Google Scholar] [CrossRef]
- Ding, D.; Xi, P.; Zhou, J.; Wang, M.; Cong, Y.S. Human telomerase reverse transcriptase regulates MMP expression independently of telomerase activity via NF-kappaB-dependent transcription. FASEB J. 2014, 27, 4375–4383. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Q.; Li, K.; Chen, L.; Li, W.; Hou, M.; Liu, T.; Yang, J.; Lindvall, C.; Bjorkholm, M.; et al. Telomerase reverse transcriptase promotes epithelial-mesenchymal transition and stem cell-like traits in cancer cells. Oncogene 2013, 32, 4203–4213. [Google Scholar] [CrossRef] [PubMed]
- Masutomi, K.; Yu, E.Y.; Khurts, S.; Ben-Porath, I.; Currier, J.L.; Metz, G.B.; Brooks, M.W.; Kaneko, S.; Murakami, S.; DeCaprio, J.A.; et al. Telomerase maintains telomere structure in normal human cells. Cell 2003, 114, 241–253. [Google Scholar] [CrossRef]
- Li, S.; Ferguson, M.J.; Hawkins, C.J.; Smith, C.; Elwood, N.J. Human telomerase reverse transcriptase protects hematopoietic progenitor TF-1 cells from death and quiescence induced by cytokine withdrawal. Leukemia 2006, 20, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sung, Y.H.; Cheong, C.; Choi, Y.S.; Jeon, H.K.; Sun, W.; Hahn, W.C.; Ishikawa, F.; Lee, H.W. TERT promotes cellular and organismal survival independently of telomerase activity. Oncogene 2008, 27, 3754–3760. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, H.; Deb, S.; Liu, J.P. TERT regulates cell survival independent of telomerase enzymatic activity. Oncogene 2002, 21, 3130–3138. [Google Scholar] [CrossRef] [PubMed]
- Ci, X.; Li, B.; Ma, X.; Kong, F.; Zheng, C.; Bjorkholm, M.; Jia, J.; Xu, D. Bortezomib-mediated down-regulation of telomerase and disruption of telomere homeostasis contributes to apoptosis of malignant cells. Oncotarget 2015, 6, 38079–38092. [Google Scholar] [PubMed]
- Liu, T.; Liang, X.; Li, B.; Bjorkholm, M.; Jia, J.; Xu, D. Telomerase reverse transcriptase inhibition stimulates cyclooxygenase 2 expression in cancer cells and synergizes with celecoxib to exert anti-cancer effects. Br. J. Cancer 2013, 108, 2272–2280. [Google Scholar] [CrossRef] [PubMed]
- Luiten, R.M.; Pene, J.; Yssel, H.; Spits, H. Ectopic hTERT expression extends the life span of human CD4+ helper and regulatory T-cell clones and confers resistance to oxidative stress-induced apoptosis. Blood 2003, 101, 4512–4519. [Google Scholar] [CrossRef] [PubMed]
- Saretzki, G. Extra-telomeric functions of human telomerase: Cancer, mitochondria and oxidative stress. Curr. Pharm. Des. 2014, 20, 6386–6403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, B.; de Jonge, N.; Bjorkholm, M.; Xu, D. The DNA methylation inhibitor induces telomere dysfunction and apoptosis of leukemia cells that is attenuated by telomerase over-expression. Oncotarget 2015, 6, 4888–4900. [Google Scholar] [CrossRef] [PubMed]
- Singhapol, C.; Pal, D.; Czapiewski, R.; Porika, M.; Nelson, G.; Saretzki, G.C. Mitochondrial telomerase protects cancer cells from nuclear DNA damage and apoptosis. PLoS ONE 2013, 8, e52989. [Google Scholar] [CrossRef] [PubMed]

| Tumor Type | Mutation Rate (%) | References |
|---|---|---|
| Skin tumor | ||
| Base cell carcinoma | 132/278 (47.4) | [112,113] |
| Squamous cell carcinoma | 75/125 (60.0) | [112,113,114,135] |
| Merkel cell carcinoma | 5/49 (10.2) | [127] |
| pleomorphic dermal sarcoma | 26/34 (76.0) | [108] |
| Atypical fibroxanthoma | 25/27 (93.0) | [108] |
| Malignant melanoma | ||
| Cutaneous melanoma | 564/1287 (43.8) | [5,6,48,49,50,51,54,57] |
| Other types of melanoma | 165/505 (32.7) | [52,55] |
| Brain tumor | ||
| Glioma (low-grade) | 929/2580 (36,0) | [57,59,62,66,67,73] |
| Glioma (high-grade) | 2171/3085 (70.4) | [57,59,62,66,67,69,70,71,72,79] |
| Meningioma | 25/337 (7.4) | [75,76] |
| Medulloblastoma | 36/182 (19.8) | [7,66] |
| Endocrine tumor | ||
| Thyroid cancer | ||
| Papillary thyroid carcinoma | 593/5380 (11.0) | [57,83,84,86,87,88,89,90,91,92,93,94,95,96,97,100,110] |
| Follicular thyroid carcinoma | 59/346 (17.1) | [57,83,92,93,94,95,96,99] |
| Anaplastic thyroid carcinoma | 93/237 (39.2) | [57,83,93,94,96,98] |
| Hurthle cell carcinoma | 8/61 (13.1) | [85] |
| Atypical follicular thyroid adenoma | 3/18 (16.7) | [99] |
| Differentiated thyroid carcinoma | 41/339 (12.1) | [94] |
| Poorly differentiated thyroid carcinoma | 73/170 (42.9) | [57,94,96,110] |
| Adrenocortical carcinoma | 4/98 (4.1) | [57,92] |
| Gynecological tumor | ||
| Ovarian clear cell carcinoma | 48/301 (15.9) | [7,143,144] |
| Ovarian low grade serous | 2/41 (4.9) | [7,144] |
| Endometrial carcinoma | 5/76 (6.6) | [7,143,144] |
| Squamous cell carcinoma of the cervix | 33/335 (9.9) | [7,131,135,144] |
| Urological tumor | ||
| Renal cell carcinoma | 22/318 (6.9) | [57,79,122] |
| Bladder cancer | 946/1511 (62.6) | [7,57,77,78,79,80,81,115] |
| Upper tract urothelial carcinomas | ||
| Renal pelvic carcinoma | 51/117 (43.6) | [7,136] |
| Ureter carcinoma | 23/122 (19) | [136] |
| Digestive system tumor | ||
| Hepatocellular carcinoma | 363/881 (41.2) | [7,61,101,103,111] |
| Gastric cancer | 0/200 (0) | [128] |
| Head and neck tumor | ||
| Laryngeal carcinoma | 64/235 (27.2) | [145] |
| Squamous cell carcinoma of head and neck | 14/86 (16.3) | [7,135] |
| Soft tissue and pleuron tumor | ||
| Myxoid liposarcoma | 50/72 (69.4) | [7,107,109] |
| Solitary fibrous tumour | 14/58 (24.1) | [7,66,107,109] |
| Chondrosarcoma | 1/2 (50) | [7] |
| Fibrosarcoma | 1/3 (33.3) | [7] |
| Malignant pleural mesothelioma | 20/132 (15.2) | [104] |
| Other tumors | ||
| Mantle cell lymphoma | 8/24 (33.3) | [106] |
| Phyllodes tumor | 30/46 (65.0) | [105] |
| Prostate cancer | 108/167 (64.7) | [129] |
| Medullary carcinoma | 0/62 (0) | [83,93,94] |
| pheochromocytoma | 1/105 (1) | [92] |
| Paraganglioma | 1/13 (7.7) | [92] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Yuan, X.; Xu, D. Cancer-Specific Telomerase Reverse Transcriptase (TERT) Promoter Mutations: Biological and Clinical Implications. Genes 2016, 7, 38. https://doi.org/10.3390/genes7070038
Liu T, Yuan X, Xu D. Cancer-Specific Telomerase Reverse Transcriptase (TERT) Promoter Mutations: Biological and Clinical Implications. Genes. 2016; 7(7):38. https://doi.org/10.3390/genes7070038
Chicago/Turabian StyleLiu, Tiantian, Xiaotian Yuan, and Dawei Xu. 2016. "Cancer-Specific Telomerase Reverse Transcriptase (TERT) Promoter Mutations: Biological and Clinical Implications" Genes 7, no. 7: 38. https://doi.org/10.3390/genes7070038